Abstract
Background
Testing for insulin dysregulation (ID) in horses is commonly performed to guide management and therapeutic strategies.
Objectives
To evaluate a newly developed glycemic pellets challenge (GPC) and compare results to those obtained using the low‐dose oral sugar test (OST).
Animals
Twenty‐four adult horses with unknown insulin status.
Methods
A randomized crossover trial was performed. Horses underwent GPC (0.5 g glycemic carbohydrates/kg body weight) and OST (0.15 mL corn syrup/kg body weight) 7 days apart. Feed was withheld before testing and blood samples were collected at T0, T60, T120, and T180 minutes for GPC and at T0, T60, and T90 minutes for OST. Blood glucose concentration was measured using a point‐of‐care glucometer and insulin by radioimmunoassay. Comparisons were made using nonparametric tests, linear regression, and Bland‐Altman agreement analysis.
Results
Eighteen horses consumed >85% of the GPC pellets within 10 minutes and had acceptable OST results. Maximum glucose (P = .02) and insulin (P = .007) concentrations were significantly higher for GPC compared with OST. Time to maximum insulin concentration (Tmax[ins]) varied within and between tests and neither Tmax[ins] (P = .28) nor maximum insulin concentration (P = .46) was correlated with the time horses took to consume pellets.
Conclusions
The GPC is well tolerated and may offer another diagnostic testing modality for ID. Blood glucose and insulin concentrations increase during GPC and reach higher concentrations than observed with low‐dose OST. The Tmax[ins] varied for GPC and OST, emphasizing the importance of identifying the optimal time range for the collection of samples to capture diagnostically relevant changes in insulin concentration.
Keywords: diagnostic testing, endocrinology, glycemic pellets challenge, insulin dysregulation, oral sugar test
Abbreviations
- BG
blood glucose
- Cmax
maximum concentration
- EDTA
ethylenediaminetetraacetic acid
- EMS
equine metabolic syndrome
- GPC
glycemic pellets challenge
- ID
insulin dysregulation
- OGT
oral glucose test
- OST
oral sugar test
- PPID
pituitary pars intermedia dysfunction
- RIA
radioimmunoassay
- Tmax
time at which maximum concentration was measured
- UMASS
University of Massachusetts
1. INTRODUCTION
Equine metabolic syndrome (EMS) is a collection of risk factors predisposing horses to the development of hyperinsulinemia‐associated laminitis. The hallmark of this syndrome is insulin dysregulation (ID), defined as basal or postprandial hyperinsulinemia, tissue insulin resistance, or any combination thereof. 1 , 2 , 3 Given the causal relationship between ID and development of laminitis, 1 , 4 determination of insulin status is critical to identify patients with ID and optimize management and therapeutic strategies to prevent laminitis.
Multiple diagnostic tests have been evaluated to determine insulin status in horses, 3 , 5 , 6 , 7 and it is important for tests to be both reliable and practical for veterinary practitioners in the field. Currently, the Equine Endocrinology Group provides interpretation criteria for the diagnosis of ID based on both basal insulin concentration as well as response to dynamic testing. 3 Tests that involve PO administration of sugars may improve clinical diagnosis as they engage metabolism at the level of the enterocyte and induce the release of incretins, which can result in a more marked insulin response than occurs with IV dextrose administration. 8 Dynamic testing can assist in identifying horses with ID that exhibit normal basal insulin concentration but have clinical signs consistent with EMS. 6 Commonly performed dynamic tests include the oral sugar test (OST) and the oral glucose test (OGT). The OST is performed by administering a measured amount of light corn syrup at a dosage of 0.15 (low‐dose test) or 0.45 (high‐dose test) mL/kg body weight PO and measuring blood glucose (BG) and insulin concentrations 60 or 90 minutes or both after dose administration. 9 When performing the OGT, dextrose powder is mixed with chaff or other low‐glycemic feed and fed to the horse after overnight fasting, and blood is collected 2 hours after the dextrose meal. 5 In some cases, the OST may not be practical because corn syrup is not available for purchase in some parts of the world, and there may be variability in the consistency of the product. There are also limitations of the OGT because horses require close monitoring to ensure full consumption of the dextrose‐containing feed, and it may be necessary to perform nasogastric intubation to administer dextrose directly into the stomach in horses unwilling to consume a glucose‐containing test meal.
