Abstract
Background
Gallbladder mucocele (GBM) is a common disease in the canine gallbladder. Although the pathogenesis of GBM remains unclear, we recently reported that the excessive accumulation of mucin in the gallbladder is not a result of overproduction by gallbladder epithelial cells (GBECs).
Hypothesis/Objectives
Changes in the function of GBECs other than the production of mucin are associated with the pathogenesis of GBM. We performed an RNA sequencing (RNA‐seq) analysis to comprehensively search for abnormalities in gene expression profiles of GBECs in dogs with GBM.
Animals
Fifteen dogs with GBM and 8 dogs euthanized for reasons other than gallbladder disease were included.
Methods
The GBECs were isolated from gallbladder tissues to extract RNA. The RNA‐seq analysis was performed using the samples from 3 GBM cases and 3 dogs with normal gallbladders, and the gene expression profiles were compared between the 2 groups. Differences in mRNA expression levels of the extracted differentially expressed genes (DEGs) were validated by quantitative reverse transcription polymerase chain reaction (RT‐qPCR) using samples of 15 GBM cases and 8 dogs with normal gallbladders.
Results
Comparison of gene expression profiles by RNA‐seq extracted 367 DEGs, including ANO1, a chloride channel associated with changes in mucin morphology, and HTR4, which regulates the function of chloride channels. The ANO1 and HTR4 genes were confirmed to be downregulated in the GBM group by RT‐qPCR.
Conclusions and Clinical Importance
Our results suggest that GBM may be associated with decreased function of chloride channels expressed in GBECs.
Keywords: ANO1, gallbladder epithelial cells, HTR4, RNA‐seq
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- DEGs
differentially expressed genes
- FDR
false discovery rate
- GBECs
gallbladder epithelial cells
- GBM
gallbladder mucocele
- GO
gene ontology
- IP3
inositol 1,4,5‐triphosphate
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PLC
phospholipase C
- RIN
RNA integrity number
- RNA‐seq
RNA sequencing
1. INTRODUCTION
Gallbladder mucocele (GBM) is a common gallbladder disease in dogs. 1 It is characterized by excessive accumulation of mucus, mainly mucin, in the gallbladder and can cause bile duct obstruction and rupture of the gallbladder. 2 , 3 The only treatment available is surgery, which has a high perioperative mortality rate of approximately 20%. 4 , 5 , 6 Several epidemiologic studies have reported that several breeds such as Shetland Sheepdogs, American Cocker Spaniels, Chihuahuas, Pomeranians, Miniature Schnauzers, and Border Terriers 7 , 8 , 9 , 10 were predisposed to develop GBM. Also, endocrinopathies such as hyperadrenocorticism, hypothyroidism, and hyperlipidemia 9 , 11 are reported to be risk factors for the development of GBM, but the pathogenesis of GBM has not yet been elucidated.
In the gallbladder, mucins are secreted by the gallbladder epithelial cells (GBECs) to protect the mucosa from the surfactant effects of bile. 12 , 13 It has been reported that the gallbladder expresses MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 in dogs, mice, and humans. 14 , 15 , 16 , 17 , 18 Furthermore, a study using mass spectrometry confirmed that MUC5AC and MUC5B are the most abundant mucin subtypes in the mucus of GBM cases and reported that MUC5AC concentrations increased compared with healthy dogs. 13 However, we previously reported that the expression levels of MUC5AC and MUC5B were not increased in the GBECs at both the gene and protein levels, 18 indicating that excess mucus accumulation might be a result of impaired mucus excretion rather than excessive production. Hence, it is necessary to investigate factors that cause abnormalities in mucus excretion.
Mucin, a large molecule consisting of a heavily glycosylated core protein, is stored within the secretory granules in a highly folded compact form before secretion (ie, packed mucins). As they are secreted into the gallbladder lumen, the packed mucins are gradually unpacked into a linear form and excreted from the gallbladder. In a previous study analyzing the molecular structure of mucin in the gallbladder of dogs, mucin from healthy dogs had a linear molecular morphology, whereas mucin from GBM had a compact morphology with many intramolecular cross‐links, 13 suggesting abnormalities in the unpacking process of mucins in GBM cases. Combined with the fact that GBECs not only secrete mucin but also are involved in the unpacking process of mucin by regulating the ionic composition of bile and water content, 17 their functions may be involved in the changes in mucin morphology observed in the mucus of the GBM. We aimed to identify candidate proteins responsible for the accumulation of mucus in GBM by comprehensively analyzing gene expression profiles in GBECs, which produce mucin and have a role in regulating mucin properties.
