Abstract
1. We have investigated whether changes in extracellular ion composition and substrate deprivation modulate basal and/or bradykinin-stimulated L-arginine transport and release of nitric oxide (NO) and prostacyclin (PGI2) in porcine aortic endothelial cells cultured and superfused on microcarriers. 2. Saturable L-arginine transport (Km = 0.14 +/- 0.03 mM; Vmax = 2.08 +/- 0.54 nmol min-1 (5 x 10(6) cells)-1) was pH insensitive and unaffected following removal of extracellular Na+ or Ca2+. 3. Cationic arginine analogues, including L-lysine and L-ornithine, inhibited L-arginine transport, whilst 2-methylaminoisobutyric acid, beta-2-amino-bicyclo[2,2.1]-heptane-2-carboxylic acid, L-phenylalanine, 6-diazo-5-oxo-norleucine, L-glutamine, L-cysteine and L-glutamate were poor inhibitors. 4. Deprivation of L-arginine (30 min to 24 h) reduced intracellular free L-arginine levels from 0.87 +/- 0.07 to 0.40 +/- 0.05 mM (P < 0.05) and resulted in a 40% stimulation of L-arginine, L-lysine and L-ornithine transport. 5. L-arginine and NG-monomethyl-L-arginine (L-NMMA), but not N omega-nitro-L-arginine methyl ester (L-NAME), trans-stimulated efflux of L-[3H]arginine. 6. Depolarization of endothelial cells with 70 mM K+ reduced L-arginine influx and prevented the stimulation of transport by 100 nM bradykinin, but agonist-induced release of NO and PGI2 was still detectable. 7. Basal rates of L-arginine transport and NO release were unaffected during superfusion of cells with a nominally Ca(2+)-free solution. Bradykinin-stimulated L-arginine transport was insensitive to removal of Ca2+, whereas agonist-induced NO release was abolished. 8. Although bradykinin-stimulated NO release does not appear to be coupled directly to the transient increase in L-arginine transport, elevated rates of L-arginine influx via system y+ in response to agonist-induced membrane hyperpolarization or substrate deprivation provide a mechanism for enhanced L-arginine supply to sustain NO generation.