Therefore, there is demand for a reliable, practical, dynamic test that can be performed worldwide. 10 To meet this demand, Boehringer Ingelheim Vetmedica GmbH (Ingelheim am Rhein, Germany) has developed a glycemic pellet (DysChEq) that can be used in place of corn syrup or dextrose powder mixed with chaff. The product can be used to conduct a glycemic pellets challenge (GPC) that is performed by feeding pellets to the horse in an amount calculated based on body weight and collecting blood at scheduled time points to assess BG and insulin responses. A comprehensive evaluation of the GPC has been performed in a multicenter trial conducted at several locations throughout the world. 10 , 11 Given that the low‐dose (0.15 mL corn syrup/kg) OST is the most commonly performed diagnostic test for ID in the United States, we compared the GPC to the low‐dose OST in a subset of horses from the larger study.
We hypothesized that BG and insulin concentrations would increase after consumption of glycemic pellets and mean maximum BG concentration (Cmax[glu]) and mean maximum insulin concentration (Cmax[ins]) would be higher for the GPC, compared with the low‐dose OST, because of the larger amount of glycemic carbohydrates delivered in the pellets. Furthermore, we hypothesized that mean Cmax[ins] would be detected at a significantly different time point for the GPC than for the low‐dose OST because of the presumed differences in time needed for the digestion of pellets compared with corn syrup.
2. MATERIALS AND METHODS
2.1. Animals
Twenty‐four adult horses from the University of Massachusetts Amherst (UMASS) teaching program were enrolled in the study. Physical examinations were performed on study days to assess the health of horses. All horses were housed in groups on pasture or dry lots with access to forage and had been under the care of UMASS personnel for a minimum of 1 year. One horse had been diagnosed with ID 5 years before (serum insulin concentration > 45 μU/mL after OST) but was not receiving medical management for ID. One horse was receiving pergolide for previously diagnosed pituitary pars intermedia dysfunction (PPID). All remaining horses (n = 22) were of unknown ID or PPID status (no prior confirmatory testing), but 4/22 (18%) exhibited ≥1 clinical signs that could be attributed to ID or PPID, including hypertrichosis, muscle wastage, changes in hoof growth patterns, and history of a laminitis episode occurring ≥2 years before the study date. Horses ranged in age from 4 to 29 years (median, 13.5 years) and included 12 mares, 11 geldings, and 1 stallion. None of the mares were pregnant or lactating at the time of the study. Multiple horse and pony breeds were represented including Appaloosa (2), Draft or Draft cross (3), Hanoverian (4), Morgan (5), Grade Pony (2), Quarter Horse (2), Standardbred (3), Thoroughbred (2), and Thoroughbred cross (1). All horses were routinely used for university‐related teaching activities.
2.2. Experimental design
Study protocols were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts. All testing was conducted in 2 periods occurring in November‐December 2019 (18 horses) and December 2020 (6 horses); the gap in sample collection was related to COVID‐19 pandemic restrictions. Each enrolled horse underwent both testing modalities (GPC and low‐dose OST) exactly 1 week apart; the order of the testing was randomly assigned and equal numbers of each test type were performed on each testing day. On the evening before performing the GPC or low‐dose OST, 0.2‐0.4 kg hay/100 kg body weight was provided, and no additional feed was offered until testing was completed the next morning; water was always available ad libitum. Testing was performed between 8 am and 11 am on testing days.
The GPC was performed using the proprietary glycemic pellets DysChEq product, which was provided by Boehringer Ingelheim Vetmedica GmbH. Pellets were formulated to provide 0.5 g carbohydrates per kg body weight (similar to the high‐dose OST protocol); absolute dosing volume changed slightly (by <15%) between the 2019 and 2020 testing periods because of a formulation change in the pellets (both pellet formulations used in the study provided a dose of 0.5 g carbohydrates per kg body weight to study horses). The GPC test performance was assessed using a palatability and tolerability score for each horse, which consisted of the amount of pellets eaten, time taken to eat the full amount of pellets, and presence of any signs of discomfort during eating (including head shaking or flehmen response). 11 Horses were allowed 10 minutes to eat the pellets and after this time, any remaining pellets were weighed. Acceptable test performance consisted of eating a minimum of 85% of the volume of offered pellets within 10 minutes of administration with no or only mild signs of discomfort while eating the pellets. Blood samples were collected for insulin and BG measurements immediately before offering the pellets and at 60, 120, and 180 minutes after pellets were offered for consumption.
The low‐dose OST was performed according to the published method. 3 , 7 Low‐dose OST was selected because this method is more commonly used in the United States than the high‐dose OST. Briefly, a dose (0.15 mL/kg body weight) of commercially available corn syrup (Karo Light corn syrup, ACH Food Companies, Inc., Oakbrook Terrace, Illinois, USA) was administered PO using dose syringes after the feed was withheld overnight. Blood samples were collected for insulin and BG measurements immediately before administering the corn syrup and at 60 and 90 minutes after dose administration. Acceptable performance of the low‐dose OST was defined as BG and insulin concentrations being higher than baseline at 60 or 90 minutes.
