Abstract
Background
Before the discovery of effective antiviral drugs, feline infectious peritonitis (FIP) was a uniformly fatal disease of cats. Multiple antiviral treatments have been recognized, but optimization of treatment protocols is needed.
Objective
To evaluate the efficacy of PO molnupiravir (MPV; EIDD‐2801) to treat effusive FIP.
Animals
Ten cats with naturally occurring effusive FIP and 10 historical control cats with effusive FIP treated with PO GS‐441524.
Methods
A single‐center, prospective, open‐label longitudinal, non‐inferiority trial with historical controls. Ten cats with FIP were enrolled and treated with PO MPV (10‐15 mg/kg PO q12h) for 84 days. Cats were evaluated at 0, 6, and 16 weeks, and the proportion of cats in clinical remission at 16 weeks was determined. Survival and clinicopathologic features were compared with historical control cats with effusive FIP treated with PO GS‐441524.
Results
Eight of the 10 cats treated with MPV survived and were in remission at 16 weeks. The 2 non‐survivors died in the first 24 hours of treatment. No adverse events that necessitated discontinuation of treatment were observed. Survival of cats treated with PO MPV was non‐inferior to historic control cats treated with PO GS‐441524 (5/9 [55%] survived), with a difference in survival of 25% (90% confidence interval, −9.3% to 59.3%). Clinicopathologic features associated with FIP normalized during the study period, and no differences in clinicopathologic data at each study time point were observed when comparing cats treated with MPV and GS‐441524.
Conclusions and Clinical Importance
Molnupiravir is an effective antiviral treatment for effusive FIP.
Keywords: antiviral, feline coronavirus, FIP, infection
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- FCoV
feline coronavirus
- FECV
feline enteric coronavirus
- FIPV
feline infectious peritonitis virus
- MCV
mean corpuscular volume
- MPV
molnupiravir
1. INTRODUCTION
Feline coronavirus (FCoV), is a large single‐stranded RNA virus in the family Coronaviridae found in cats worldwide. 1 The virus exists in 2 biotypes. The first is a ubiquitous, nonpathogenic biotype feline enteric coronavirus (FECV) with infection rates of up to 90% of cats in multi‐cat housing. 2 The second biotype, feline infectious peritonitis virus (FIPV), arises after a change in viral tropism to macrophages, which is thought to occur because of spike mutations. 3 The clinical spectrum of FIP is variable, with some cats presenting with high protein concentration cavitary effusions and some with pyogranulomatous infiltrates that can affect nearly any organ system. Without treatment, both forms of the disease are essentially 100% fatal. 1 , 3
Safe and effective antiviral treatments for cats with FIP have been discovered. The nucleoside analog GS‐441524 has been most widely studied, and survival rates range from 55% to 100%. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Other antiviral drugs also have been effective in treating FIP. 13 , 14 , 15 The nucleoside analog, EIDD‐2801 or molnupiravir (MPV), is an antiviral prodrug that is orally bioavailable and was developed for treating COVID‐19. Molnupiravir inhibits FCoV replication in vitro, and a pharmacokinetic study in cats determined that PO dosing at 10 mg/kg q12h results in plasma concentrations above the minimum effective concentration for the inhibition of FCoV replication. 16 Indeed, some promising clinical outcomes indicate that MPV may be an effective treatment for FIP, with survival rates of 78% to 92%. 13 , 17 These reports include cats that have failed other antiviral treatments, were treated with unlicensed owner‐sourced medications, or were derived from limited field trials. Further assessment of MPV as a first‐line treatment for effusive FIP is needed to determine efficacy compared with other antiviral treatments.
Our objective was to evaluate the efficacy of PO MPV in treating naturally occurring effusive FIP in a prospective clinical trial compared with historical controls from previous clinical trials treating cats with effusive FIP with GS‐441524 using identical enrollment criteria, methodology, and study endpoints.
2. MATERIALS AND METHODS
2.1. Study design
A single‐center, prospective, unblinded longitudinal trial with a single treatment arm was performed. A historical control group of cats with effusive FIP recruited between November 2021 and November 2022, and treated with PO GS‐441524 (12.5‐15 mg/kg PO q24h), was used for comparison. 5 Cats confirmed to have effusive FIP were considered for enrollment between February and June 2023, and assigned to the single treatment arm. Our study followed ethical guidelines and was approved by the University of California, Davis Institutional Animal Care and Use Committee (Protocol number 23282, approval date February 15, 2023).
