ABSTRACT
Background and Aims
There are still no useful biomarkers for the prognosis of esophageal squamous cell carcinoma (ESCC). In the prognosis of some kinds of cancer, soluble programmed death 1 (sPD‐1) and programmed death ligand 1 (sPD‐L1) have demonstrated statistical significance, but the prognostic value of serum sPD‐L1 and sPD‐1 remains unclear in ESCC.
Methods
Here, a meta‐analysis was performed to estimate the prognostic value of sPD‐L1 and sPD‐1 in ESCC. To obtain eligible studies, we searched mainstream databases (PubMed, Cochrane, Embase, Web of Science, Wanfang Data, and CNKI), and the survival data including hazard ratios (HR) and its 95% confidence intervals (95% CI) from included literature were extracted.
Results
Six articles were included, including 645 patients with ESCC. The statistical result of this meta‐analysis indicated that serum sPD‐1 had no significant correlation with overall survival (OS) of patients with ESCC (p > 0.05). Patients with ESCC with high concentrations of serum sPD‐L1 demonstrated a significantly poor prognosis (HR = 1.73, 95% CI: 1.42–2.11, p < 0.001).
Conclusion
Higher levels of serum sPD‐L1 may predict poor OS in ESCC patients, which may be a promising and credible prognostic biomarker for esophageal cancer.
Keywords: biomarkers, ESCC, meta‐analysis, prognosis, sPD‐1, sPD‐L1
Abbreviations
- 95% CI
95% confidence interval
- ELISA
enzyme‐linked immunosorbent assay
- ESCC
esophageal squamous cell carcinoma
- HR
hazard ratios
- NOS
Newcastle–Ottawa Quality Assessment Scale
- OS
overall survival
- PD‐1
programmed cell death 1
- PD‐L1
programmed cell death ligand 1
- sPD‐1
soluble programmed cell death 1
- sPD‐L1
soluble programmed cell death ligand 1
1. Introduction
Esophageal cancer (EC) is the fourth leading cancer‐related cause of death in China [1], and ranks sixth among cancer‐related deaths worldwide [2]. Squamous cell carcinoma (ESCC) and adenocarcinoma (EA) are two principal pathological types of EC with the former accounting for approximately 90% of the global pathological types. Although the main treatment of EC uses multidisciplinary treatment, including chemotherapy, surgery, and radiotherapy, the 5‐year survival rate of EC is only about 29.7% [3, 4, 5]. To improve the overall survival (OS) of patients with ESCC and to assist in choosing the most treatment strategy, it is imperative to identify credible biomarkers.
The immune escape mechanism mediated by programmed cell death 1 (PD‐1) and programmed cell death ligand 1 (PD‐L1) pathways, plays an important role in the occurrence development, and metastasis of various tumors [6]. PD‐1 and PD‐L1 exist in two forms: soluble and membrane‐bound forms. Numerous studies have demonstrated that the expression level of PD‐L1 in tumor tissue of ESCC is higher than that in normal tissue, and serves as an independent predictor of poor prognosis [7, 8, 9, 10, 11]. However, the relationship between serum levels of soluble PD‐1 (sPD‐1) and PD‐L1 (sPD‐L1) and the prognosis of ESCC remains unclear. Existing studies on lung cancer [12] and liver cancer [13] have shown that higher concentrations of serum sPD‐L1 is associated with poorer prognosis of patients, while studies on esophageal cancer are limited. Therefore, the prognostic value of serum sPD‐1 and sPD‐L1 in ESCC was evaluated by meta‐analysis to explore whether these could be promising and reliable biomarkers of ESCC.