For all samples, blood was collected by jugular venipuncture directly into 10 mL vacuum tubes containing a clot activator (BD Vacutainer Serum Blood Collection Tubes, BD‐367820, Becton Dickinson, Franklin Lakes, New Jersey, USA) and 6 mL vacuum tubes containing ethylenediaminetetraacetic acid (EDTA; BD Vacutainer EDTA Tubes, BD‐367863, Becton Dickinson). Blood collection was performed using a 20‐gauge Vacutainer needle. All horses were monitored for adverse reactions for the duration of the testing protocol.
2.3. Sample processing and analysis
Blood glucose concentrations were measured in fresh whole blood immediately after blood collection using a handheld glucometer (Accu‐Chek, Roche Diabetes Care, Indianapolis, Indiana, USA). 12 Plasma and serum were separated by centrifugation within 6 hours of collection and samples were stored in 1 mL aliquots at −80°C until batch analysis. Samples were shipped overnight on dry ice to the Animal Health Diagnostic Center Laboratory at Cornell University (Ithaca, New York, USA) for analysis. Insulin concentrations were measured using a radioimmunoassay (RIA; Millipore Human Insulin Specific RIA, EMD Millipore Corporation, Billerica, Massachusetts, USA) in serum samples collected during the GPC (T0, T60, T120, T180) and EDTA plasma samples collected during the low‐dose OST (T0, T60, T90). The difference in sample types used for insulin measurement was necessitated by the sample collection requirements of the larger multicenter trial. The laboratory routinely measures insulin concentrations in serum and EDTA plasma samples using the same RIA and identical reference ranges are used for serum and plasma insulin concentrations.
2.4. Test interpretation
Current recommendations for interpretation of the low‐dose OST utilize an insulin cutoff of >45 μIU/mL insulin measured by RIA at 60 or 90 minutes as positive for ID (>65 μIU/mL insulin cutoff for the high‐dose OST). The recent publication describing the GPC established a cutoff of 83 μIU/mL insulin measured using IMMULITE 2000xpi at 120 minutes as positive for ID. IMMULITE is a chemiluminescent immunoassay system; its results are well correlated with RIA results but bias exists between test methods, necessitating individual diagnostic cutoffs for each method. 13 , 14 As yet, diagnostic cutoffs for the GPC using RIA have not been established. For purposes of comparison between methods used in our study, in the absence of RIA‐specific diagnostic cutoffs for the GPC, the >65 μIU/mL insulin diagnostic cutoff for the high‐dose (0.45 mL/kg corn syrup) OST (which provides a similar amount of glycemic carbohydrates) was employed to interpret the GPC insulin response.
2.5. Data and statistical analyses
Demographic data for all horses are reported descriptively. Only data from the 18 horses that completed both GPC and low‐dose OST with acceptable results were included in comparisons of the 2 tests.
Maximum BG (Cmax[glu]) and maximum insulin (Cmax[ins]) were identified and used for comparisons between tests. The time at which the Cmax[glu] was measured was defined as (Tmax[glu]), and the time at which the Cmax[ins] was measured was defined as (Tmax[ins]). The percentage increase in BG and insulin concentrations was calculated by comparing Cmax[glu] and Cmax[ins] to their respective basal concentrations.
Shapiro‐Wilk tests were performed to evaluate data for normality. Because of small sample size and nonnormally distributed data, nonparametric testing was used for all comparisons, and median and range values were reported. Wilcoxon matched‐pairs signed rank tests were used to compare magnitude of differences in these nonnormally distributed data. Fisher's exact test and linear regression were used to compare groups. Bland‐Altman plots were used to examine bias between testing methods. A P‐value of .05 was considered significant. GraphPad Prism (GraphPad 9.2.0 for Windows, GraphPad Software, San Diego, California, USA) was used for all statistical analyses.
3. RESULTS
3.1. Animals
Physical examinations were performed before all study procedures to confirm that heart rate, respiratory rate, and rectal temperature were within normal range for all horses. No health problems were reported by the staff caring for the horses at the facility. All horses tolerated the GPC and low‐dose OST with no adverse events.
3.2. Test performance
Nineteen horses consumed >85% of the offered pellets within 10 minutes when the GPC was performed, and data from these horses was considered acceptable. The pellet formulation was changed between the first and second testing period but the majority of horses in each group voluntarily consumed the pellets, with >85% of pellets consumed within 10 minutes in 78% (14/18) of horses in the first testing period and in 83% (5/6) of horses in the second testing period. For the low‐dose OST, acceptable results were obtained for all horses except 1, which demonstrated maximum BG and insulin concentrations at T0, and data from this horse were excluded from analyses. Results from the GPC and low‐dose OST therefore were compared using data obtained from 18 horses with acceptable data from both tests.