2.2. Inclusion criteria
Cats suspected of having effusive FIP were considered for enrollment if pleural, peritoneal, or pericardial effusion was observed ultrasonographically by a study veterinarian (K.L. Reagan, T. Brostoff). To be included, a diagnosis of FIP had to be achieved by (a) immunohistochemical or immunocytochemical detection of FCoV antigen in the context of pyogranulomatous inflammation as determined by a veterinary pathologist or (b) positive FCoV RNA using quantitative RT‐PCR from a commercial laboratory or University of California, Davis Veterinary Clinical Diagnostic Laboratory obtained from an effusion specimen with ≥3 of the following clinical criteria: rectal temperature >102.5 °F documented twice over ≥12 hours, whole blood lymphocyte count below the lower limit of normal, serum globulin concentration above the upper limit of normal, serum albumin : globulin ratio <0.6, serum bilirubin concentration above the upper limit of normal, cavitary effusion with total protein concentration >3.5 g/dL and cytology findings assessed by a veterinary clinical pathologist to be consistent with FIP, or positive FCoV antibody serology tested by a commercial laboratory or the University of California, Davis Veterinary Clinical Diagnostic Laboratory. Blood test results obtained using in‐house analyzers at the referring veterinarian or a commercial laboratory were utilized for patient screening.
To be included in the study, cats were required to have negative feline leukemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody test results in the preceding month. Cats were excluded from the clinical trial if they had been treated with any anti‐coronaviral medications before enrollment. Additionally, cats were excluded if the cat or caretaker was not able to fulfill the requirements of the study, were assessed to have a high likelihood of noncompliance, or if the caretaker was not able to administer PO medications reliably. Further, cats that were critically ill and not expected to survive for at least 48 hours were excluded (moribund, agonal, nonresponsive, or stuporous on physical examination), cats with neutrophil count <2000/μL, platelet count <75 000/μL without platelet clumps, serum creatinine concentration above the upper limit of normal, serum alanine aminotransferase (ALT) activity > twice the upper limit of normal, cats assessed by the study veterinarian as needing blood transfusion, or cats with marked hypothermia (rectal temperature <97 °F). These exclusion criteria were identical for the historical control group.
2.3. Treatment and study protocol
Cats were treated with PO MPV 10 to 15 mg/kg PO q12h for 84 days. The drug was sourced from Natural Micron Pharm Tech (NM PharmTech; Tai'an, China) with a purity of >99% and compounded into gelatin capsules. High‐pressure liquid chromatography was used to confirm the identity of the drug. 16 Caretakers weighed the cats weekly between visits using a baby scale, and the dose was adjusted to remain within the prescribed range.
Cats were examined by study veterinarians (K.L. Reagan, T. Brostoff) at enrollment (week 0), week 6, and week 16. Physical examination, CBC (Advia 120; Siemens), and serum biochemistry panel (Cobas c501/6000; Roche) were performed at each visit. The FCoV antibody indirect immunofluorescence serology assay (University of California, Davis Veterinary Clinical Diagnostic Laboratory) was performed at weeks 0 and 16. Serial dilutions of patient serum specimens were tested starting at a dilution of 1 : 20 to a maximum dilution of 1 : 20 480. Specimens with no staining at 1 : 20 were recorded as negative. Effusion volume was approximated at each visit. Peritoneal effusion was quantified by a point‐of‐care ultrasound scan score recorded from 0 to 4. 18 Pleural effusion was monitored using a thoracic point‐of‐care ultrasound scan, and the fluid depth at the area of greatest accumulation was recorded in centimeters. 19 Disease remission was assessed at 16 weeks and was defined as the resolution of clinical signs associated with initial presentation, lack of relapse of clinical signs after study drug discontinuation, resolution of cavitary effusion, and normalization of serum globulin and bilirubin concentrations.
Supportive care, hospitalization, and additional reevaluations were allowed at the discretion of the attending veterinarian. All adverse events were recorded. Cats that died or were euthanized during the study underwent complete necropsy examination with caretaker consent within 24 hours of death. Tissue was collected and placed in 10% buffered formalin for >24 hours. Tissue from necropsied cats was trimmed, paraffin‐embedded, and processed for histologic examination following standard protocols. Immunohistochemistry (IHC) to detect FCoV antigen (FIPV3‐70, Custom Monoclonals International, Sacramento, CA) was performed as previously described. 20 Interpretation of histopathology and IHC was performed by a single veterinary anatomic pathologist (B.G. Murphy).