2. Materials and Methods
2.1. Searching Strategy and Inclusion/Exclusion Criteria
Potential eligible studies from mainstream databases (PubMed, Cochrane, Embase, Web of Science, Wanfang Data, and CNKI) were collected and reviewed. The deadline for searching was December 31, 2023. Literature retrieval was based on the combination of free words and subject words, using the keywords (“EC” OR “ESCC” OR “esophageal cancer” OR “esophageal squamous cell carcinoma”) AND (“sPD‐L1” OR “sPD‐1” OR “soluble programmed cell death 1” OR “soluble programmed cell death ligand 1”).
Inclusion criteria included: (1) Chinese and English literature; (2) Patients were pathologically detected as esophageal squamous cell carcinoma; (3) The detection method in studies was only based on enzyme‐linked immunosorbent assay (ELISA); (4) anti‐PD‐L1 or anti‐PD‐1 immunotherapy had not been applied to the patient; (5) Complete survival data or Kaplan‐Meier survival curve.
Exclusion criteria included: (1) Repeated clinical studies; (2) animal experiments, cell experiments, case reports, conference abstracts, and literature review; (3) patients with other pathological types of esophageal cancer; and (4) incomplete literature data or low‐quality literature.
Ethical approval details were observed in this article, and informed consent was not required as this article is a meta‐analysis.
2.2. Data Extraction and Quality Assessment
All retrieved documents were evaluated by two independent investigators (Wenjie Mao and Jie Li), and if any discrepancies, were resolved by another investigator (Zheng Li). The following information was recorded: first author, country, publication year, sample size, age, gender, tumor stage, concentrations of serum sPD‐L1 and sPD‐1 of controls and patients, overall survival rate hazard ratios (HR) and 95% confidence interval (CI), and so forth. If the original text only provides the Kaplan–Meier curve and the author cannot be contacted, the Engauge Digitizer 4.1 software was used for extracting the survival data as was reported in the study by Zhou et al. [14]. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used for evaluating the quality of the literature. The included literature with an NOS score ≥ 6 was deemed to be a high‐quality study.
2.3. Statistical Analyses
Review Manager 5.3 software was used to analyze the relationship between the prognosis of ESCC and the concentrations of serum sPD‐1 and sPD‐L1. The heterogeneity among studies was assessed by Higgins' I 2 statistic. If I 2 ≤ 50%, the fixed effects model was used, otherwise, the random effects model was used. One‐by‐one deletion method was used to detect heterogeneity. To detect publication bias, Egger's test was performed using Stata SE 15 software. When a meta‐analysis was carried out using statistical software, it was repeated at least three times. p‐value < 0.05 was considered statistically significant.
3. Results
3.1. Literature Search, Studies Characteristics, and Quality Evaluation
As shown in Table 1, six eligible articles that met quality evaluation were included, including four case‐control studies and two cohort studies, with a total of 203 healthy controls and 645 patients, all from China and Japan. Among them, 421 were male patients and 224 were female patients, and most of them have received adjuvant radiotherapy and chemotherapy. All the following are one‐sided tests. The specific process of the included literature is shown in Figure 1, and the basic characteristics and methodological quality scores are shown in Table 1.
Table 1.
The characteristics of included studies.
| First author(y) | Country | Sample size | Age | Gender (M/F) | Type of Study | Tumor stage | Treatment | Reagent brand for ELISA | Cutoff (IQR) | Indicators | HR for OS | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akutsu (2018) [15] | Japan | 85 | 67.8 | 73/12 | Array | I–IV | Mixeda | CUSABIO | P50 | sPD‐1 sPD‐L1 | 0.77 [0.32, 1.83] 1.49 [1.00, 2.22] | 6 |
| Fu (2021) [16] | China | 190 | N/A | 87/103 | Case–control | III–IV | Chemotherapy | Heguo | N/A | sPD‐L1 | 3.71 [2.05, 6.71] | 7 |
| Ito (2019) [17] | Japan | 150 | N/A | 122/28 | array | I–IV | Surgery and NCT | RandD | P75 | sPD‐L1 | 1.70 [1.03, 2.81] | 7 |
| Liu (2019) [18] | China | 127 | N/A | 77/50 | Case–control | N/A | Chemotherapy | RandD | P50 | sPD‐L1 | 1.53 [1.06, 2.21] | 7 |
| Yoshida (2018) [19] | Japan | 47 | 66 | 42/5 | Case–control | II–IV | Mixeda | Ray Biotech | P50 | sPD‐1 | 1.98 [0.62, 6.31] | 6 |
| Zheng (2019) [20] | China | 46 | 67.2 | 20/26 | Case–control | II–IV | Chemoradiotherapy | Joe feather | P50 | sPD‐1 sPD‐L1 | 1.27 [0.52, 3.10] 1.63 [1.01, 2.63] | 7 |
Abbreviations: IQR, interquartile range; N/A, not applicable; NCT, neoadjuvant chemotherapy; NOS, Newcastle–Ottawa Quality Assessment Scale; OS, overall survival.