3.3. Blood glucose and insulin concentrations
Basal (T0) BG concentrations did not differ significantly between GPC and low‐dose OST groups (Figure 1; P = .20). All 18 horses experienced an increase in BG concentration with both the GPC and the low‐dose OST. A significant difference in Cmax[glu] was detected between tests (Figure 1; GPC Cmax[glu] median, 110 mg/dL; OST Cmax[glu] median, 105 mg/dL; P = .02).
FIGURE 1.
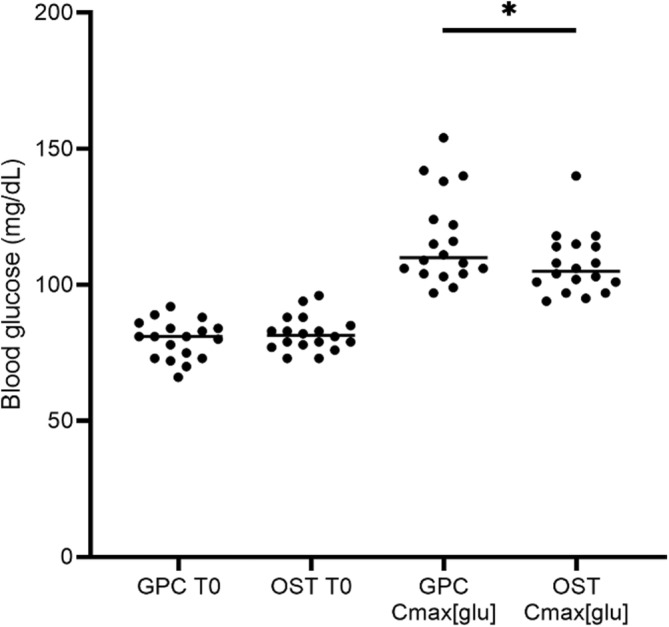
Blood glucose concentrations measured during the OST and GPC. Blood glucose concentrations (mg/dL) at baseline (T0) and maximum concentration measured (Cmax[glu]) during the glycemic pellets challenge (GPC) and oral sugar test (OST). Baseline blood glucose concentrations (GPC T0 BG median 81 mg/dL, range 66‐92 mg/dL; OST T0 BG median 81.5 mg/dL, range 73‐96 mg/dL) did not differ significantly between tests (P = .20). Cmax[glu] values (GPC median 110 mg/dL, range 97‐154 mg/dL; OST median 105 mg/dL, range 94‐140 mg/dL) differed significantly (P = .02, denoted by asterisk) between tests.
Basal (T0) serum or plasma insulin concentrations were not significantly different between the GPC and low‐dose OST (Figure 2: P = .52). All horses experienced an increase in insulin concentration with both the GPC and low‐dose OST. A significant difference in Cmax[ins] was detected between tests (Figure 2; GPC Cmax[ins] median, 37.4 μIU/mL; OST Cmax[ins] median, 25.8 μIU/mL; P < .01). The Cmax[ins] values were correlated between test types (P < .01; Figure 3). However, for each individual test type, Cmax[ins] and Cmax[glu] did not show a significant correlation when compared using linear regression (GPC, P = .23; OST, P = .14).
FIGURE 2.
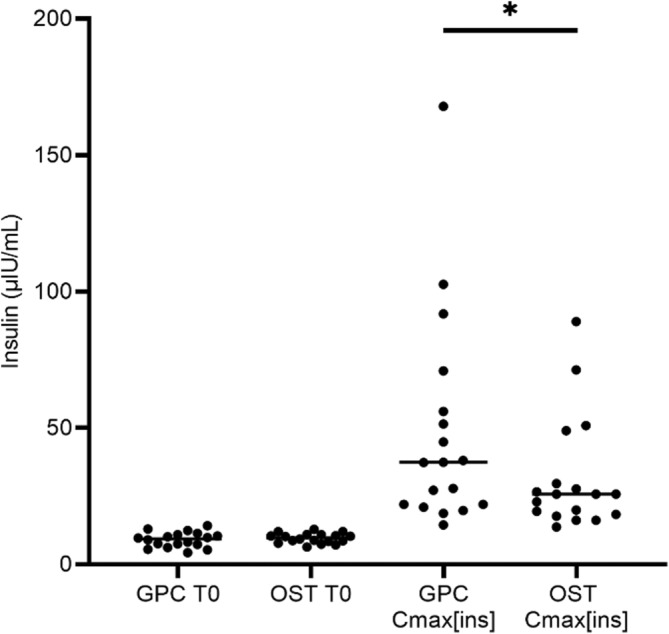
Insulin concentrations measured during the OST and GPC. Insulin concentrations (μIU/mL) at baseline (T0) and maximum insulin concentration detected (Cmax[ins]) during the glycemic pellets challenge (GPC) and oral sugar test (OST). Baseline insulin concentrations (GPC T0 insulin median 9.3 μIU/mL, range 4.3‐14.1; OST T0 insulin median 9.7 μIU/mL, range 6.3‐12.8) did not differ significantly between tests (P = .52). Cmax[ins]values (GPC Cmax[ins] median 37.4 μIU/mL, range 14.4‐167.9 μIU/mL; OST Cmax[ins] median 25.8 μIU/mL, range 13.7‐89.0 μIU/mL) differed significantly (P = .02, denoted by asterisk) between tests.