2.4. Statistical analysis
The study was designed to test a non‐inferiority hypothesis compared with a historical control population of cats treated with PO GS‐441524 with a primary outcome measure of disease remission at 16 weeks. A non‐inferiority limit of −15% was chosen, and a power calculation determined a minimum of 10 cats were needed to achieve 90% power and alpha of 5% based on a survival rate of 55% in the control group and an anticipated survival rate of 92.3% in the experimental group. 5 , 13 , 21 Demographic data and secondary outcome measures including clinicopathologic data were summarized with descriptive statistics. Parametric or nonparametric testing was performed based on normality testing using a Shapiro‐Wilk test. A mixed‐effects model was utilized, with Tukey's multiple comparisons test, to evaluate clinicopathologic variables throughout the study for cats treated with MPV. At each study time point, clinicopathologic features also were compared between cats in the MPV group and the control group using Šídák's multiple comparisons test. Commercially available software was utilized for statistical analysis (Prism Version 10.0.0, GraphPad).
3. RESULTS
3.1. Study population
Seventy‐one cats were screened for enrollment, and 61 were excluded because they did not meet the study inclusion criteria. Ten cats met the enrollment criteria and were enrolled in the trial. These included 1 purebred Burmese cat and 9 domestic short or medium‐haired cats (Table 1). All cats had positive FCoV real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) obtained from a cavitary effusion specimen. All cats also fulfilled ≥3 minor criteria for enrollment, including 10 with serum albumin : globulin ratio <0.6, 9 with positive FCoV serology, 8 with lymphopenia, 7 with cavitary effusion cytology performed that was consistent with FIP, 7 with hyperbilirubinemia, 6 with persistent fever, and 6 with hyperglobulinemia. All cats were FeLV and FIV negative.
TABLE 1.
Baseline demographics.
| Cats treated with MPV | Historical controls treated with GS‐441524 | |
|---|---|---|
| Total number | 10 | 9 |
| Purebred | 1 | 3 |
| Age (in months) a | 11 (5‐86) | 7 (3‐164) |
| Sex | ||
| Intact female | — | 2 |
| Spayed female | 4 | 5 |
| Intact male | 1 | 1 |
| Neutered male | 5 | 1 |
| Body weight (kg) b | 3.6 (1.7) | 2.8 (1.2) |
| Effusion location | ||
| Abdominal | 8 | 8 |
| Pleural | 2 | 1 |
Non‐normal results presented as median and range.
Normal results presented as mean and SD.
Eight cats had peritoneal effusion, and 2 cats had pleural effusion (Table 2). Of the 8 cats with peritoneal effusion, 6 had an abdominal‐focused assessment with sonography for tracking (AFAST) score of 4/4, and 2 had a score of 3/4 at enrollment. The peritoneal effusion had a median total nucleated cell count of 990 cells/μL (range, 180‐31 000 cells/μL) and a total protein concentration of 5.5 g/dL (range, 4.8‐5.7 g/dL). Two cats had pleural effusion, with a maximum fluid accumulation of 2 and 3 cm. Pleural effusion nucleated cell count and total protein concentration were 2100 and 3800 cells/μL and 7.6 and 5.6 g/dL, respectively. A veterinary clinical pathologist reviewed effusion cytology in 7/10 cases, and no other underlying cause of effusion was noted in any of those specimens.
TABLE 2.
Baseline clinicopathologic features.
| Cats treated with MPV | Historical controls treated with GS‐441524 | |
|---|---|---|
| Total number | 10 | 9 |
| Effusion | ||
| Cell count (/μL) a | 1545 (180‐30 820) | 2530 (200‐26 960) |
| Protein (g/dL) a | 5.6 (4.8‐7.6) | 6.6 (4.5‐8.4) |
| Hematologic | ||
| Hematocrit (%) b | 29 (4.5) | 27 (8.0) |
| Lymphocytes (/μL) b | 510 (498) | 900 (563) |
| Neutrophils (/μL) a | 10 281 (6652‐33 852) | 15 576 (8414‐52 882) |
| Bands (/μL) a | 228 (0‐823) | 0 (0‐960) |
| Biochemical | ||
| Albumin (g/dL) b | 2.3 (0.8) | 2.1 (0.4) |
| Globulin (g/dL) b | 4.9 (0.86) | 5.7 (1.1) |
| Albumin : globulin b | 0.48 (0.17) | 0.39 (0.10) |
| Bilirubin (mg/dL) b | 1.9 (1.9) | 1.4 (1.2) |
Non‐normal results presented as median and range.
Normal results presented as mean and SD.