More than two treatments.
Figure 1.
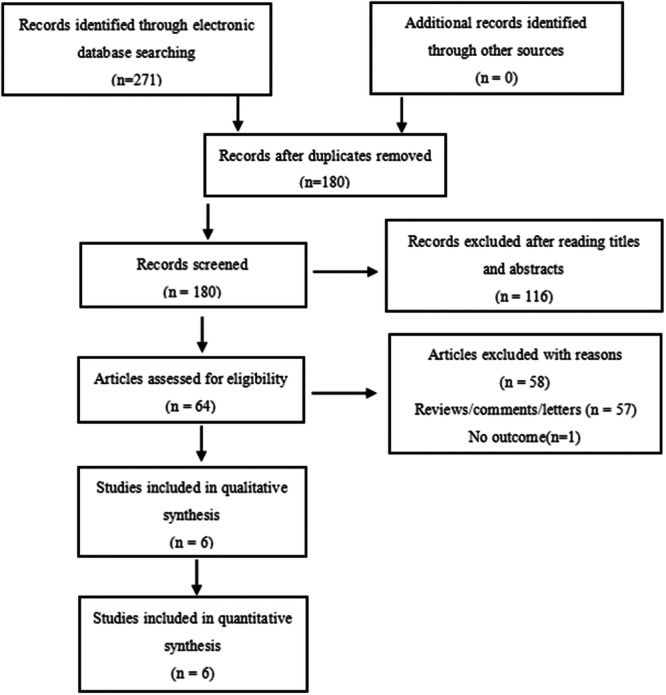
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). Six studies were included in the meta‐analysis.
3.1.1. Correlation of the Level of sPD‐1 With OS
Based on three studies [15, 19, 20], the correlation between the OS of ESCC and the level of serum sPD‐1 in patients with ESCC was not statistically significant (HR = 1.15, 95% CI: 0.66–1.98, p > 0.05) (Figure 2A).
Figure 2.
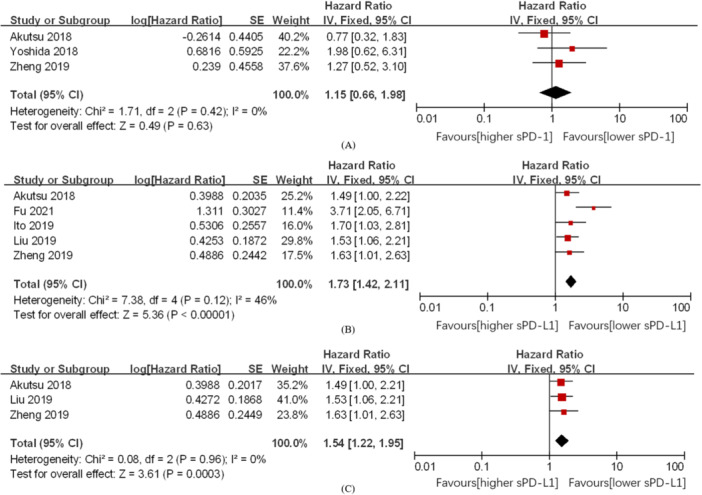
Forest plot of HR for the association between a high level of serum sPD‐1 and OS in patients with ESCC (A). Forest plot of HR for the association between high level of serum sPD‐L1 and OS in patients with ESCC (B). Take P50 as the cutoff point, forest plot of HR for the association between high level of serum sPD‐L1 and OS in patients with ESCC (C). CI, confidence interval; ESCC, esophageal squamous cell carcinoma; HR, hazard ratios; OS, overall survival; sPD‐L1, soluble programmed cell death ligand 1.