FIGURE 3.
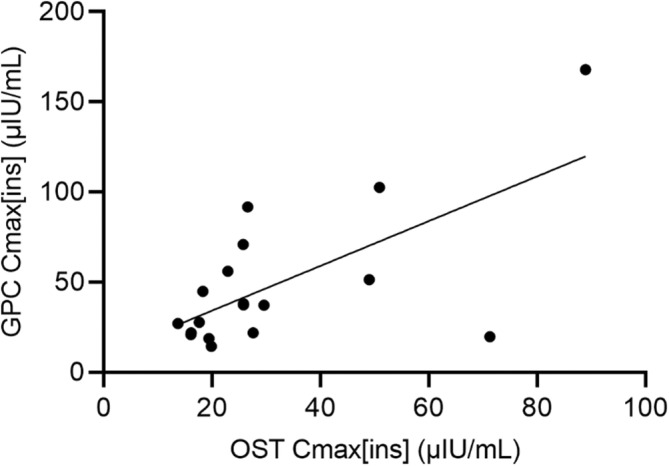
Correlation of maximum insulin concentration (Cmax[ins]) measured during the glycemic pellets challenge (GPC) and oral sugar test (OST). Cmax[ins]values were significantly correlated between the 2 test types when compared using linear regression (R2 = 0.43, P < .01).
For the GPC, Tmax[glu] was detected at 120 minutes in 67% (12/18) of horses, with Tmax[glu] occurring at 60 minutes or 180 minutes in 11% (2/18) and 22% (4/18) of horses, respectively. For the low‐dose OST, Tmax[glu] was detected at 90 minutes for most horses (56%, 10/18), and at 60 min for 39% (7/18) of horses. One horse had the same BG concentration recorded at 60 and 90 minutes and thus a Tmax[glu] could not be determined.
For the GPC, the Tmax[ins] was 60 minutes for most horses (44%, 8/18), with Tmax[ins] occurring at 120 or 180 minutes in 33% (6/18) and 22% (4/18) of horses, respectively. For the low‐dose OST, Tmax[ins] was detected at 90 minutes for most horses (56%, 10/18), and at 60 minutes in 44% (8/18) of horses. The Tmax[glu] and Tmax[ins] were the same (maximum concentrations of both insulin and glucose recorded at the same time point) in 44% (8/18) GPC and 61% (11/18) low‐dose OST results. The Tmax[glu] occurred earlier than Tmax[ins] in 17% (3/18) GPC and 17% (3/18) low‐dose OST results. The Tmax[glu] occurred later than Tmax[ins] in the remaining 39% (7/18) GPC and 17% (3/18) low‐dose OST results.
A significant (P < .01) difference in the percentage increase in BG concentration was detected between test modalities. A median increase of 146% (range, 112%‐203%) from T0 to Cmax[glu] was detected for the GPC, compared with 131% (range, 119%‐151%) for the low‐dose OST. A significant (P < .01) difference in the percentage increase in insulin concentration also was detected between test modalities. A median increase of 451% (range, 193%‐1298%) from T0 to Cmax[ins] was detected for the GPC compared with 252% (range, 132%‐1016%) for the low‐dose OST.
Bland‐Altman plots indicated that the GPC resulted in a higher Cmax[glu] (bias, 9.1 mg/dL; Figure 4A) and higher Cmax[ins] (bias, 17.0 μIU/mL; Figure 4B) than the low‐dose OST in the study population.
FIGURE 4.