3.2. Survival analysis
Two of the 10 enrolled cats died of their disease during the study period, resulting in overall survival and disease remission at 16 weeks of 8/10 (80%; 95% confidence interval [CI], 44.4%‐97.5%). One cat presented with severe hypoglycemia and generalized seizure activity within 24 hours of study enrollment and was euthanized. A second cat died at home within 24 hours of study enrollment. The remaining 8 cats survived and were assessed to be in remission at 16 weeks. A median survival time was not reached in this group. All of the surviving cats continue to be in clinical remission at the time of manuscript submission, ranging from 7 to 11 months posttreatment initiation. Historical control cats with effusive FIP treated with PO GS‐441524 had a survival rate of 55% (5/9 cats; 95% CI, 21.2%‐86.3%). The difference in survival between the groups was 25% (90% CI, −9.3% to 59.3%). 5 Molnupiravir fulfilled non‐inferiority criteria as compared with PO GS‐441524 (Figure 1).
FIGURE 1.
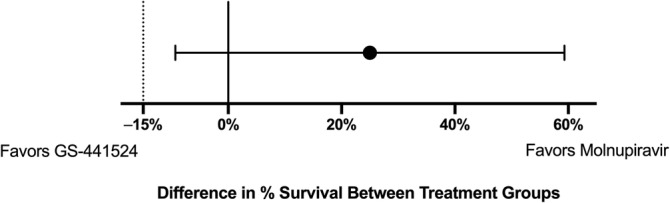
The percent difference in overall survival between cats treated with MPV versus historical control cats treated with GS‐441524. The black dot represents the difference in survival between groups, and the horizontal line represents the 90% CI of the difference. A dotted vertical line at −15% represents the predetermined non‐inferiority limit.
Two cats that did not survive were submitted for necropsy evaluation. One was a 9‐month‐old, spayed female domestic short‐haired cat that presented for effusive FIP characterized by peritoneal effusion. The day after study enrollment and administration of 1 dose of MPV, the caretaker noted seizure activity, and the cat was presented for euthanasia. Serum blood glucose concentration was 16 mg/dL (reference interval, 63‐118 mg/dL). Upon necropsy evaluation, moderate to severe multifocal pyogranulomatous and lymphoplasmacytic peritonitis with positive FCoV immunoreactivity and lesions scattered throughout the kidneys, spleen, urinary bladder serosa, and lymph nodes were noted. Histopathology of the cardiac tissue disclosed multifocally extensive acute myodegeneration. No obvious inflammatory lesions consistent with FIP were identified in the brain. The second cat that did not survive was a 5‐month‐old male Burmese cat diagnosed with peritoneal effusive FIP. Upon study enrollment, the cat was noted to have abnormal pulmonary sounds auscultated in the caudodorsal lung fields, and thoracic radiographs indicated a multilobar alveolar pulmonary pattern consistent with bronchopneumonia. The cat was noted to be in respiratory distress the day after enrollment and, after 1 dose of MPV, died at home. On necropsy, peritoneal and pleural effusion was noted. Lung histopathology indicated severe bronchopneumonia with intralesional bacteria and foreign material in the airways, consistent with aspiration pneumonia. Furthermore, extensive pyogranulomatous, fibrinous interstitial pneumonia, and pleuritis were noted as consistent with FIP. Moderate pyogranulomatous pericarditis also was noted. Severe, multifocal pyogranulomatous serositis was noted in the liver, spleen, gastrointestinal tract, adrenal glands, lymph nodes, urinary bladder, and kidneys with positive immunoreactivity for FCoV. The pericardium was not tested for FCoV immunoreactivity.
3.3. Adverse events and comorbidities
No adverse events that necessitated withdrawal from the clinical trial were noted. During the study period, 1 cat was diagnosed with Enterococcus sp. bacteremia on treatment day 1 and was hospitalized for 5 days on IV antimicrobials and supportive care. One cat with pleural effusion was hospitalized on treatment days 1 and 2 for thoracocentesis and oxygen support. Two cats initially experienced hyporexia and were treated with transdermal mirtazapine. One cat had intermittent small bowel diarrhea throughout the study period, which was actively monitored without direct intervention. Two cats had ocular discharge; 1 cat had historical herpesvirus keratitis treated with PO lysine supplementation during the study period, and 1 cat had 1 day of serous ocular discharge that resolved without intervention. One cat had a single episode of vomiting during the car ride to the hospital for a reevaluation, and 1 other cat had an episode of vomiting after MPV administration on treatment day 51 that was self‐limiting.