2. MATERIALS AND METHODS
2.1. Animals
Dogs that underwent cholecystectomy at the Veterinary Medical Center of the University of Tokyo and were histopathologically diagnosed with GBM at the Laboratory of Veterinary Pathology at the University of Tokyo were included in the study. The diagnosis of GBM was made based on gross examination and light microscopy of gallbladder, as in previous studies, 10 , 13 , 19 which identified abundant mucus accumulation in the lumen, hyperplasia of the mucosal epithelial cells, and their elongation into a columnar shape in all cases included in the present study. All owners provided written informed consent for the use of gallbladder tissues. Normal canine gallbladders were obtained from research animals maintained at Yamaguchi University that were euthanized for other experimental purposes approved by the Yamaguchi University Ethics Committee (Approval No. 209). The age of these healthy dogs ranged from 6 to 8 years. The experimental procedures and animal care were conducted in conformity with the Yamaguchi University Animal Care and Use guidelines.
2.2. Collection of GBECs
Isolation of GBECs was performed as previously reported. 18 Gallbladder tissues were obtained during surgical cholecystectomy from GBM cases or collected from research dogs immediately after euthanasia. The gallbladder was cut open and washed with ice‐cold phosphate‐buffered saline (PBS). Any remaining mucus gel was removed using forceps. Then, gallbladder tissues were digested using 0.25% w/v trypsin (Fujifilm Wako, Osaka, Japan) or 2000 U/mL of dispase (Goudou Shusei, Tokyo, Japan) at 37°C for 45 minutes to collect GBECs. After inactivation of trypsin by adding 10% fetal bovine serum, remaining debris was removed by passing through a 100‐μm cell strainer, followed by centrifugation at 1500 rpm for 5 minutes. After another wash with PBS, the pelleted cells were stored at −80°C until utilized. The viability of GBECs was confirmed to be >95% using the trypan blue exclusion test.
2.3. RNA sequencing analysis
Total RNA was extracted from GBECs using RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. The RNA integrity number (RIN) was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA), and RNA with an RIN >7 was used for RNA sequencing (RNA‐seq). Sequencing libraries were prepared with the total RNA using NEBNext UltraTM ll Directional RNA Library Prep Kit (NEW ENGLAND Biolabs, Ipswich, Massachusetts, USA). RNA‐seq (150 bp paired‐end) was conducted using a NovaSeq 6000 (Illumina, San Diego, California, USA).
Quality controls and adaptor trimmings of the obtained fastq files were conducted using Fastp. Trimmed fastq data were mapped to canine genomes (CanFam3.1) by STAR 2.7.3a, 20 and transcript abundance was estimated using RSEM v.1.3.3 21 with gene transfer file for Ensembl (CanFam3.1.98, https://www.ensembl.org). These gene count data were used to normalize and extract differential gene expressions with an R package (DEseq2 V.1.32.0). Differentially expressed genes (DEGs) were identified by comparison between the 2 groups, normal and GBM, at a false discovery rate (FDR) < 0.2. To understand the underlying biological mechanisms and pathways associated with the identified DEGs, we performed gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis using all DEGs, which were extracted from the comparison between the 2 groups by the Database for Annotation, Visualization, and Integrated Discovery (DAVID). The analysis using DAVID was conducted with the background gene list of standard option. To correct for multiple comparisons, the P‐values were adjusted by employing the Benjamini‐Hochberg procedure with a threshold of FDR < 0.05.
The datasets used and analyzed are available at the DDBJ Sequenced Read Archive repository with accession number PRJDB17836.
