Abstract
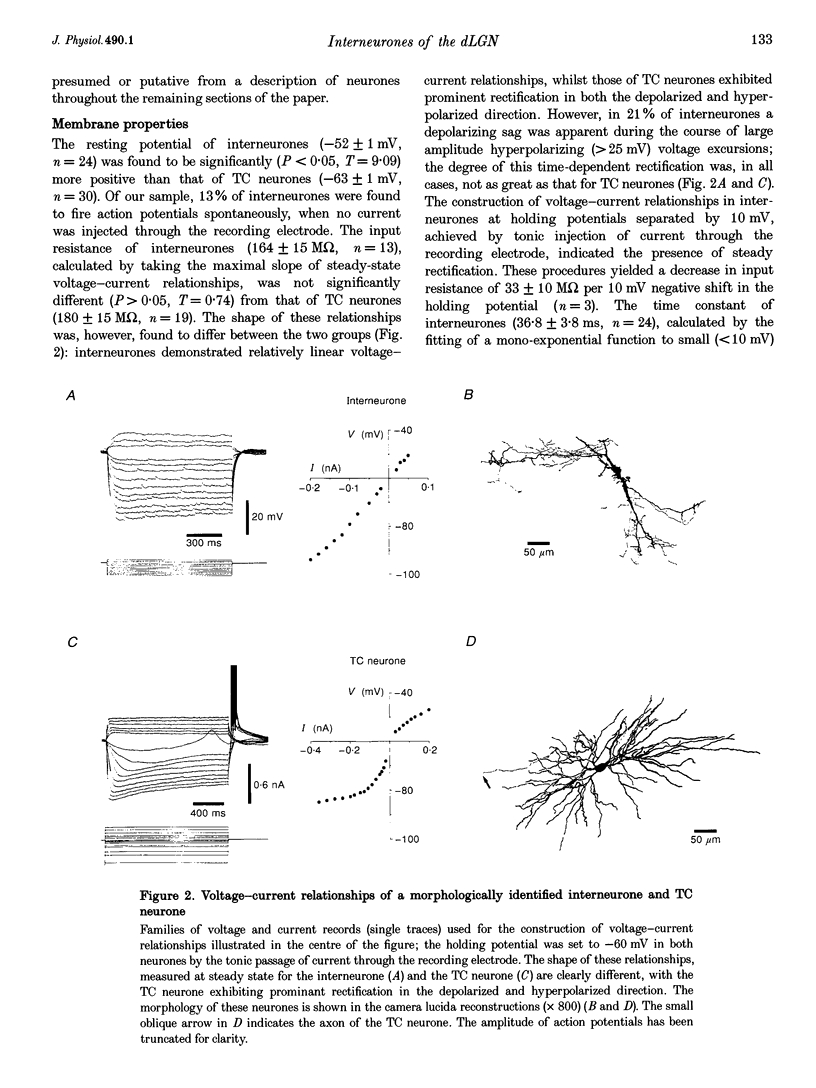
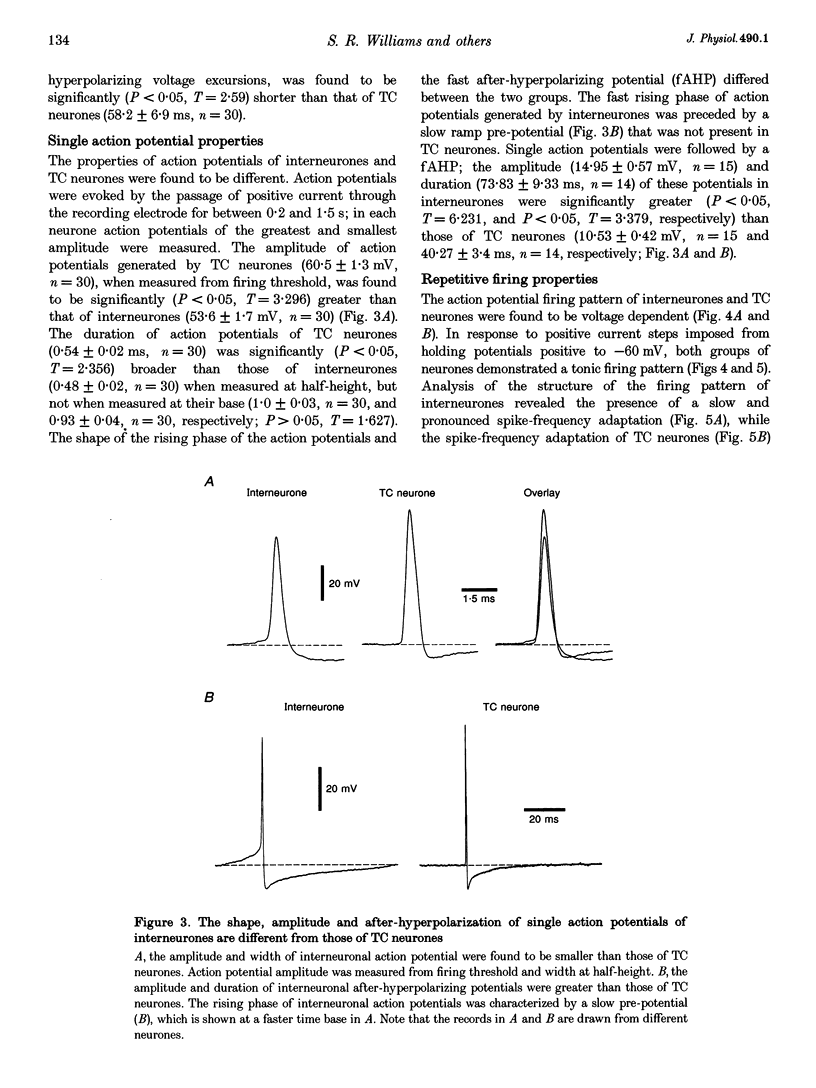
1. Intracellular recordings were made from putative interneurones (n = 24) and thalamocortical (TC) projection neurones (n = 45) in slice preparations of the rat dorsal lateral geniculate nucleus (dLGN) in order to compare the electrophysiological properties of these neuronal types. 2. Intracellular injection of biocytin to electrophysiologically identified neurones (n = 34) revealed the morphology of putative interneurones (n = 4) to be similar to class B and that of TC neurones (n = 30) to be similar to class A Golgi-impregnated neurones. 3. Interneurones had resting membrane potentials (-52 mV) relatively positive to those of TC neurones (-63 mV), shorter time constants (36.8 and 58.2 ms, respectively), but similar steady-state input resistances (164 and 180 M omega, respectively). Steady-state voltage-current relationships were nearly linear in interneurones, but highly non-linear in TC neurones. 4. The structure of action potential firing evoked at the break of hyperpolarizing voltage transients was dependent upon neuronal type. Interneurones fired a single action potential or a burst of action potentials with a maximum frequency of < 130 Hz, whilst TC