No biochemical or hematologic adverse events that necessitated discontinuation of the study drug were noted during the study period. Hematologic abnormalities noted at the 6 week reevaluation included 2 cats that developed microcytosis of 41.9 and 40.6 fL (reference interval, 42‐53 fL), 1 cat with basophilia of 287 cells/μL (reference interval, 0‐200 cells/μL), and 1 cat with eosinophilia of 1527 cells/μL (reference interval, 150‐1100 cells/μL) that persisted after study completion. One cat had neutrophilia of 11 180 cells/μL at the 16‐week reevaluation (reference interval, 2000‐9000 cells/μL).
Biochemical abnormalities that were noted at the 6‐week reevaluation included 1 cat with hypocholesterolemia of 71 mg/dL (reference interval, 89‐258 mg/dL), 1 cat with an increased BUN : Cr ratio with a BUN concentration of 36 mg/dL (reference interval, 18‐33 mg/dL) with serum creatinine concentration within the normal reference interval. Both were resolved at the 16‐week reevaluation. Hepatopathy was noted in 2 cats at the 6‐week reevaluation. One cat had ALT activity of 105 IU/L (reference interval, 27‐101 IU/L). Serum ALT activity was normal at study conclusion. The second cat had serum ALT activity of 165 IU/L, aspartate aminotransferase (AST) activity of 107 IU/L (reference interval, 17‐58 IU/L), and alkaline phosphatase (ALP) activity of 73 IU/L (reference interval, 14‐71 IU/L) at the 6‐week reevaluation. This cat had vomited en route to the hospital before blood collection. The cat was reevaluated 1 week later and treated with maropitant before car rides. At reevaluation, normal ALT and AST activities were noted, with mildly increased ALP activity of 82 IU/L. At study conclusion, all liver enzyme activities were normal for this cat. Hyperglycemia was noted in 2 cats (129 and 136 mg/dL; reference interval, 63‐118 mg/dL) at the 6‐week reevaluation.
3.4. Secondary outcomes
Weight loss in the first 2 weeks of treatment occurred in all 6 cats for which weights at enrollment and week 1 were recorded, and all surviving cats gained weight during the 16‐week study period (Figure 2). Effusion resolved in 7/8 surviving cats at the week 6 reevaluation. One cat with pleural effusion had a scant amount of fluid noted ultrasonographically that could not be sampled safely. The cat also had a scant volume of pleural effusion noted at week 16.
FIGURE 2.
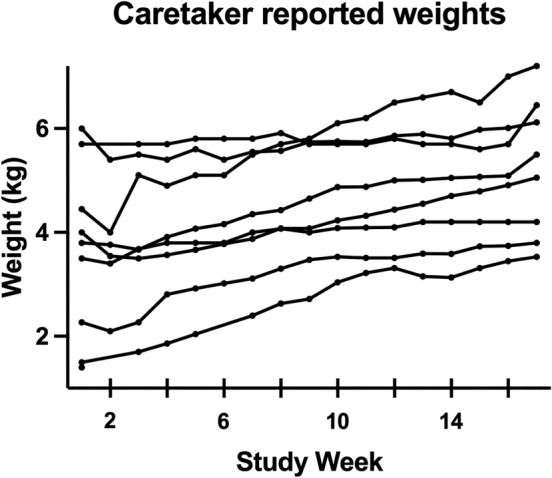
Caretaker reported weekly weights. Each line represents weekly weights for individual cats.
During the study period, mean hematocrit increased by 8.6% (95% CI, 2.4%‐14.8%), and lymphocyte count increased by 2209 cells/μL (95% CI, 982‐3435 cells/μL). No significant changes in mean corpuscular volume (MCV) or neutrophil count were observed during the study period. When compared with historical control cats treated with GS‐441524, no differences in hematocrit, neutrophil count, MCV, or lymphocyte count were noted between groups at any study time point (data not shown). During the study period, serum albumin concentration increased by 1.8 g/dL (95% CI, 0.7‐3.0 g/dL), serum globulin concentration decreased by 1.2 g/dL (95% CI, 0.5‐1.9 g/dL), serum albumin : globulin ratio increased by 0.7 (95% CI, 0.3‐1.1), and serum total bilirubin concentration decreased by 1.8 mg/dL (95% CI, 0.06‐3.6 mg/dL; Figure 3). When compared with historical controls treated with GS‐441524, no significant differences in albumin, globulin, or bilirubin concentrations were observed between groups at any study time point (data not shown).
FIGURE 3.