3.1.2. Correlation of the Level of sPD‐L1 With OS
Based on five studies [15, 16, 17, 18, 20], a high concentration of sPD‐L1 significantly reduced the overall survival rate of ESCC (HR = 1.73, 95% CI: 1.42–2.11, p < 0.001). This result was statistically significant. There was a certain degree of heterogeneity (I 2 = 46%) when the fixed‐effects model was used (Figure 2B).
3.2. Sensitivity Analyses and Subgroup Analysis
Sensitivity analysis was carried out in Review Manager 5.3 software using one‐by‐one deletion method to assess the stability of sPD‐L1 in ESCC for predicting OS. No significant differences were found outside the 95% CI of the pooled results. Taking P50 as the best cutoff point and after excluding the studies by Fu et al. [16] and Ito et al. [17], correlational analysis between the concentration of sPD‐L1 and the OS in ESCC showed that high concentration of sPD‐L1 and poor prognosis were significantly related (HR = 1.52, 95% CI: 1.22–1.95, p < 0.001). This result was statistically significant. No heterogeneity was detected (I 2 = 0) when the fixed effects model was used (Figure 2C).
3.3. Publication Biases
In this meta‐analysis, to detect publication bias, Egger's test was performed using Stata SE 15 software. There was no evidence of apparent publication bias between sPD‐L1 and OS. (Egger's test: Pr > |z | = 0.086 (continuity corrected)) (Figure 3).
Figure 3.
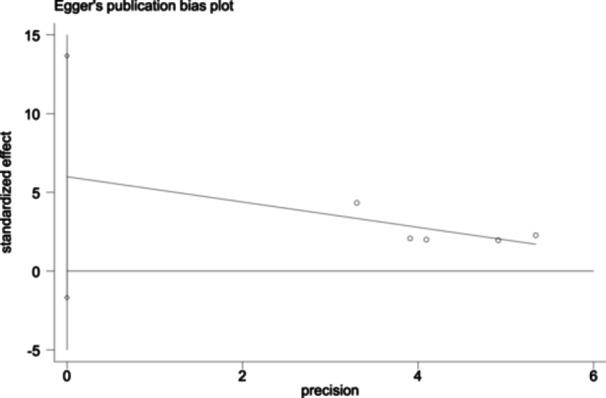
Egger's publication bias plot for the analysis of serum sPD‐L1 in OS in patients with ESCC: Egger's test for OS, Pr > |z| = 0.086 (continuity corrected). ESCC, esophageal squamous cell carcinoma; OS, overall survival; sPD‐L1, soluble programmed cell death ligand 1.