2.4. Quantitative reverse transcription polymerase chain reaction
Extracted total RNA was reverse transcribed using ReverTra Ace (TOYOBO, Osaka, Japan). The quantitative reverse transcription polymerase chain reaction (RT‐qPCR) was performed using StepOnePlus (Applied Biosystems, Waltham, Massachusetts, USA) with PowerUP SYBR Green Master Mix (Applied Biosystems). Relative mRNA amounts for each gene were calculated by standard curves and normalization was conducted by dividing them by the relative mRNA amount of GAPDH as the internal control. Each PCR reaction was performed in duplicate. The primers used are listed in Table S1.
2.5. Statistical analysis
Statistical analysis was performed using R v4.1.0 (R core team, Vienna, Austria). The Wilcoxon rank‐sum test with the exact test option was used for the comparison of relative amounts of mRNAs between the 2 groups. A P‐value of < .05 was considered significant.
3. RESULTS
3.1. Animals
Fifteen dogs with GBM and 8 normal dogs were included (Table S2). All of the cases included were diagnosed as GBM by pathological examination, in which 14 were also clinically diagnosed as GBM by ultrasound examination (Figure S1), but 1 case was clinically diagnosed as gallbladder sludge before histopathological examination. The GBM cases included 5 intact males, 1 intact female, 4 neutered males, and 5 spayed females. There were 5 Toy Poodles, 2 Chihuahuas, 2 Shetland Sheepdogs, 2 Papillons, 1 Miniature Schnauzer, 1 Beagle, 1 Maltese, and 1 Parson Russell terrier. In all cases, there was no rupture of the gallbladder, and no necrosis of the gallbladder wall was observed in histopathological examination. Normal gallbladders were collected from 8 beagles, which included 6 intact females and 2 intact males.
3.2. Extractions of DEGs in GBM and enrichment analysis
The RNA‐seq analysis generated at least 27.93 million total raw reads for each paired end, with a mapping rate of at least 95.17% (Table S3). In a comparison between the 2 groups, normal and GBM, 367 DEGs were extracted at FDR < 0.2, of which 192 genes were upregulated and 175 genes were downregulated in the GBM group (Figure 1 and Table S4). Based on q‐values, the top 5 upregulated genes were RIPPLY1, CCKAR, BICC1, PLK2, and MNDA, whereas the top 5 downregulated genes were CLEC3A, MT1E, ZDHHC7, TSPAN5, and HIF3A. The expression levels of mucins were not different between the 2 groups except for MUC1, which was consistent with our previous study. 18
FIGURE 1.
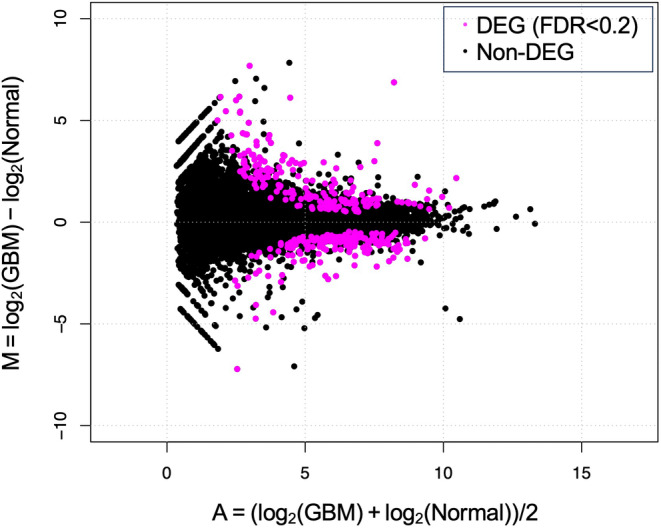
M‐A plot of differentially expressed genes (DEGs). Differentially expressed genes extracted from the comparison of 2 groups, dogs with gallbladder mucocele (GBM) and normal gallbladder, are plotted by M‐A plot. DEGs with false discovery rate (FDR) <0.2 are plotted in magenta.
The GO enrichment analysis using all DEGs was performed to investigate the associated biological functions according to 3 categories: biological process, cellular component, and molecular function (Table 1). Two and 8 significant terms were extracted for the biological process and cellular component, respectively, whereas no significant term was extracted for the molecular function. “Cholesterol biosynthetic process” and “Sterol biosynthetic process” were enriched in the biological process, but terms related to mucus or mucin were not enriched. In the KEGG pathway analysis, only the pathway “Steroid biosynthesis” was extracted (Gene count, 7; FDR = 0.00018).