neurones fired a high frequency burst with a minimum frequency of > 250 Hz. In addition, well-defined burst firing of action potentials in response to depolarizing voltage excursions, from membrane potentials negative to -65 mV, could be evoked in TC neurones, but not in interneurones. 5. The directly evoked action potentials of interneurones were characterized by an initial slow pre-potential preceding the fast upstroke of the action potential. The amplitude and width of interneurones' action potentials were smaller than those of TC neurones and the amplitude and duration of the single action potential after-hyperpolarization were greater in interneurones. Both interneurones and TC neurones fired action potentials repetitively in response to suprathreshold voltage excursions, with interneurones demonstrating a greater degree of spike-frequency adaptation. Following a train of action potentials, interneurones and TC neurones generated a slow after-hyperpolarizing potential: in interneurones but not TC neurones this potential was followed by a slow depolarizing potential. 6. An intrinsic, subthreshold membrane potential oscillatory activity with a mean frequency of approximately 8 Hz was observed in interneurones. 7. Electrical stimulation of the optic tract evoked in interneurones apparently pure EPSPs, pure IPSPs or a mixture of EPSPs and IPSPs. EPSPs were found to be biphasic and mediated by the activation of non-N-methyl-D-aspartate (NMDA) and NMDA excitatory amino acid receptors. IPSPs and the response to the iontophoretic application of GABA were found to reverse between -65 and -70 mV. The application of GABAB receptor agonists failed to affect the membrane properties of six of seven interneurones tested. In addition spontaneous EPSPs and IPSPs were recorded in interneurones. 8. These results demonstrate that the electrophysiological properties of putative interneurones are distinct from those of TC neurones of the rat dLGN. The implications of these findings for the control of visual responsiveness of TC neurones are discussed.
Full text
PDF


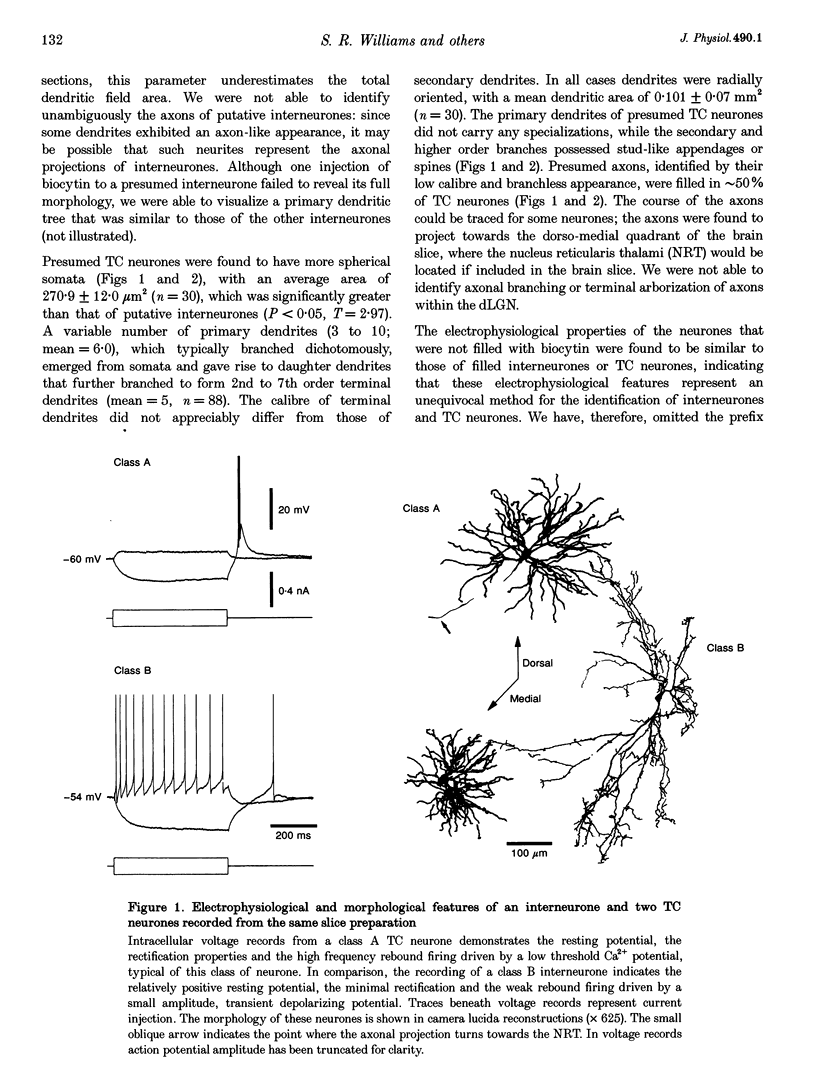
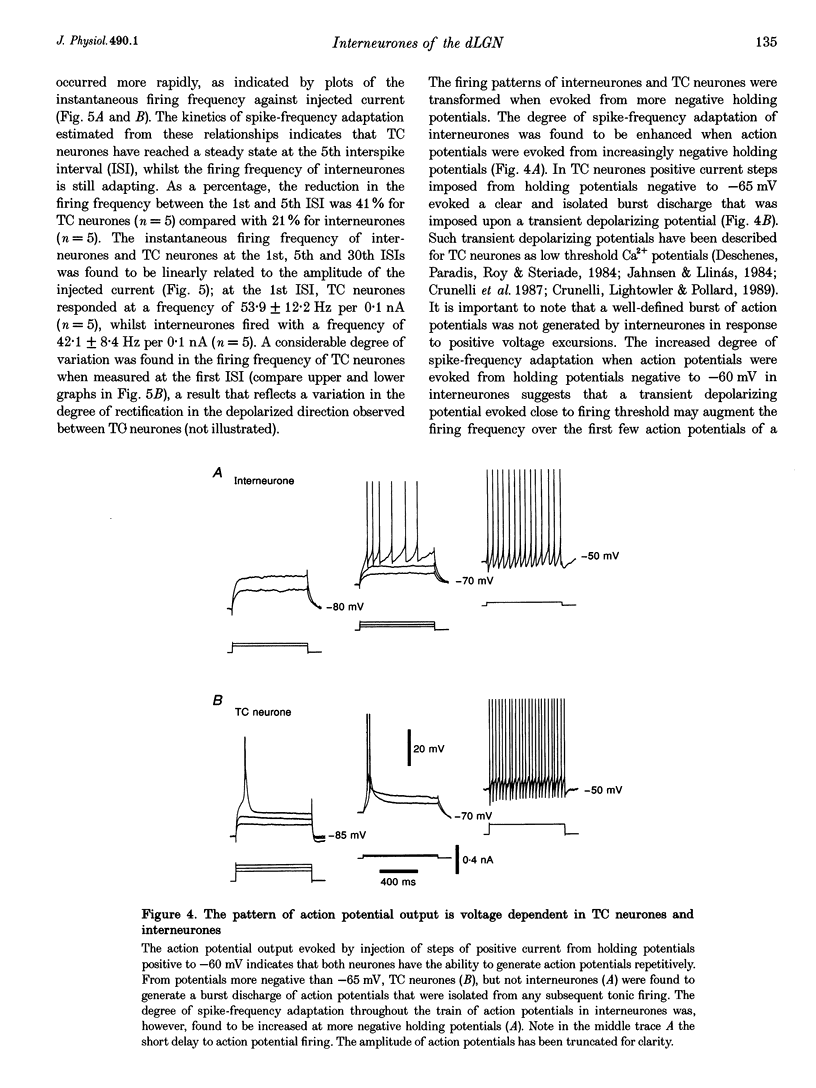
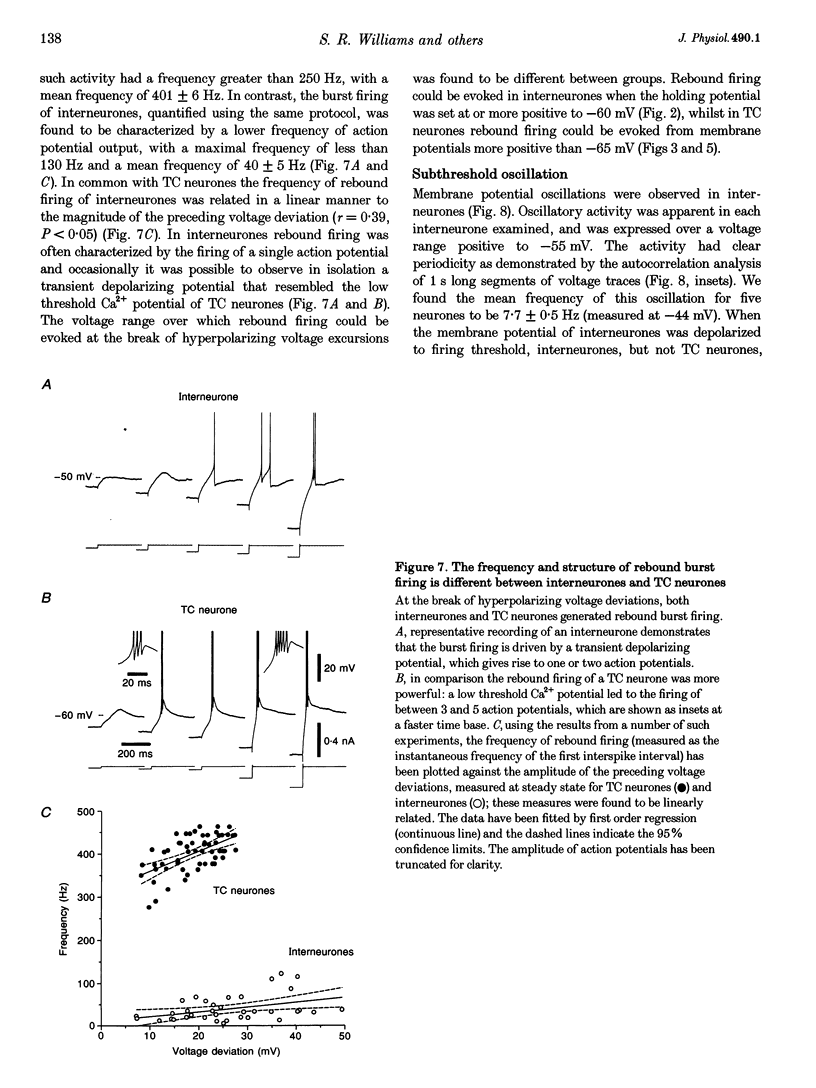





Selected References
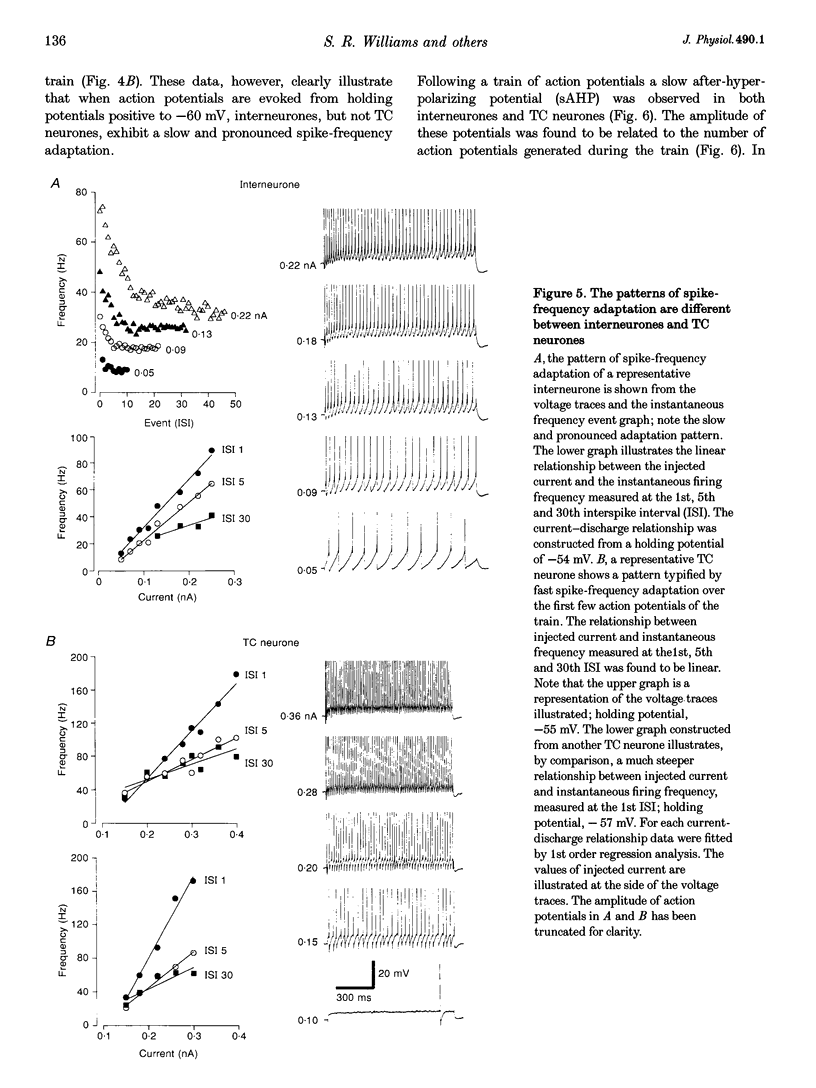
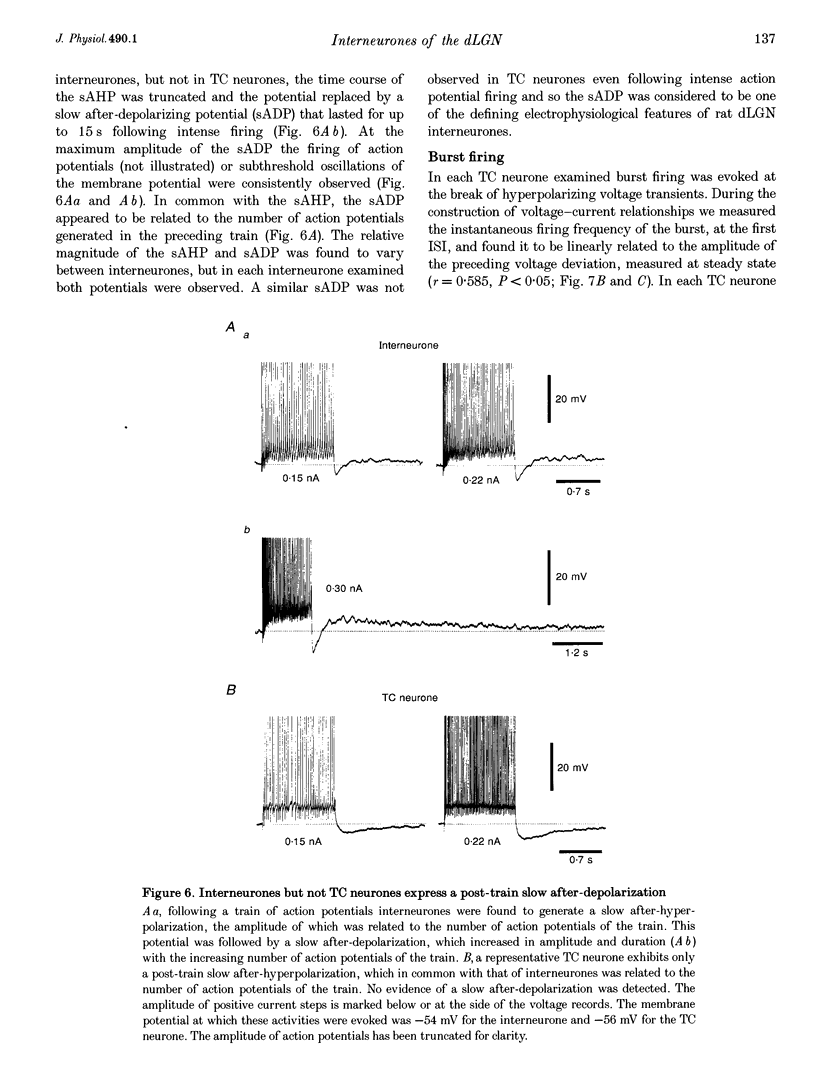
These references are in PubMed. This may not be the complete list of references from this article.
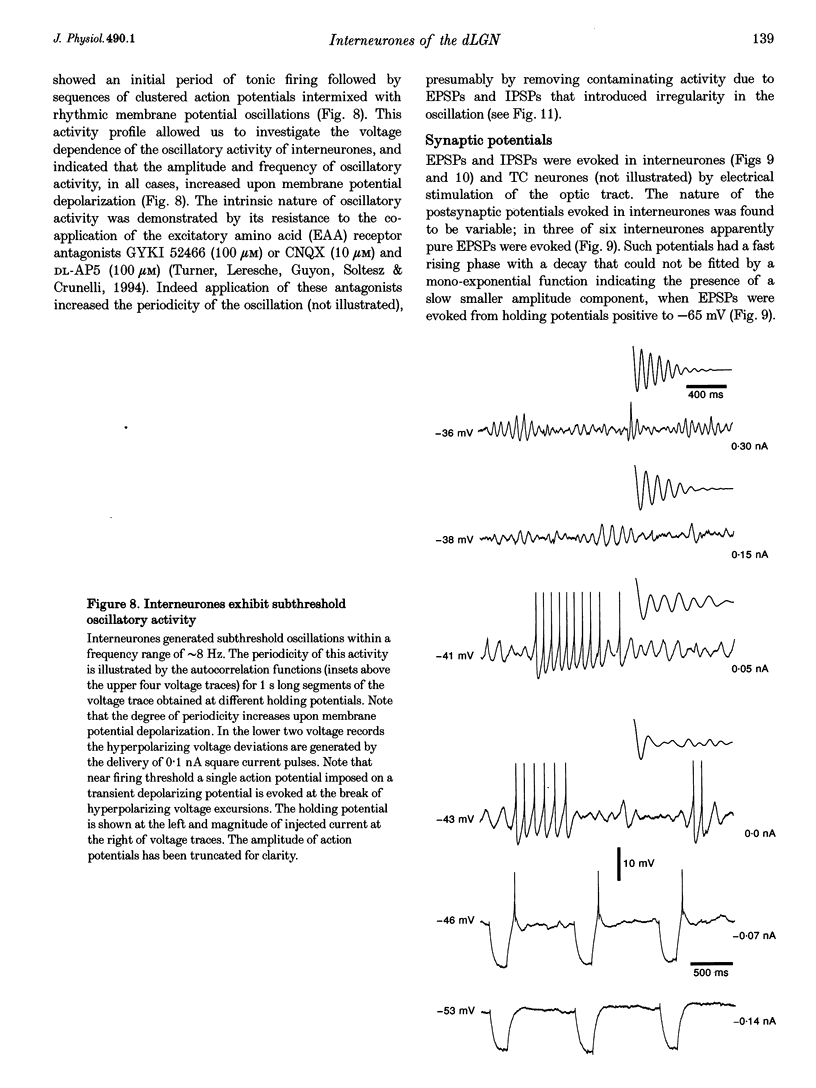