4. Discussion
Serum sPD‐L1 is perceived as deriving primarily from PD‐L1 positive cells. The combining of PD‐1 on activated lymphoid T cells binds to PD‐L1 expressed on cancer cells initiate the transmission of inhibitory signals to these T cells to prevent them from eliminating target malignant tumor cells [21]. To some extent, the PD‐1/PD‐L1 pathway is deemed to preserve tumor cells from T cell offensive [22]. The combining of serum sPD‐L1 to cell surface PD‐1 can influence T cells and increase complexity of PD‐1/PD‐L1 co‐inhibitory pathway [23]. Some studies have demonstrated that the level of sPD‐L1 can not only stand for the activity of the immune suppression axis, but also the degree of T cell response in tumor tissue [12, 24]. Therefore, sPD‐L1 is expected to serve as a credible biomarker. Here, a meta‐analysis was conducted by us to estimate the effect of serum sPD‐L1 and sPD‐1 on the prognosis of ESCC. In this meta‐analysis, 645 patients were screened through a series of inclusion and exclusion criteria, and serum sPD‐L1's prognostic value was systematically evaluated. The comprehensive results demonstrated that a higher concentration of serum sPD‐L1 was prominently related to unfavorable prognosis. In addition, we also performed a subgroup analysis with a preset cut‐off point (taking P50 as the best cut‐off point), and found that a high concentration of serum sPD‐L1 was also related to the poor prognosis, and the heterogeneity of stratification was not obvious (HR = 1.52, 95% CI: 1.22–1.95, p = 0.0003). Furthermore, we discovered that the serum concentrations of sPD‐L1 of healthy people were significantly lower than those of ESCC patients [16, 18, 20], consistent with the fact that the expression level of PD‐L1 in normal tissue was lower than that in tumor tissue of ESCC [8, 10]. However, studies have demonstrated that there is no significant relationship between the expression level of PD‐L1 in tumor tissue and the level of serum sPD‐L1 [25, 26, 27]. However, by comparing with Guo's study [28], we were surprised to find that the correlation between the OS of ESCC and the concentration of sPD‐L1 was more significant than that between the OS of ESCC and the PD‐L1 in tumor, and serum sPD‐L1 was easier to obtain (sPD‐L1: HR = 1.73, p < 0.00001; PD‐L1:HR = 1.38, p = 0.04). There are some reasons that high concentrations of serum sPD‐L1 can be related to the patient's immune status, and the increase in serum inflammatory cytokines may lead to increased concentrations of serum sPD‐L1 [18]. Not only that, a high concentration of sPD‐L1 can also be associated to the large volume of the tumor and/or the high malignancy of the tumor [15, 17].
ELISA, the known antigen or antibody is adsorbed on the surface of the solid phase carrier, so that the enzyme‐labeled antigen–antibody reaction is carried out on the surface of the solid phase, and the free components in the liquid phase are removed. Because different ELISA reagents were used in the six studies, there may be some heterogeneity in the results. Due to practical reasons, various reagents cannot be fully investigated, which is left to future researchers to investigate and analyze. However, I believe that the results of this article will attract the interest of future researchers and conduct research in this direction.
However, it has to be admitted that this article may be biased due to the small sample size and the nationality of the authors is mainly from China and Japan. I want to declare that the incidence of esophageal squamous cell carcinoma in China and Japan is higher than that in European and American countries, so it attracts more interest and attention of scientists in the two countries, leading to more research on esophageal squamous cell carcinoma in China and Japan. Therefore, I hope that some European and American researchers can find and devote themselves to this related research in the future, and add new research results.
Our meta‐analysis presented several valuable findings. First, compared with low concentrations of sPD‐L1, patients with ESCC with higher concentrations of serum sPD‐L1 had unfavorable prognosis, indicating that serum sPD‐L1 may be a useful predictor for the survival and prognosis of ESCC. Second, serum sPD‐L1 provided an effective and easy‐to‐detect method to predict the prognosis of ESCC. A lot of studies have demonstrated that PD‐L1 in tumor tissue is an appropriate prognostic biomarker. Nevertheless, to detect the expression level of PD‐L1, it is requisite to obtain tumor tissue in invasive procedures, especially in solid tumors. Therefore, detection of sPD‐L1 may be a better option for predicting the prognosis of ESCC due to its more convenient and less invasive ways.