TABLE 1.
Results of gene ontology analysis using differentially expressed genes.
| Category | Term | Count | FDR |
|---|---|---|---|
| Biological process | Cholesterol biosynthetic process | 10 | 6.16E−06 |
| Biological process | Sterol biosynthetic process | 7 | 2.20E−04 |
| Cellular component | Apical plasma membrane | 23 | 5.54E−05 |
| Cellular component | Endoplasmic reticulum | 40 | 2.40E−04 |
| Cellular component | Endoplasmic reticulum membrane | 35 | 6.40E−03 |
| Cellular component | Cytoplasm | 116 | 6.49E−03 |
| Cellular component | Cytosol | 113 | 6.49E−03 |
| Cellular component | Extracellular exosome | 53 | 2.35E−02 |
| Cellular component | Basolateral plasma membrane | 13 | 2.35E−02 |
| Cellular component | Integral component of plasma membrane | 38 | 2.98E−02 |
Abbreviation: FDR, false discovery rate.
3.3. Validations of expression levels of DEGs using RT‐qPCR
Because no pathways involving mucus or mucin were extracted in the enrichment analysis, we focused on DEGs included in “mucus secretion” in the biological process of the GO term, although it was not included in the terms significantly enriched with DEGs. Among the DEGs, 3 genes were included in “mucus secretion”: AGR2, ANO1, and HTR4, and their mRNA expression levels were compared using RT‐qPCR between the 2 groups: normal and GBM groups. Although no difference was found in AGR2 mRNA expression between the 2 groups (P = .19), ANO1 and HTR4 mRNA expression was significantly decreased in the GBM group (P = .04, and P < .01, respectively; Figure 2), consistent with the RNA‐seq results.
FIGURE 2.
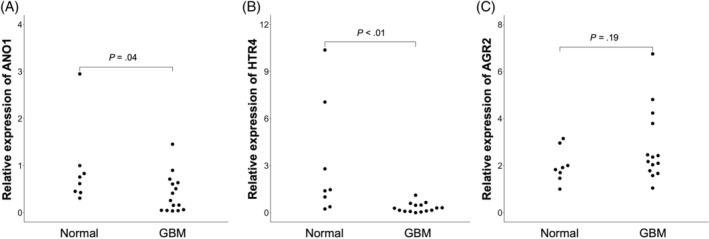
Comparison of the gene expression levels of differentially expressed genes using quantitative reverse transcription polymerase chain reaction (RT‐qPCR). Among differentially expressed genes extracted by RNA‐seq, gene expression levels of ANO1 (A), HTR4 (B), and AGR2 (C), which were included in the term “mucus secretion” in gene ontology, were quantified by RT‐qPCR using 8 normal dogs and 15 gallbladder mucocele (GBM) cases.
4. DISCUSSION
Gallbladder mucocele is characterized by excessive mucus accumulation in the gallbladder, substantially impacting its function. To identify the cause of excessive mucin accumulation in GBM cases, we performed a comprehensive analysis of gene expression profiles in GBECs of GBM cases and healthy dogs, that secrete mucin and are responsible for promoting its morphological changes. In a comparison between GBM cases and normal dogs, 367 genes were extracted as DEGs, which included genes involved in mucus secretion, such as ANO1, HTR4, and AGR2. In the validation using RT‐qPCR, expression levels of ANO1 and HTR4 were confirmed to be downregulated in dogs with GBM compared with normal dogs.