Full text
PDF



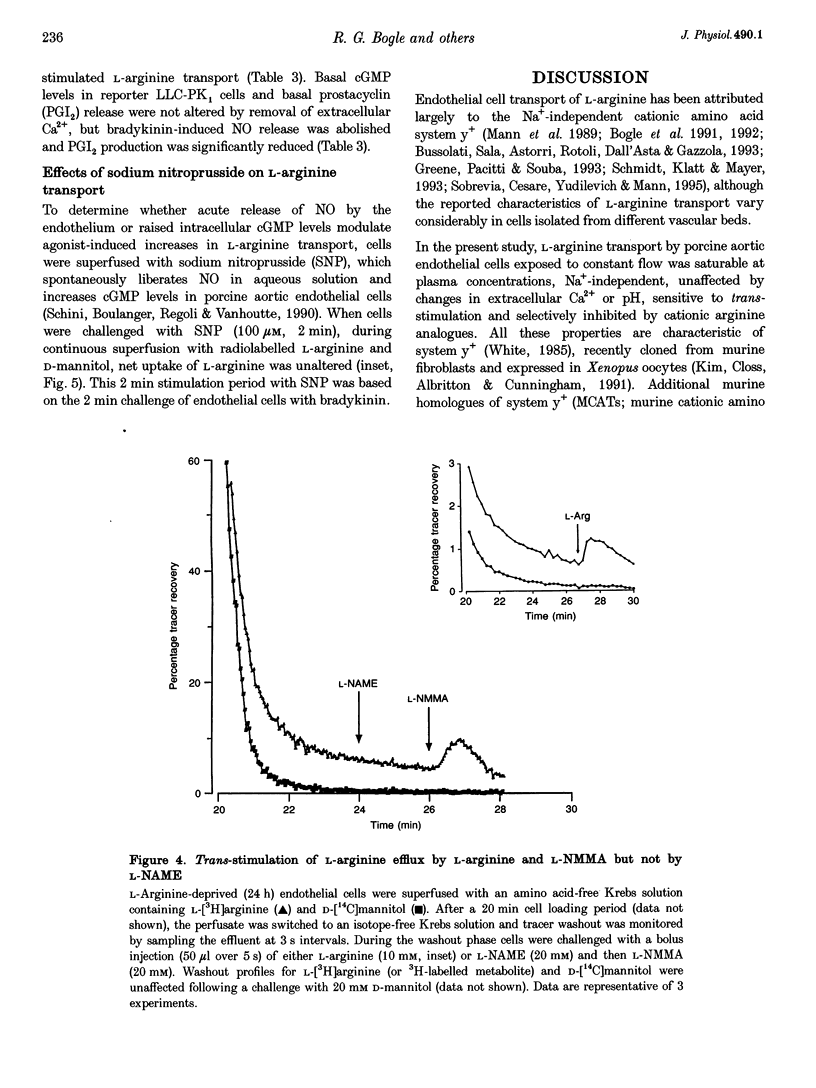
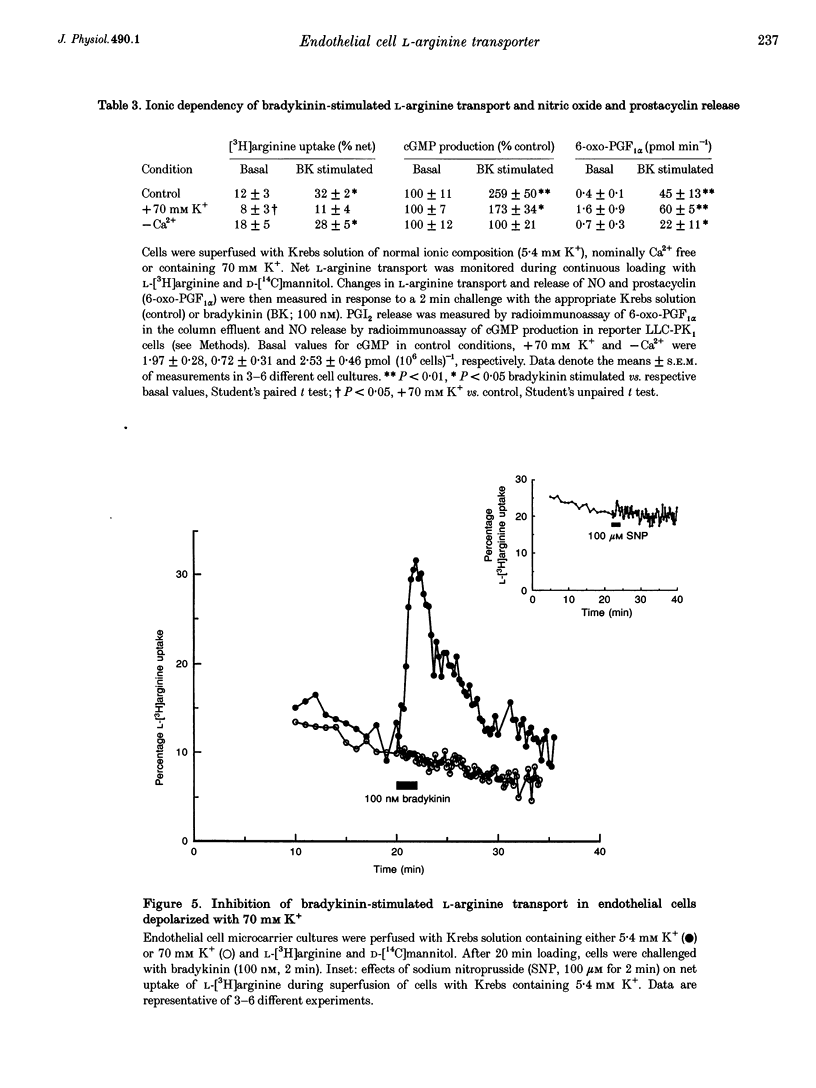

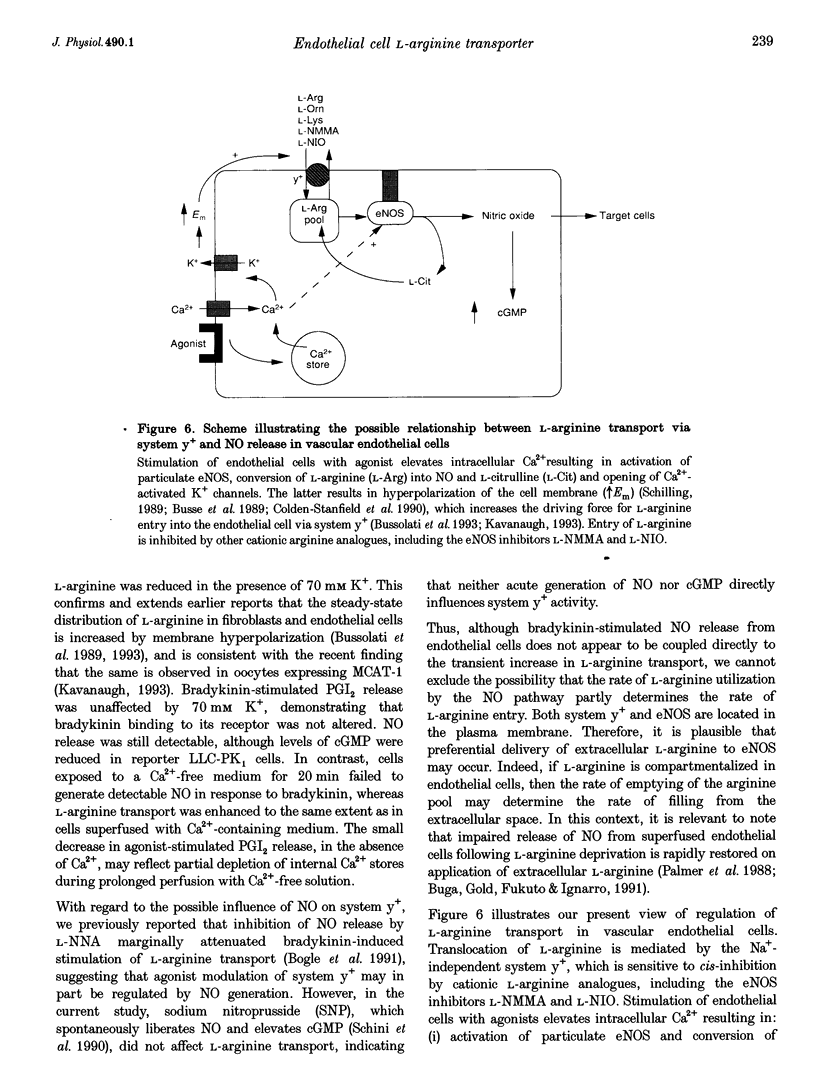


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Bogle R. G., Pearson J. D., Mann G. E. Discrimination between citrulline and arginine transport in activated murine macrophages: inefficient synthesis of NO from recycling of citrulline to arginine. Br J Pharmacol. 1994 Jun;112(2):487–492. doi: 10.1111/j.1476-5381.1994.tb13099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Emery P. W., Pearson J. D., Mann G. E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990 Dec 31;173(3):940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Coade S. B., Moncada S., Pearson J. D., Mann G. E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Bussolati O., Laris P. C., Nucci F. A., Dall'Asta V., Franchi-Gazzola R., Guidotti G. G., Gazzola G. C. Influx of L-arginine is an indicator of membrane potential in human fibroblasts. Am J Physiol. 1989 Apr;256(4 Pt 1):C930–C935. doi: 10.1152/ajpcell.1989.256.4.C930. [DOI] [PubMed] [Google Scholar]
- Bussolati O., Sala R., Astorri A., Rotoli B. M., Dall'Asta V., Gazzola G. C. Characterization of amino acid transport in human endothelial cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C1006–C1014. doi: 10.1152/ajpcell.1993.265.4.C1006. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Bogle R. G., Bjaaland T. Spiking of intracellular calcium ion concentration in single cultured pig aortic endothelial cells stimulated with ATP or bradykinin. Biochem J. 1991 Sep 15;278(Pt 3):697–704. doi: 10.1042/bj2780697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs E. I., Lyons C. R., Kelly C., Cunningham J. M. Characterization of the third member of the MCAT family of cationic amino acid transporters. Identification of a domain that determines the transport properties of the MCAT proteins. J Biol Chem. 1993 Oct 5;268(28):20796–20800. [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Possani L. D., Kunze D. L. Bradykinin-induced potassium current in cultured bovine aortic endothelial cells. J Membr Biol. 1990 Jul;116(3):227–238. doi: 10.1007/BF01868462. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Andon N. A., Girerd X. J., Hirsch A. T., Creager M. A. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation. 1991 Mar;83(3):1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- Devés R., Chavez P., Boyd C. A. Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol. 1992 Aug;454:491–501. doi: 10.1113/jphysiol.1992.sp019275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I., Hecker M., Busse R. Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ Res. 1994 Jun;74(6):1220–1226. doi: 10.1161/01.res.74.6.1220. [DOI] [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3191–3198. [PubMed] [Google Scholar]
- Gold M. E., Bush P. A., Ignarro L. J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Greene B., Pacitti A. J., Souba W. W. Characterization of L-arginine transport by pulmonary artery endothelial cells. Am J Physiol. 1993 Apr;264(4 Pt 1):L351–L356. doi: 10.1152/ajplung.1993.264.4.L351. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mülsch A., Bassenge E., Förstermann U., Busse R. Subcellular localization and characterization of nitric oxide synthase(s) in endothelial cells: physiological implications. Biochem J. 1994 Apr 1;299(Pt 1):247–252. doi: 10.1042/bj2990247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M. P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry. 1993 Jun 8;32(22):5781–5785. doi: 10.1021/bi00073a009. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Norman P. S., Smith I. C. Amino acid efflux in the isolated perfused rat pancreas: trans-stimulation by extracellular amino acids. J Physiol. 