- Ahlsén G., Lindström S. Excitation of perigeniculate neurones via axon collaterals of principal cells. Brain Res. 1982 Mar 25;236(2):477–481. doi: 10.1016/0006-8993(82)90730-2. [DOI] [PubMed] [Google Scholar]
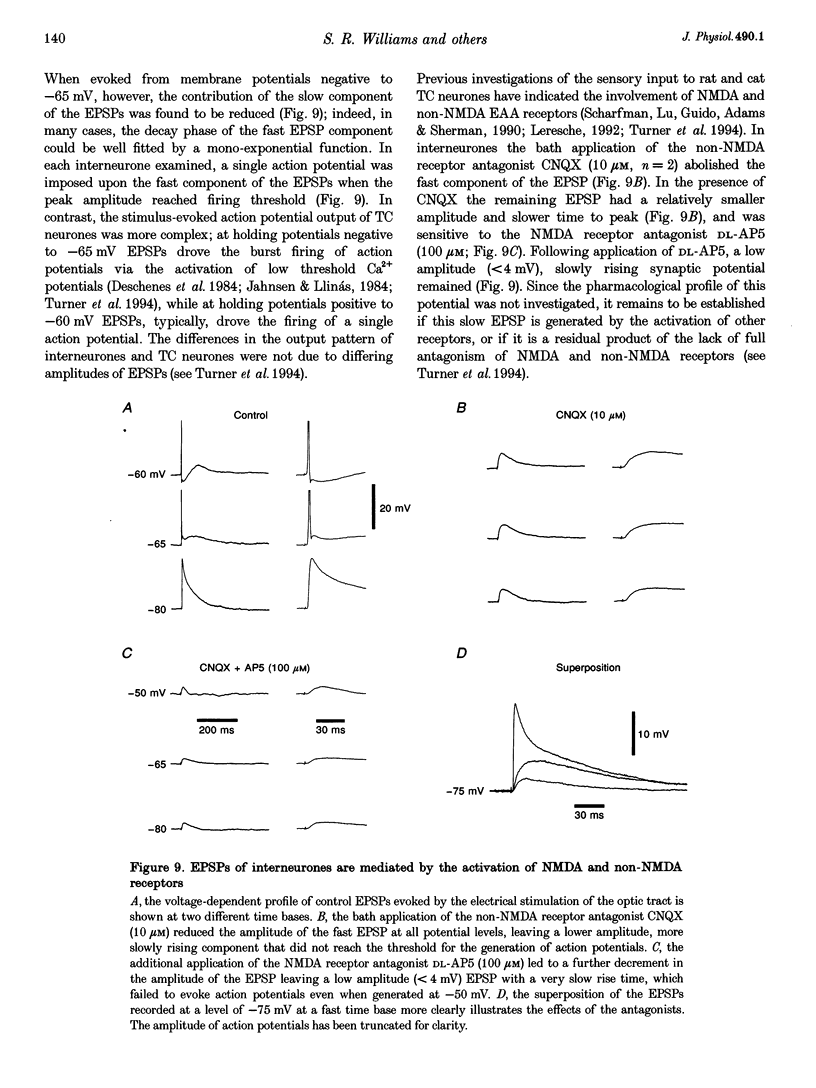
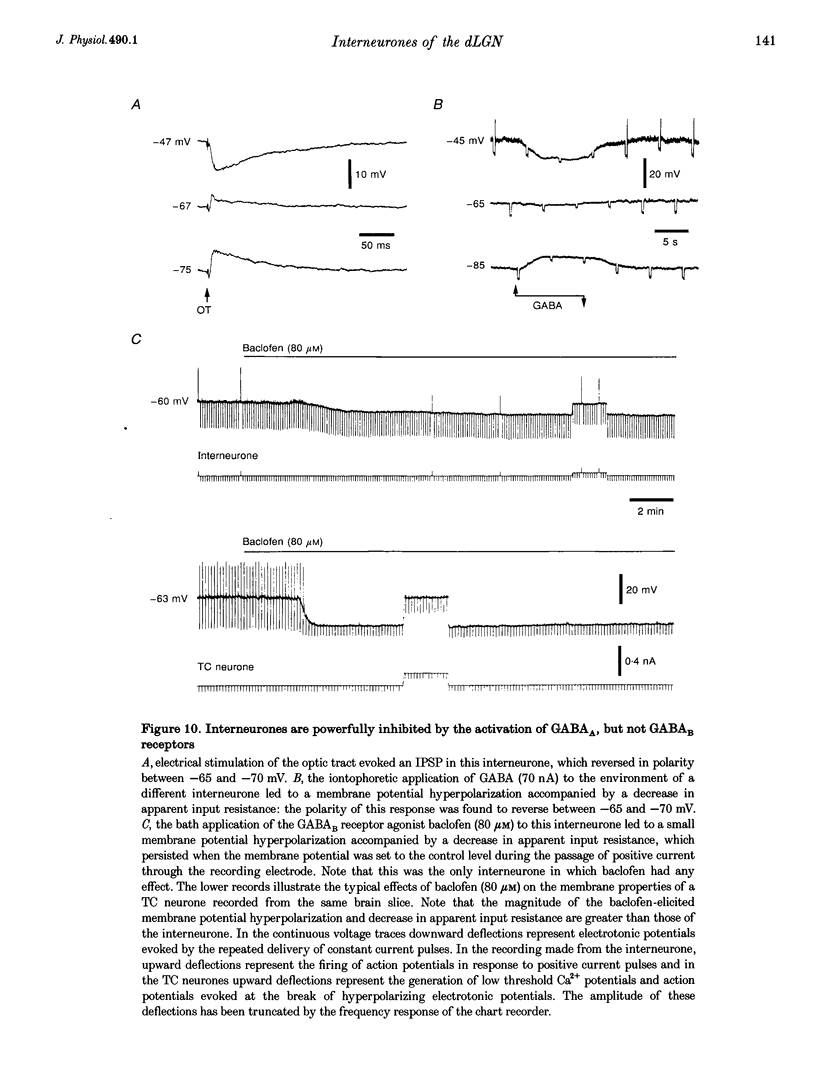
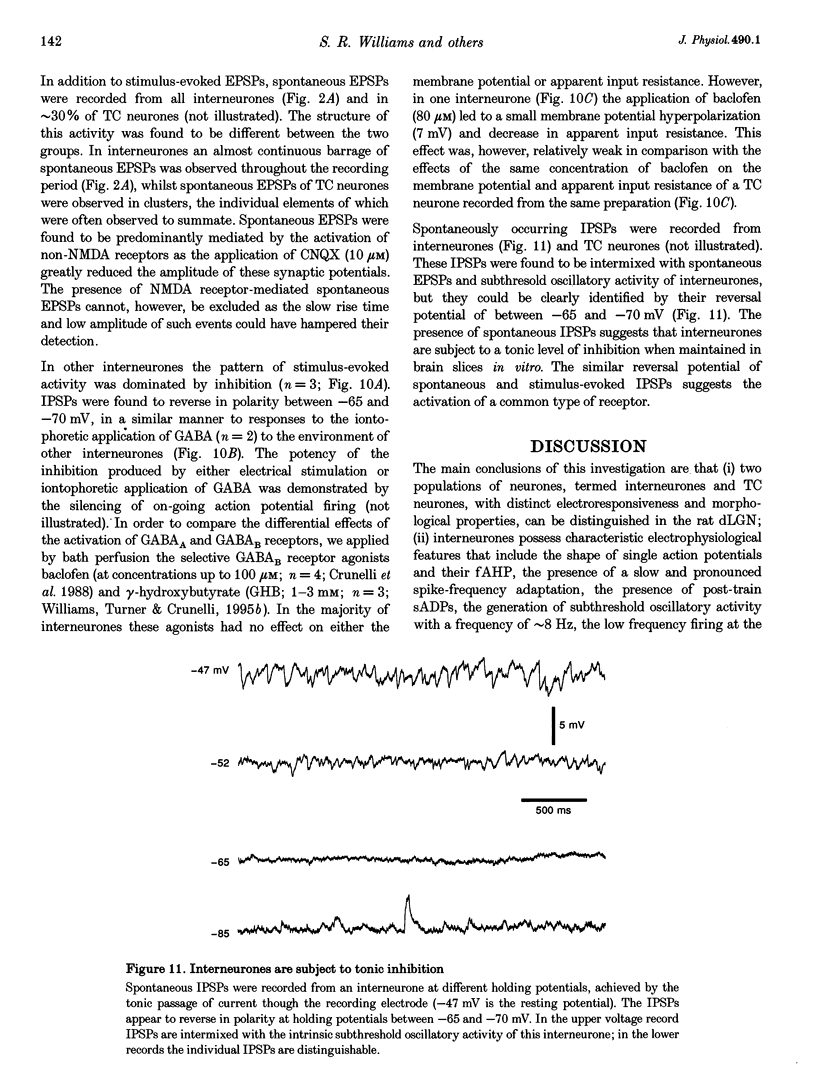
- Ahlsén G., Lindström S., Lo F. S. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58(1):134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Berardi N., Morrone M. C. The role of gamma-aminobutyric acid mediated inhibition in the response properties of cat lateral geniculate nucleus neurones. J Physiol. 1984 Dec;357:505–523. doi: 10.1113/jphysiol.1984.sp015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila M., Milleret C., Rougeul A., Buser P. Alpha rhythm in the cat thalamus. C R Acad Sci III. 1993;316(1):51–58. [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. The ventral and dorsal lateral geniculate nucleus of the rat: intracellular recordings in vitro. J Physiol. 1987 Mar;384:587–601. doi: 10.1113/jphysiol.1987.sp016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiaro J. B., Uhlrich D. J., Sherman S. M. Electron-microscopic analysis of synaptic input from the perigeniculate nucleus to the A-laminae of the lateral geniculate nucleus in cats. J Comp Neurol. 