Anoctamin 1, encoded by ANO1, is a Ca2+‐activated chloride channel, which is an ion channel that secretes anions such as chloride and bicarbonate from the intracellular to the extracellular space upon an increase of intracellular calcium ion concentration. 22 Because Anoctamin 1 is responsible for anion secretion in epithelial cells, 23 , 24 decreased expression of ANO1 may lead to decreased anion secretion from GBECs. Mucin is packed in a folded structure in intracellular secretory granules with the calcium ions cross‐linking between the sugar chains of mucin. 17 As mucin is secreted, calcium ions are replaced by sodium ions and chelated by bicarbonate ions in bile, transforming the packed mucins into linear‐formed fluid mucins with cleaved cross‐links. 25 It has been reported that dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR), another major chloride channel, causes insufficient anion secretion and defects in the unpacking process of mucin, resulting in mucus accumulation in the gastrointestinal tract and airways in humans with cystic fibrosis (CF). 26 Dysfunction of CFTR or decreased expression in the gallbladder has not been reported in GBM before, and the expression level of CFTR was not different between GBM and normal canine gallbladder in our study. However, our results suggest that similar changes as observed in CF patients also may have occurred in the gallbladder of GBM cases because of decreased expression of ANO1. Furthermore, a report analyzing the mucus secreted from the gallbladder showed that linear‐formed fluid mucin constitutes the majority in healthy dogs, whereas granular mucin with a compact morphology accounts for the majority of the mucin in GBM patients, 13 which indicates defects in the unpacking process of mucin. Hence, a decrease in ANO1 expression may cause a reduction in chloride ion concentrations in the bile, leading to abnormalities in the process of mucin unpacking.
5‐hydroxytryptamine receptor 4 (HTR4), which is encoded by HTR4, is the G protein‐coupled receptor (GPCR) that stimulates cAMP production in response to serotonin binding. 27 Because our study showed a decrease in HTR4 expression, a concomitant decrease in intracellular cAMP concentration can be inferred, which is consistent with the significantly decreased cAMP concentration in bile of dogs with GBM compared with healthy dogs in a study that analyzed the metabolites in bile of dogs with GBM. 28 It has been reported that cAMP produced by HTR4 activates CFTR, a cAMP‐activated chloride channel, promoting anion secretion. 29 , 30 In addition, GPCR induces the release of calcium from the endoplasmic reticulum through phospholipase C (PLC)/inositol 1,4,5‐triphosphate (IP3) signaling, which increases intracellular calcium concentration and evokes activation of anoctamin 1. 22 , 31 Therefore, decreased expression of HTR4 may suppress cAMP‐mediated activation of CFTR and PLC/IP3 pathway‐mediated activation of anoctamin 1, resulting in decreased anion secretion into the bile.
Several studies have pointed out the similarity between GBM and CF, where decreased anion secretion caused by abnormal CFTR function results in excessive mucus accumulation. It has been reported that CFTR‐knock‐out models in pigs and ferrets, similar to GBM, show mucus accumulation in the gallbladder and characteristic papillary elongation of the mucosal epithelium on pathological examination. 32 , 33 However, because CF is a disease caused by congenital genetic mutations, it often develops at a young age, but GBM develops at a middle to older age. 8 , 34 Furthermore, it is known that people with CF do not show enlargement of the gallbladder associated with mucus retention as in GBM. Rather, microgallbladders with an accumulation of highly gelled mucus often are observed. 35 , 36 Our results suggest that GBM could be caused by abnormal ion secretory function, which is similar to CF in humans, but it is necessary to investigate GBM‐specific mechanisms considering these differences between CF and GBM.
In our study, the number of DEGs extracted by RNA‐seq analysis was 367 at FDR <0.2. This result indicated that the gene expression profiles did not change markedly despite the severe pathological changes in the gallbladder tissues when compared with the results of other studies that performed RNA‐seq in dogs with various diseases and in healthy dogs. 37 , 38 , 39 Possible causes of this result include a large variation in gene expression profiles of the GBECs among the cases, which masks GBM‐specific changes in gene expression. Although GBM cases included in the present RNA‐seq analysis did not have any concomitant diseases, it is possible that diet and medications, which are reported to affect the composition of bile, 40 , 41 , 42 , 43 might have changed gene expression in GBECs through alterations in the composition of the bile. It also might be possible that alterations related to GBM pathogenesis cause little change in the gene expression profiles of GBECs. An RNA‐seq analysis using GBECs of CFTR knockout pigs has reported only 163 DEGs. 44 In this previous study, pathways involving CFTR gene were not extracted in the gene enrichment analysis, suggesting little impact of the knocked‐out of CFTR gene expression on comprehensive gene expression profiles although alteration of ion exchange procedures occurred. 44 Because ANO1, detected as a DEG in our study, is also a gene involved in anion exchange as is CFTR, changes in ANO1 expression may have as little effect on the expression profiles of other genes as in CFTR knock‐out pigs.