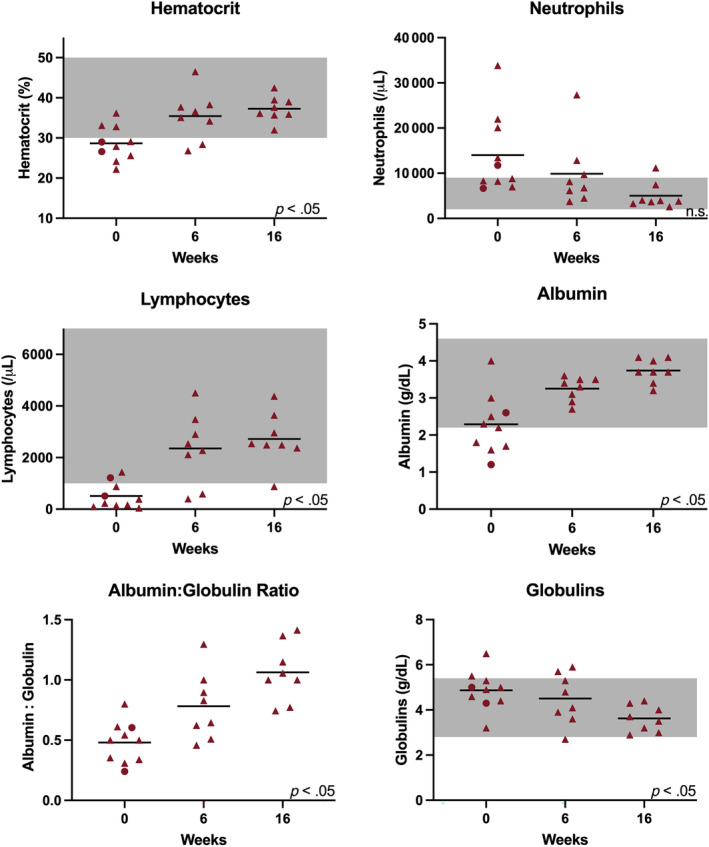
Clinicopathologic features of cats treated with MPV (survivors are represented with red triangles and non‐survivors are represented with red circles) at study weeks 0, 6, and 16. A black horizontal line represents the mean of the group. The gray shaded regions correspond to the normal reference interval where available. Significant changes in clinicopathologic features during the study period are depicted by P < .05. Features without significant changes are depicted by n.s. (not significant).
Anti‐FCoV serology titers were measured at enrollment for 9/10 cats. Of these cats, 8 had titers ≥1 : 20 480, and 1 had a titer of 1 : 2560 at the time of diagnosis. Antibody titers measured at the study conclusion were identical to enrollment titers for all surviving cats (data not shown).
4. DISCUSSION
In this open label, prospective trial evaluating MPV as a first line treatment for cats with naturally occurring effusive FIP, MPV treatment resulted in clinical remission in 8/10 enrolled cats. The non‐surviving cats both died or were euthanized within 24 hours of treatment initiation. The treatment was safe, with no adverse events noted that necessitated discontinuation of the medication. When MPV was compared with historical control cats treated with GS‐441524, using the same enrollment criteria, MPV met non‐inferiority criteria for the primary endpoint of survival and disease remission at 16 weeks.
Survival rates observed here were similar to the other report of treating cats with MPV as a first‐line drug for FIP. 17 Two cats succumbed to their disease in our trial. Both cats died within 24 hours of study enrollment, and necropsy identified pathology associated with FIP and positive IHC staining for FCoV antigen, indicating the initial diagnosis was correct and the cats likely died from FIP‐related disease. In 1 case, signs of aspiration pneumonia were present that likely occurred after force feeding and probably contributed to the clinical decline. Of the 8 surviving cats, all were documented to be in clinical remission at 16 weeks without any documented relapses at the time of manuscript preparation, at minimum, 5 months since discontinuation of MPV. One cat had a scant volume of pleural effusion noted at the study's conclusion. The fluid could not be safely collected, and all other clinical and pathologic signs associated with FIP had resolved, and thus the cat was assessed to be in clinical remission. The cat was still in clinical remission at the time of manuscript submission, 9 months after study conclusion.
The FCoV antibody titers were measured at study initiation and study conclusion. As in other studies, all cats remained seropositive at the end of the treatment period. 22 Long term follow‐up of cats treated for FIP has shown positive titers months beyond disease remission, which may be a result of long‐lasting humoral immunity or reinfection with enteric FCoV. Regardless, FCoV serology should not be relied upon to gauge treatment success.