1989 Sep;416:485–502. doi: 10.1113/jphysiol.1989.sp017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Pearson J. D., Sheriff C. J., Toothill V. J. Expression of amino acid transport systems in cultured human umbilical vein endothelial cells. J Physiol. 1989 Mar;410:325–339. doi: 10.1113/jphysiol.1989.sp017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Anggård E. E., Vane J. R. Cultured endothelial cells maintain their L-arginine level despite the continuous release of EDRF. Eur J Pharmacol. 1990 Jul 17;182(3):573–576. doi: 10.1016/0014-2999(90)90058-e. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakache M., Gaub H. E. Hydrodynamic hyperpolarization of endothelial cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1841–1843. doi: 10.1073/pnas.85.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Boulanger C., Regoli D., Vanhoutte P. M. Bradykinin stimulates the production of cyclic GMP via activation of B2 kinin receptors in cultured porcine aortic endothelial cells. J Pharmacol Exp Ther. 1990 Feb;252(2):581–585. [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Klatt P., Mayer B. Characterization of endothelial cell amino acid transport systems involved in the actions of nitric oxide synthase inhibitors. Mol Pharmacol. 1993 Sep;44(3):615–621. [PubMed] [Google Scholar]
- Sobrevia L., Cesare P., Yudilevich D. L., Mann G. E. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: association with membrane hyperpolarization. J Physiol. 1995 Nov 15;489(Pt 1):183–192. doi: 10.1113/jphysiol.1995.sp021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle L. J. Amino acid transport in developing animal oocytes and early conceptuses. Biochim Biophys Acta. 1988 Feb 24;947(1):173–208. doi: 10.1016/0304-4157(88)90024-x. [DOI] [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Wu G., Meininger C. J. Regulation of L-arginine synthesis from L-citrulline by L-glutamine in endothelial cells. Am J Physiol. 1993 Dec;265(6 Pt 2):H1965–H1971. doi: 10.1152/ajpheart.1993.265.6.H1965. [DOI] [PubMed] [Google Scholar]