In our study, RNA‐seq was performed on 3 dogs with GBM and 3 healthy dogs, and qPCR validation also was conducted on 15 dogs with GBM and 8 healthy dogs. All healthy dogs were Beagles, whereas the dogs with GBM consisted of 8 different breeds. Because the predicted pathogenesis of the disease was based on only 8 breeds in our study, a limitation of the study is that breed‐specific factors might have been masked because of the different genetic backgrounds of the breeds. Therefore, a larger number of GBM cases should be studied and breed‐specific analysis should be conducted in the future.
In conclusion, we found that expression of genes involved in anion channel function, such as ANO1 and HTR4, were downregulated in GBM cases, suggesting that decreased anion secretion may lead to an abnormal mucin unpacking process and mucus accumulation. Ion channel function should be further evaluated using a cell culture system to confirm that anion secretion is decreased in GBM cases.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC of Yamaguchi University (Approval Number 209).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1. Representative images of ultrasound and histopathological examinations of GBM cases included in the present study. Results of ultrasound and histopathological examinations of the gallbladders of GBM2, GBM5, and GBM14.
Table S1. Primer pairs used in RT‐qPCR for the 4 genes.
Table S2. Signalment of the cases with gallbladder mucocele included in the present study. Abbreviation: GBM, gallbladder mucocele.
Table S3. Total read counts and Mapping rate obtained by RNA sequencing.
Abbreviations: GBM, gallbladder mucocele.
Table S4. Differentially expressed genes extracted by comparison between gallbladder mucocele cases and healthy dogs. Abbreviation: FDR, false discovery rate.
ACKNOWLEDGMENTS
Funding was provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan Society for the Promotion of Science (24KJ0651). We are grateful to Dr. Ryohei Nishimura, Dr. Takayuki Nakagawa, Dr. Yosuke Takahashi, and Dr. Yuko Hahsimoto for sample collection at the University of Tokyo. Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics.
Nagao I, Motegi T, Goto‐Koshino Y, et al. Comprehensive gene expression analysis in gallbladder mucosal epithelial cells of dogs with gallbladder mucocele. J Vet Intern Med. 2024;38(6):3031‐3037. doi: 10.1111/jvim.17157
REFERENCES
- 1. Crews LJ, Feeney DA, Jessen CR, Rose ND, Matise I. Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture in dogs: 45 cases (1997–2007). J Am Vet Med Assoc. 2009;234(3):359‐366. [DOI] [PubMed] [Google Scholar]
- 2. Mizutani S, Torisu S, Kaneko Y, Naganobu K. Retrospective analysis of canine gallbladder contents in biliary sludge and gallbladder mucoceles. J Vet Med Sci. 2017;79(2):366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39(3):543‐598. [DOI] [PubMed] [Google Scholar]
- 4. Jaffey JA, Graham A, VanEerde E, et al. Gallbladder mucocele: variables associated with outcome and the utility of ultrasonography to identify gallbladder rupture in 219 dogs (2007–2016). J Vet Intern Med. 2018;32(1):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parkanzky M, Grimes J, Schmiedt C, Secrest S, Bugbee A. Long‐term survival of dogs treated for gallbladder mucocele by cholecystectomy, medical management, or both. J Vet Intern Med. 2019;33(5):2057‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galley M, Lang J, Mitchell M, Fletcher J. Factors affecting survival in 516 dogs that underwent cholecystectomy for the treatment of gallbladder mucocele. Can Vet J. 2022;63(1):63‐66. [PMC free article] [PubMed] [Google Scholar]
- 7. Allerton F, Barker FSL, Kathrani VBA, Kent A, Tivers M. Gall bladder mucoceles in Border terriers. J Vet Intern Med. 2018;32(5):1618‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaffey JA, Pavlick M, Webster CR, et al. Effect of clinical signs, endocrinopathies, timing of surgery, hyperlipidemia, and hyperbilirubinemia on outcome in dogs with gallbladder mucocele. Vet J. 2019;251:105350. [DOI] [PubMed] [Google Scholar]