The MPV dosage utilized in our study was determined based on previous feline cell‐based in vitro assays, pharmacokinetic studies conducted in healthy laboratory cats, and anecdotal reports that indicated favorable outcomes at 10 to 20 mg/kg PO q12h. 13 , 16 , 17 We chose a dosage of 10 to 15 mg/kg PO q12h for our trial, which appeared to be effective. The minimal effective duration of treatment needed has yet to be systematically determined for MPV. Treatment for 12 weeks has been reported but additional studies evaluating shorter courses of treatment are needed to better define the optimal treatment protocol.
A group of cats with effusive FIP treated with PO GS‐441524 in a previous clinical trial was utilized as a historical control population. 5 Enrollment criteria, study protocol other than the drug administered, and outcome measures were held constant between these trials to preserve the integrity of the control group. Doing so allowed comparisons between survival rates and clinicopathologic findings, but may be a limitation because the study was unblinded and occurred at different times. Nonetheless, MPV met the non‐inferiority criteria compared with PO GS‐441524, and no significant treatment effects were observed in any clinicopathologic data between survivors in each group.
No clinically relevant adverse events necessitating drug discontinuation were observed in our trial. Previous reports have indicated that hepatopathy, panleukopenia, and folded ear tips may be adverse effects associated with MPV treatment. Two cats at the 6‐week reevaluation were noted to have hepatopathy develop during the study period. Hepatopathy was characterized by mild increases in ALT activity in both cats, with increased AST and ALP activity also reported in 1 cat. The cat with increased ALT, AST, and ALP activity had vomited on the car ride to the hospital before a blood specimen was collected. On reevaluation 1 week later, with maropitant pretreatment before the car ride, the hepatopathy resolved without intervention. The other cat also had spontaneous resolution of its increased ALT activity. No documented panleukopenia, hypersalivation, pruritus, or curled ear tips were noted during the study. Molnupiravir's mechanism of action includes introduction of mutations into the viral genome during replication, which raises concern for genetic selection of antiviral resistant strains of FIPV. 23 No cats in our study exhibited relapse of their disease to indicate that a mutated resistant strain was present, but this aspect of treatment was not evaluated in a comprehensive manner in our study. Furthermore, host mutagenicity has been a concern, and long term safety studies monitoring for adverse outcomes and teratogenicity are indicated. 24
A limitation of our study was reliance on a historical control group rather than a blinded prospective study. The study design was implemented because there were no prospective clinical trials evaluating MPV at study initiation, and the full extent of adverse effects had not been determined. Therefore, blinding to the study drug was not determined to be in the patients' best interest, and an open‐label trial was commenced. Future studies comparing MPV to other antiviral treatments should be performed in a randomized, blinded fashion for further evaluation of efficacy.
Other limitations of our study include the limited number of reevaluations. If transient hepatopathies or leukopenias occurred, they may not have been identified. Furthermore, the drug utilized in our study was compounded in‐house from the bulk chemical compound and is not a commercially available medication. Severely ill cats and those not expected to live for at least 48 hours were excluded from the study. This design feature may have biased the population toward cats with a higher chance of survival. However, FIP is considered a highly fatal, progressive disease, and therefore all cats enrolled in the study had a grave prognosis without treatment. Cats enrolled in our study all were diagnosed based on identifying effusion‐associated FCoV RNA in the context of the appropriate clinical presentation, similar to other clinical trials, but no cats had antemortem tissue biopsy with IHC as a confirmatory diagnostic test at study enrollment. 4 , 5 , 8
In conclusion, MPV administered at 10 to 15 mg/kg PO q12h for 12 weeks is an effective treatment for naturally occurring effusive FIP. The medication is well tolerated without observation of any adverse events that necessitated drug discontinuation. Additional studies are needed to determine optimal treatment duration and if shorter antiviral courses are adequate for certain patients and to further understand the drug safety profile. Additionally, the efficacy of MPV for treating other clinical presentations of FIP, such as neurologic FIP, should be explored.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Molnupiravir (EIDD‐2801) was used off‐label.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of California, Davis IACUC. All clients gave their informed consent.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Funding provided by EveryCat Health Foundation grant EC22‐007 and SOCK FIP at the Center for Companion Animal Health at the University of California, Davis School of Veterinary Medicine.