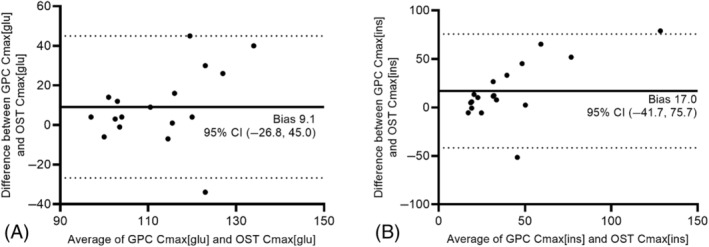
Bland‐Altman plots showing the effect of test modality on maximum glucose concentration (Cmax[glu]; A) and maximum insulin concentration (Cmax[ins]; B) in 18 horses that underwent both tests. Calculated bias is shown on all graphs as a solid line; the dotted lines mark the limits of each 95% confidence interval (CI).
Time to adequate (>85%) consumption of pellets (measured in minutes) was not correlated with Tmax[ins] or Cmax[ins] when evaluated by linear regression (P = .28, P = .46, respectively). Median time to adequate consumption of pellets was similar for all horses, taking 4 minutes for horses with Tmax[ins] occurring at 60 and 120 minutes, and taking 3 minutes for horses with Tmax[ins] occurring at 180 minutes.
3.4. Test interpretation
Based on low‐dose OST results, 22% (4/18) of horses had insulin concentrations above the reference interval for this test (>45 μIU/mL at 60 or 90 minutes). Of these 4 horses, 2 (50%) also had GPC insulin concentrations >65 μIU/mL at 120 minutes. Two horses with positive low‐dose OST results had GPC Cmax[ins] results <65 μIU/mL (GPC Cmax[ins] 19.7 and 51.4 μIU/mL). Additionally, 2 horses with normal low‐dose OST results (OST Cmax[ins] 25.8 and 26.6 μIU/mL) had GPC Cmax[ins] >65 μIU/mL (GPC Cmax[ins] 70.9 and 91.8 μIU/mL, measured at 180 minutes for both horses). Twelve (67%) of the 18 horses had both low‐dose OST (Cmax[ins] <45 μIU/mL and GPC Cmax[ins] <65 μIU/mL).
4. DISCUSSION
We found that feeding horses glycemic pellets to conduct a GPC elicits expected increases in BG and insulin concentrations. These findings are consistent with the results of the larger international multicenter trial evaluating the use of carbohydrate pellets in horses. 11 When compared with the low‐dose OST, higher BG and insulin concentrations were detected during the GPC, which was attributed to the larger amount of glycemic carbohydrates delivered with the pellets. Time to maximum insulin concentration varied within and between tests, and differences could not be attributed to variability in the time taken for horses to consume pellets.
In our study cohort, most (79%) horses voluntarily consumed >85% of the offered pellets within 10 minutes, indicating good overall palatability. This factor is important because when GPC is performed in the field, owners will be instructed to feed their horse a measured amount of pellets and schedule the veterinarian to arrive in time to collect blood at specific time points afterward. To achieve test success, pellets must be palatable and supplied in an amount that can be readily consumed. Results of this study show that most horses voluntarily consume the pellets, and the amount of glycemic carbohydrate ingested is sufficient to increase BG and insulin concentrations above those observed with the low‐dose (0.15 mL corn syrup/kg) OST. Voluntary consumption of pellets may be affected by the feeds that the horse is accustomed to receiving or dental abnormalities. Horses that are unaccustomed to being fed concentrate feeds or less tolerant of diet changes may refuse to eat the pellets. Although the mass of pellets fed to an average 450 kg horse (<400 g of pellets) is small enough to be readily consumed by most horses within 10 minutes, this timeframe may not be achievable for horses with dental disease or other conditions affecting mastication or deglutition. Although horse owners may be concerned about feeding a concentrated source of sugars to horses with known or suspected ID, the amount of sugars contained in a dose of pellets is comparable to the amounts administered for the high‐dose OST and OGT. 3 , 5
As described in the results, initial (T0) BG and insulin concentrations were not significantly different when the GPC and low‐dose OST were performed in the same horses. However, significant differences were observed between tests for Cmax[glu] and Cmax[ins], which was expected given the difference in the amount of glycemic carbohydrate ingested. Bland‐Altman plots confirmed these biases, with higher Cmax[glu] and Cmax[ins] values recorded for the GPC compared with the low‐dose OST. The percentage increase from baseline (T0) to Cmax[glu] and Cmax[ins] also was found to be significantly higher for the GPC compared with the low‐dose OST. These biases are not surprising, because the PO dose of carbohydrates administered was higher in the GPC and thus could be anticipated to provoke more exaggerated responses. The low‐dose OST commonly used in the United States was selected for our study. Comparing the GPC to the high‐dose OST used in the United Kingdom and Europe would more closely match the total dose of glycemic carbohydrate administered. However, although the use of the high‐dose OST can elicit significantly higher insulin responses than the low‐dose OST in ponies diagnosed with ID, 9 the same was not found to be true for horses. 15 Thus, it is uncertain whether comparison of the GPC to high‐dose OST would be likely to provide different results from those presented in our study.