It is undeniable that this article has certain limitations: first, there are too few existing relevant studies and fewer patients are included, which is susceptible to some bias to some extent, and continuous attention needs to be paid to the update of the articles in this field. Secondly, the ELISA reagents used in these included studies are different, making it hard to have a common standard and creating a certain bias. Thirdly, the best cutoff point for the detection of the concentration of serum sPD‐L1 has not yet been determined, and P50 is now more commonly used. Therefore, it is necessary to further design prospective large‐sample studies to verify the results in the future.
5. Conclusion
In conclusion, this is the first meta‐analysis to research the prognostic value of sPD‐1 and sPD‐L1 in ESCC and our results demonstrate that a high concentration of sPD‐L1 is obviously related to the unfavorable prognosis of ESCC. And sPD‐L1 is expected to be a new potential prognostic biomarker for ESCC and it provides a new therapeutic strategy for patients due to its advantages of easy acquisition, detection, and non‐invasiveness.
Author Contributions
Qiyao Yu: conceptualization, methodology, writing–original draft, visualization, data curation, writing–review and editing, software, formal analysis, validation, investigation. Jie Li: investigation, supervision, software, data curation. Wenjie Mao: investigation, supervision, software, data curation. Zheng Li: investigation, supervision, software, data curation. Xuan Li: methodology, investigation, formal analysis, software. Bin Li: writing–review and editing, funding acquisition, supervision, project administration, resources, validation, conceptualization.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Bin Li affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
This work was supported by the Science and Technology Key Research and Development Program of Gansu Province (grant number 20YF3FA032).
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Chen W., Zheng R., Baade P. D., et al., “Cancer Statistics in China, 2015,” CA: A Cancer Journal for Clinicians 66 (2016): 115–132, 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2. Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71 (2021): 209–249, 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3. Rustgi A. K. and El‐Serag H. B., “Esophageal Carcinoma,” New England Journal of Medicine 371 (2014): 2499–2509, 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 4. Cohen D. J. and Leichman L., “Controversies in the Treatment of Local and Locally Advanced Gastric and Esophageal Cancers,” Journal of Clinical Oncology 33 (2015): 1754–1759, 10.1200/JCO.2014.59.7765. [DOI] [PubMed] [Google Scholar]
- 5. Allemani C., Matsuda T., Di Carlo V., et al., “Global Surveillance of Trends in Cancer Survival 2000‐14 (CONCORD‐3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population‐Based Registries in 71 Countries,” The Lancet 391 (2018): 1023–1075, 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sznol M. and Chen L., “Antagonist Antibodies to PD‐1 and B7‐H1 (PD‐L1) in the Treatment of Advanced Human Cancer,” Clinical Cancer Research 19 (2013): 1021–1034, 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen K., Cheng G., Zhang F., et al., “Prognostic Significance of Programmed Death‐1 and Programmed Death‐Ligand 1 Expression in Patients With Esophageal Squamous Cell Carcinoma,” Oncotarget 7 (2016): 30772–30780, 10.18632/oncotarget.