- 9. Kutsunai M, Kanemoto H, Fukushima K, Fujino Y, Ohno K, Tsujimoto H. The association between gall bladder mucoceles and hyperlipidaemia in dogs: a retrospective case control study. Vet J. 2014;199(1):76‐79. doi: 10.1016/j.tvjl.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 10. Aguirre AL, Yeager AE, Keegan AM, Harvey HJ, Erb HN. Gallbladder disease in Shetland sheepdogs: 38 cases (1995–2005). J Am Vet Med Assoc. 2005;231(1):79‐88. [DOI] [PubMed] [Google Scholar]
- 11. Mesich MLL, Mayhew PD, Paek M, Holt DE, Brown DC. Gall bladder mucoceles and their association with endocrinopathies in dogs: a retrospective case‐control study. J Small Anim Pract. 2009;50(12):630‐635. [DOI] [PubMed] [Google Scholar]
- 12. Lee SP, Scott AJ. Dihydrocholesterol‐induced gallstones in the rabbit: evidence that bile acids cause gallbladder epithelial injury. Br J Exp Pathol. 1979;60(3):231‐238. [PMC free article] [PubMed] [Google Scholar]
- 13. Kesimer M, Cullen J, Cao R, et al. Excess secretion of gel‐forming mucins and associated innate defense proteins with defective mucin un‐packaging underpin gallbladder mucocele formation in dogs. PLoS One. 2015;10(9):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buisine M p, Devisme L, Degand P, et al. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000;48(12):1667‐1676. [DOI] [PubMed] [Google Scholar]
- 15. Moniaux N, Buisine M p, Debailleul V, et al. Mucin gene expression in biliary epithelial cells. J Hepatol. 1997;27(6):1057‐1066. [DOI] [PubMed] [Google Scholar]
- 16. Yoo K s, Choi HS, Jun DW, et al. MUC expression in gallbladder epithelial tissues in cholesterol‐associated gallbladder disease. Gut Liver. 2016;10(5):851‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demouveaux B, Gouyer V, Gottrand F, Narita T, Desseyn JL. Gel‐forming mucin interactome drives mucus viscoelasticity. Adv Colloid Interface Sci. 2018;252:69‐82. [DOI] [PubMed] [Google Scholar]
- 18. Nagao I, Tsuji K, Goto‐Koshino Y, et al. MUC5AC and MUC5B expression in canine gallbladder mucocele epithelial cells. J Vet Med Sci. 2023;85(12):1269‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malek S, Sinclair E, Hosgood G. Clinical findings and prognostic factors for dogs undergoing cholecystectomy for gall bladder mucocele. Vet Surg. 2013;42:418‐426. [DOI] [PubMed] [Google Scholar]
- 20. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA‐seq aligner. Bioinformatics. 2013;29(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA‐Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Liu Z, Wang KW. The Ca2+‐activated chloride channel ANO1/TMEM16A: an emerging therapeutic target for epithelium‐originated diseases? Acta Pharm Sin B. 2021;11(6):1412‐1433. doi: 10.1016/j.apsb.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorrieri G, Scudieri P, Caci E, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016;6(October):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung J, Lee MG. Role of calcium signaling in epithelial bicarbonate secretion. Cell Calcium. 2014;55(6):376‐384. doi: 10.1016/j.ceca.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 25. Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel‐forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol. 2010;298(1):15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066‐1073. [DOI] [PubMed] [Google Scholar]
- 27. Yoshikawa T, DuPont BR, Leach RJ, Detera‐Wadleigh SD. New variants of the human and rat nuclear hormone receptor, TR4: expression and chromosomal localization of the human gene. Genomics. 1996;35(2):361‐366. [DOI] [PubMed] [Google Scholar]