1991 Aug 15;310(3):316–336. doi: 10.1002/cne.903100304. [DOI] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Sumitomo I., Sugitani M., Iwama K. Receptive-field properties of cells in the dorsal part of the albino rat's lateral geniculate nucleus. Jpn J Physiol. 1979;29(3):283–307. doi: 10.2170/jjphysiol.29.283. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Somogyi J., Stewart M. G., Hámori J. GABA-immunoreactive neurons in the dorsal lateral geniculate nucleus of the rat: characterisation by combined Golgi-impregnation and immunocytochemistry. Exp Brain Res. 1986;61(2):311–322. doi: 10.1007/BF00239521. [DOI] [PubMed] [Google Scholar]
- Grossman A., Lieberman A. R., Webster K. E. A Golgi study of the rat dorsal lateral geniculate nucleus. J Comp Neurol. 1973 Aug;150(4):441–466. doi: 10.1002/cne.901500404. [DOI] [PubMed] [Google Scholar]
- Hale P. T., Sefton A. J., Baur L. A., Cottee L. J. Interrelations of the rat's thalamic reticular and dorsal lateral geniculate nuclei. Exp Brain Res. 1982;45(1-2):217–229. doi: 10.1007/BF00235781. [DOI] [PubMed] [Google Scholar]
- Hamos J. E., Van Horn S. C., Raczkowski D., Uhlrich D. J., Sherman S. M. Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature. 1985 Oct 17;317(6038):618–621. doi: 10.1038/317618a0. [DOI] [PubMed] [Google Scholar]