Reagan KL, Brostoff T, Pires J, Rose A, Castillo D, Murphy BG. Open label clinical trial of orally administered molnupiravir as a first‐line treatment for naturally occurring effusive feline infectious peritonitis. J Vet Intern Med. 2024;38(6):3087‐3094. doi: 10.1111/jvim.17187
REFERENCES
- 1. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg. 2009;11:225‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen NC, Sato R, Foley JE, Poland AM. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J Feline Med Surg. 2004;6:83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vennema H, Poland A, Foley J, Pedersen NC. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coggins SJ, Norris JM, Malik R, et al. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS‐441524. J Vet Intern Med. 2023;37:1772‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosaro E, Pires J, Castillo D, Murphy BG, Reagan KL. Efficacy of oral remdesivir compared to GS‐441524 for treatment of cats with naturally occurring effusive feline infectious peritonitis: a blinded, non‐inferiority study. Viruses. 2023;15:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy BG, Perron M, Murakami E, et al. The nucleoside analog GS‐441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet Microbiol. 2018;219:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickinson PJ, Bannasch M, Thomasy SM, et al. Antiviral treatment using the adenosine nucleoside analogue GS‐441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J Vet Intern Med. 2020;34:1587‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krentz D, Zenger K, Alberer M, et al. Curing cats with feline infectious peritonitis with an oral multi‐component drug containing GS‐441524. Viruses. 2021;13:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Green J, Syme H, Tayler S. Thirty‐two cats with effusive or non‐effusive feline infectious peritonitis treated with a combination of remdesivir and GS‐441524. J Vet Intern Med. 2023;37:1784‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katayama M, Uemura Y. Prognostic prediction for therapeutic effects of Mutian on 324 client‐owned cats with feline infectious peritonitis based on clinical laboratory indicators and physical signs. Vet Sci. 2023;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones S, Novicoff W, Nadeau J, Evans S. Unlicensed GS‐441524‐like antiviral therapy can be effective for at‐home treatment of feline infectious peritonitis. Animals. 2021;11:2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedersen NC, Perron M, Bannasch M, et al. Efficacy and safety of the nucleoside analog GS‐441524 for treatment of cats with naturally occurring feline infectious peritonitis. J Feline Med Surg. 2019;21:271‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy M, Jacque N, Novicoff W, Li E, Negash R, Evans SJM. Unlicensed molnupiravir is an effective rescue treatment following failure of unlicensed GS‐441524‐like therapy for cats with suspected feline infectious peritonitis. Pathogens. 2022;11:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen NC, Kim Y, Liu H, et al. Efficacy of a 3C‐like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J Feline Med Surg. 2018;20:378‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lv J, Bai Y, Wang Y, Yang L, Jin Y, Dong J. Effect of GS‐441524 in combination with the 3C‐like protease inhibitor GC376 on the treatment of naturally transmitted feline infectious peritonitis. Front Vet Sci. 2022;9:1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cook S, Wittenburg L, Yan VC, et al. An optimized bioassay for screening combined anticoronaviral compounds for efficacy against feline infectious peritonitis virus with pharmacokinetic analyses of GS‐441524, remdesivir, and molnupiravir in cats. Viruses. 2022;14:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sase O. Molnupiravir treatment of 18 cats with feline infectious peritonitis: a case series. J Vet Intern Med. 2023;37:1876‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lisciandro GR, Lagutchik MS, Mann KA, et al. Evaluation of an abdominal fluid scoring system determined using abdominal focused assessment with sonography for trauma in 101 dogs with motor vehicle trauma. J Vet Emerg Crit Care. 2009;19:426‐437. [DOI] [PubMed] [Google Scholar]
- 19. Lisciandro GR, Lagutchik MS, Mann KA, et al. Evaluation of a thoracic focused assessment with sonography for trauma (TFAST) protocol to detect pneumothorax and concurrent thoracic injury in 145 traumatized dogs. J Vet Emerg Crit Care. 2008;18:258‐269. [Google Scholar]
- 20. Pedersen NC, Eckstrand C, Liu H, Leutenegger C, Murphy B. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet Microbiol. 2015;175:157‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sealed Envelope Ltd . Power calculator for binary outcome non‐inferiority trial [Online]. Accessed May 17, 2024. https://www.sealedenvelope.com/power/binary-noninferior/
- 22. Zwicklbauer K, Krentz D, Bergmann M, et al. Long‐term follow‐up of cats in complete remission after treatment of feline infectious peritonitis with oral GS‐441524. J Feline Med Surg. 2023;25. doi: 10.1177/1098612X231183250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanderson T, Hisner R, Donovan‐Banfield I, et al. A molnupiravir‐associated mutational signature in global SARS‐CoV‐2 genomes. Nature. 2023;623:594‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waters MD, Warren S, Hughes C, Lewis P, Zhang F. Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir. Environ Mol Mutagen. 2022;63:37‐63. [DOI] [PubMed] [Google Scholar]