- 28. Gookin JL, Mathews KG, Cullen J, Seiler G. Qualitative metabolomics profiling of serum and bile from dogs with gallbladder mucocele formation. PLoS One. 2018;13(1):1‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut microbiota‐produced tryptamine activates an epithelial G‐protein‐coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23(6):775‐785.e5. doi: 10.1016/j.chom.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moran O. Model of the cAMP activation of chloride transport by CFTR channel and the mechanism of potentiators. J Theor Biol. 2010;262(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 31. Wang P, Zhao W, Sun J, et al. Inflammatory mediators mediate airway smooth muscle contraction through a G protein‐coupled receptor–transmembrane protein 16A–voltage‐dependent Ca2+ channel axis and contribute to bronchial hyperresponsiveness in asthma. J Allergy Clin Immunol. 2018;141(4):1259‐1268.e11. doi: 10.1016/j.jaci.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 32. Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR‐knockout ferrets. Am J Pathol. 2014;184(5):1309‐1322. doi: 10.1016/j.ajpath.2014.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176(3):1377‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nasr SZ. Cystic fibrosis in adolescents and young adults. Adolesc Med. 2000;11(3):589‐603. [PubMed] [Google Scholar]
- 35. Nagel RA, Westaby D, Javaid A, et al. Liver disease and bile duct abnormalities in adults with cystic fibrosis. Lancet (London, England). 1989;2(8677):1422‐1425. [DOI] [PubMed] [Google Scholar]
- 36. Rovsing H, Sloth K. Micro‐gallbladder and biliary calculi in mucoviscidosis. Acta Radiol Diagn (Stockh). 1973;14(5):588‐592. [DOI] [PubMed] [Google Scholar]
- 37. Wang A, Neill SG, Newman S, et al. The genomic profiling and MAMLD1 expression in human and canines with Cushing's disease. BMC Endocr Disord. 2021;21(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bannoehr J, Balmer P, Stoffel MH, et al. Abnormal keratinocyte differentiation in the nasal planum of labrador retrievers with hereditary nasal parakeratosis (HNPK). PLoS One. 2020;15(3):e0225901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeda S, Tomiyasu H, Tsuboi M, et al. Comprehensive gene expression analysis of canine invasive urothelial bladder carcinoma by RNA‐Seq. BMC Cancer. 2018;18(1):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shikano R, Ohno K, Nagahara T, et al. Effects of proportions of carbohydrates and fats in diets on mucin concentration and bile composition in gallbladder of dogs. J Vet Med Sci. 2022;84(11):1465‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagahara T, Ohno K, Kanemoto H, et al. Effect of prednisolone administration on gallbladder emptying rate and gallbladder bile composition in dogs. Am J Vet Res. 2018;79(10):1050‐1056. [DOI] [PubMed] [Google Scholar]
- 42. Kook PH, Schellenberg S, Rentsch KM, Reusch CE, Glaus TM. Effect of twice‐daily oral administration of hydrocortisone on the bile acids composition of gallbladder bile in dogs. Am J Vet Res. 2011;72(12):1607‐1612. [DOI] [PubMed] [Google Scholar]
- 43. Kakimoto T, Kanemoto H, Fukushima K, Ohno K, Tsujimoto H. Effect of a high‐fat‐high‐cholesterol diet on gallbladder bile acid composition and gallbladder motility in dogs. Am J Vet Res. 2017;78(12):1406‐1413. [DOI] [PubMed] [Google Scholar]
- 44. Zarei K, Stroik MR, Gansemer ND, et al. Early pathogenesis of cystic fibrosis gallbladder disease in a porcine model. Lab Investig. 2020;100(11):1388‐1399. doi: 10.1038/s41374-020-0474-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative images of ultrasound and histopathological examinations of GBM cases included in the present study. Results of ultrasound and histopathological examinations of the gallbladders of GBM2, GBM5, and GBM14.
Table S1. Primer pairs used in RT‐qPCR for the 4 genes.
Table S2. Signalment of the cases with gallbladder mucocele included in the present study. Abbreviation: GBM, gallbladder mucocele.
Table S3. Total read counts and Mapping rate obtained by RNA sequencing.
Abbreviations: GBM, gallbladder mucocele.
Table S4. Differentially expressed genes extracted by comparison between gallbladder mucocele cases and healthy dogs. Abbreviation: FDR, false discovery rate.