- Heggelund P., Hartveit E. Neurotransmitter receptors mediating excitatory input to cells in the cat lateral geniculate nucleus. I. Lagged cells. J Neurophysiol. 1990 Jun;63(6):1347–1360. doi: 10.1152/jn.1990.63.6.1347. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Weller R. E. Structural correlates of functionally distinct X-cells in the lateral geniculate nucleus of the cat. J Comp Neurol. 1988 Feb 15;268(3):448–468. doi: 10.1002/cne.902680312. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R., Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J Neurophysiol. 1993 Jul;70(1):144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Leresche N., Lightowler S., Soltesz I., Jassik-Gerschenfeld D., Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche N. Synaptic Currents in Thalamo-cortical Neurons of the Rat Lateral Geniculate Nucleus. Eur J Neurosci. 1992;4(7):595–602. doi: 10.1111/j.1460-9568.1992.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res. 1982 Feb 25;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988 Jul 21;334(6179):246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero V. M. Ultrastructural identification of synaptic terminals from the axon of type 3 interneurons in the cat lateral geniculate nucleus. J Comp Neurol. 1987 Oct 8;264(2):268–283. doi: 10.1002/cne.902640210. [DOI] [PubMed] [Google Scholar]
- Norton T. T., Godwin D. W. Inhibitory GABAergic control of visual signals at the lateral geniculate nucleus. Prog Brain Res. 1992;90:193–217. doi: 10.1016/s0079-6123(08)63615-8. [DOI] [PubMed] [Google Scholar]
- Ohara P. T., Lieberman A. R., Hunt S. P., Wu J. Y. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience. 1983;8(2):189–211. doi: 10.1016/0306-4522(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Pape H. C., Budde T., Mager R., Kisvárday Z. F. Prevention of Ca(2+)-mediated action potentials in GABAergic local circuit neurones of rat thalamus by a transient K+ current. J Physiol. 1994 Aug 1;478(Pt 3):403–422. doi: 10.1113/jphysiol.1994.sp020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E., Lu S. M., Guido W., Adams P. R., Sherman S. M. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Crunelli V. On the properties and origin of the GABAB inhibitory postsynaptic potential recorded in morphologically identified projection cells of the cat dorsal lateral geniculate nucleus. Neuroscience. 1989;33(1):23–33. doi: 10.1016/0306-4522(89)90307-2. [DOI] [PubMed] [Google Scholar]
- Spreafico R., Schmechel D. E., Ellis L. C., Jr, Rustioni A. Cortical relay neurons and interneurons in the N. ventralis posterolateralis of cats: a horseradish peroxidase, electron-microscopic, Golgi and immunocytochemical study. Neuroscience. 1983 Jul;9(3):491–509. doi: 10.1016/0306-4522(83)90168-9. [DOI] [PubMed] [Google Scholar]
- Staley K. J., Otis T. S., Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992 May;67(5):1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991 Oct;11(10):3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Paré D., Oakson G. Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. P., Leresche N., Guyon A., Soltesz I., Crunelli V. Sensory input and burst firing output of rat and cat thalamocortical cells: the role of NMDA and non-NMDA receptors. J Physiol. 1994 Oct 15;480(Pt 2):281–295. doi: 10.1113/jphysiol.1994.sp020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington M. A., Traub R. D., Jefferys J. G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995 Feb 16;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Turner J. P., Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB, receptor-mediated hyperpolarization. Neuroscience. 1995 May;66(1):133–141. doi: 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]