The Tmax[glu] and Tmax[ins] values were highly variable for both tests. Limited sampling was performed during the GPC, and it is conceivable that the insulin peak was missed as a result. However, the observation that Cmax[ins] was not consistently observed at any single time point indicates that more frequent sampling would not be likely to yield different findings caused by horse‐to‐horse and within‐horse variability. In addition, the time at which maximum values could be detected was constrained by the testing modalities used, and sample collections were performed according to established testing protocol to maximize the clinical applicability of the results. Alterations in gastric emptying time, 16 differences in intestinal absorption capacity, and the effect of incretins stimulated by each discrete testing modality 8 could have contributed to the variability in Tmax[glu] and Tmax[ins] observed for both tests and may be a factor that cannot be standardized. Multiple sampling times are recommended for the OST, and the same approach may enhance diagnostic utility of the GPC. 3 , 5 , 17 A delay in Tmax[ins] was not observed during the GPC, which may have been associated with greater digestibility of the pellets than predicted, leading to comparable timing to the OST for transit of test materials to the small intestine for glucose absorption and subsequent insulin increase. 18
In our study, the low‐dose OST and GPC were performed in the same horses to compare insulin and glucose concentrations during each test. Although Cmax[ins] was correlated between the 2 testing types, Cmax[ins] was not significantly correlated with Cmax[glu] within each individual test modality. When comparing the results of 2 different tests, it is useful to consider the within‐test variability reported for each individual test. Only reasonable agreement is noted in test results when the OST is repeated in the same horses 7 to 14 days apart, with significant inter‐test variability in insulin concentrations reported. 19 , 20 In our study, tests were separated by 7 days and all efforts were made to minimize errors arising from sample collection and processing. Sample processing was performed by the same investigator using standardized methods, and all samples were analyzed at a commercial veterinary diagnostic laboratory using previously validated testing methods. 13 , 21 Because season may have an effect on basal insulin concentrations 22 , 23 as well as results of dynamic testing, 9 all testing was performed in the winter months (November/December) to minimize that source of variability. Throughout the study, BG measurements were performed on whole blood using a hand‐held glucometer. Measurements obtained using a glucometer can be less accurate than results obtained from chemistry analyzers or using different sample collection and processing techniques and thus may have introduced minor variability or imprecision to BG results. 24
A limitation of our study is the low number of horses that would be classified as having ID based on the results of the low‐dose OST. Evaluation of the GPC test in a population of horses with confirmed ID (based on OST or other testing) could provide more information about the magnitude of glycemic and insulin responses provoked by the GPC test in insulin‐dysregulated horses. Additionally, comparison of the GPC and high‐dose OST should be considered, because these 2 tests provide more similar glycemic challenges and may potentially provide better correlation between test results. Another limitation is the lack of RIA measurement‐specific interpretation guidelines for the GPC, which would be an asset for interpreting results of this method for horses in the United States, in which RIA testing is more commonly performed. Finally, the use of serum for insulin measurements in the GPC and plasma for the OST could have contributed to some of the variability observed between the OST and GPC methods, although both serum and plasma are commonly measured using the same RIA, and identical reference intervals are used.
In summary, our results show that the BG and insulin concentrations increase during the GPC and are higher than those detected during the low‐dose OST. The GPC offers a promising new modality to evaluate insulin status in horses that are less amenable to traditional testing modalities. The GPC was well tolerated by the horses and mimics natural feeding behavior and digestion. Further work is required to optimize the GPC for the diagnosis of ID in horses and to establish specific test interpretation criteria for multiple assay methods. The variability in Tmax[ins] observed for both the GPC and OST emphasizes that diagnostic utility of these tests may be improved by collecting multiple blood samples to capture peak insulin concentration, which can vary because of within‐horse factors.
CONFLICT OF INTEREST DECLARATION
Nicholas Frank consults for, and Johanna Sonntag, Dania Reiche, and Tobias Warnken are or were employed by, Boehringer Ingelheim, which provided funds to support this study; these authors did not contribute to data collection or analysis. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Massachusetts IACUC.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This study was supported by Boehringer Ingelheim Vetmedica GmbH.
Thane K, Sonntag J, Warnken T, Reiche D, Uricchio C, Frank N. Comparison of a customized glycemic pellets challenge with the oral sugar test to measure glycemic and insulinemic responses in horses. J Vet Intern Med. 2024;38(6):3281‐3287. doi: 10.1111/jvim.17191
REFERENCES
- 1. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J Vet Intern Med. 2010;24:467‐475. [DOI] [PubMed] [Google Scholar]
- 3. Frank N, Bailey SR, Bertin FR, et al. Recommendations for the diagnosis and treatment of equine metabolic syndrome (EMS). 2020. https://sites.tufts.edu/equineendogroup/
- 4. de Laat MA, Reiche DB, Sillence MN, McGree JM. Incidence and risk factors for recurrence of endocrinopathic laminitis in horses. J Vet Intern Med. 2019;33:1473‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith S, Harris PA, Menzies‐Gow NJ. Comparison of the in‐feed glucose test and the oral sugar test. Equine Vet J. 2016;48:224‐227. [DOI] [PubMed] [Google Scholar]
- 6. McFarlane D. Diagnostic testing for equine endocrine diseases: confirmation versus confusion. Vet Clin North Am Equine Pract. 2019;35:327‐338. [DOI] [PubMed] [Google Scholar]
- 7. Schuver A, Frank N, Chameroy KA, Elliott SB. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet Sci. 2014;34:465‐470. [Google Scholar]
- 8. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310:E61‐E72. [DOI] [PubMed] [Google Scholar]
- 9. Jocelyn NA, Harris PA, Menzies‐Gow NJ. Effect of varying the dose of corn syrup on the insulin and glucose response to the oral sugar test. Equine Vet J. 2018;50:836‐841. [DOI] [PubMed] [Google Scholar]
- 10. Warnken T, Schaub C, Delarocque J, et al. Palatability, glycemic, and insulinemic responses to various carbohydrate formulations: alternatives for the diagnosis of insulin dysregulation in horses? J Vet Intern Med. 2023;37:282‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Laat MA, Warnken T, Delarocque J, et al. Carbohydrate pellets to assess insulin dysregulation in horses. J Vet Intern Med. 2023;37:302‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2017;49:238‐243. [DOI] [PubMed] [Google Scholar]
- 13. Warnken T, Huber K, Feige K. Comparison of three different methods for the quantification of equine insulin. BMC Vet Res. 2016;12:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carslake HB, Pinchbeck GL, McGowan CM. Evaluation of a Chemiluminescent immunoassay for measurement of equine insulin. J Vet Intern Med. 2017;31:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyn Macon E, Harris P, Partridge E, Day Barker V, Adams A. Effect of dose and fasting on oral sugar test responses in insulin dysregulated horses. J Equine Vet Sci. 2021;107:103770. [DOI] [PubMed] [Google Scholar]
- 16. Metayer N, Lhote M, Bahr A, et al. Meal size and starch content affect gastric emptying in horses. Equine Vet J. 2004;36:436‐440. [DOI] [PubMed] [Google Scholar]
- 17. Mair TS, Hillyer MH, Taylor FG, et al. Small intestinal malabsorption in the horse: an assessment of the specificity of the oral glucose tolerance test. Equine Vet J. 1991;23:344‐346. [DOI] [PubMed] [Google Scholar]
- 18. Dyer J, Fernandez‐Castano Merediz E, Salmon KS, et al. Molecular characterisation of carbohydrate digestion and absorption in equine small intestine. Equine Vet J. 2002;34:349‐358. [DOI] [PubMed] [Google Scholar]
- 19. Frank N, Walsh DM. Repeatability of oral sugar test results, glucagon‐like peptide‐1 measurements, and serum high‐molecular‐weight adiponectin concentrations in horses. J Vet Intern Med. 2017;31:1178‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knowles EJ, Harris PA, Elliott J, Menzies‐Gow NJ. Use of the oral sugar test in ponies when performed with or without prior fasting. Equine Vet J. 2017;49:519‐524. [DOI] [PubMed] [Google Scholar]
- 21. Borer‐Weir KE, Bailey SR, Menzies‐Gow NJ, Harris PA, Elliott J. Evaluation of a commercially available radioimmunoassay and species‐specific ELISAs for measurement of high concentrations of insulin in equine serum. Am J Vet Res. 2012;73:1596‐1602. [DOI] [PubMed] [Google Scholar]
- 22. Hart KA, Wochele DM, Norton NA, McFarlane D, Wooldridge AA, Frank N. Effect of age, season, body condition, and endocrine status on serum free cortisol fraction and insulin concentration in horses. J Vet Intern Med. 2016;30:653‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams CA, Kenny LB, Burk AO. Effects of grazing system, season, and forage carbohydrates on glucose and insulin dynamics of the grazing horse. J Anim Sci. 2019;97:2541‐2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rendle DI, Armstrong SK, Heller J, Hughes KJ. Precision and accuracy of a point‐of‐care glucometer in horses and the effects of sample type. Vet J. 2019;252:105359. [DOI] [PubMed] [Google Scholar]