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M. F., Chen P. T., Chen W. C., Lu M. S., Lin P. Y., and Lee K. D., “The Role of PD‐L1 in the Radiation Response and Prognosis for Esophageal Squamous Cell Carcinoma Related to IL‐6 and T‐Cell Immunosuppression,” Oncotarget 7 (2016): 7913–7924, 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J. J., Zhou Z. Q., Wang P., et al., “Orchestration of Immune Checkpoints in Tumor Immune Contexture and Their Prognostic Significance in Esophageal Squamous Cell Carcinoma,” Cancer Management and Research 10 (2018): 6457–6468, 10.2147/CMAR.S181949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu Y., Li M., Mu D., et al., “CD8+/FOXP3+ Ratio and PD‐L1 Expression Associated With Survival in pT3N0M0 Stage Esophageal Squamous Cell Cancer,” Oncotarget 7 (2016): 71455–71465, 10.18632/oncotarget.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsutsumi S., Saeki H., Nakashima Y., et al., “Programmed Death‐Ligand 1 Expression at Tumor Invasive Front Is Associated With Epithelial‐Mesenchymal Transition and Poor Prognosis in Esophageal Squamous Cell Carcinoma,” Cancer Science 108 (2017): 1119–1127, 10.1111/cas.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okuma Y., Hosomi Y., Nakahara Y., Watanabe K., Sagawa Y., and Homma S., “High Plasma Levels of Soluble Programmed Cell Death Ligand 1 Are Prognostic for Reduced Survival in Advanced Lung Cancer,” Lung Cancer 104 (2017): 1–6, 10.1016/j.lungcan.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 13. Finkelmeier F., Canli Ö., Tal A., et al., “High Levels of the Soluble Programmed Death‐Ligand (sPD‐L1) Identify Hepatocellular Carcinoma Patients With a Poor Prognosis,” European Journal of Cancer 59 (2016): 152–159, 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Z. R., Zhang T. S., li B., Mao Z., Zeng X. T., and Liu S. X., “Extracting and Transforming of Appropriate Data of Meta‐Analysis in Survival Curve,” Chinese Journal of Evidence‐Based Cardiovascular Medicine 6 (2014): 243–247, 10.3969/j.1674-4055.2014.03.02. [DOI] [Google Scholar]
- 15. Akutsu Y., Murakami K., Kano M., et al., “The Concentration of Programmed Cell Death‐Ligand 1 in the Peripheral Blood Is a Useful Biomarker for Esophageal Squamous Cell Carcinoma,” Esophagus 15 (2018): 103–108, 10.1007/s10388-018-0604-1. [DOI] [PubMed] [Google Scholar]
- 16. Fu R., Jing C. Q., Li X. R., Tan Z. F., and Li H. J., “Prognostic Significance of Serum PD‐L1 Level in Patients With Locally Advanced or Metastatic Esophageal Squamous Cell Carcinoma Treated With Combination Cytotoxic Chemotherapy,” Cancer Management and Research 13 (2021): 4935–4946, 10.2147/CMAR.S312690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito M., Yajima S., Suzuki T., et al., “High Serum PD‐L1 Level Is a Poor Prognostic Biomarker in Surgically Treated Esophageal Cancer,” Cancer Medicine 9 (2020): 1321–1327, 10.1002/cam4.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C., Chen D. J., Yu J. L., et al, “Detection of Serum sPD‐L1 and Tissue PD‐L1 in Patients With Advanced Esophageal Squamous Cell Carcinoma and Prognostic Value,” Journal of Zhengzhou University: Medical Sciences 54 (2019): 500–503, 10.13705/j.issn. [DOI] [Google Scholar]
- 19. Yoshida J., Ishikawa T., Doi T., et al., “Clinical Significance of Soluble Forms of Immune Checkpoint Molecules in Advanced Esophageal Cancer,” Medical Oncology 36 (2019): 60, 10.1007/s12032-019-1285-x. [DOI] [PubMed] [Google Scholar]
- 20. Zheng Z. Y., Zhou F. Y., Zhang A. P., et al., “Changes of sPD‐1 and sPD‐L1 in Patients With Esophageal Squamous Cell Carcinoma (ESCC) Before and After Chemoradiotherapy and Their Clinical Significance,” China Medical Engineering 27 (2019): 18–23, 10.19338/j.issn.1672-2019.2019.02.004. [DOI] [Google Scholar]
- 21. Zou W. and Chen L., “Inhibitory B7‐Family Molecules in the Tumour Microenvironment,” Nature Reviews Immunology 8 (2008): 467–477, 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 22. Sharma P. and Allison J. P., “The Future of Immune Checkpoint Therapy,” Science 348 (2015): 56–61, 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y., Wang Q., Shi B., et al., “Development of a Sandwich ELISA for Evaluating Soluble PD‐L1 (CD274) in Human Sera of Different Ages as Well as Supernatants of PD‐L1+ Cell Lines,” Cytokine 56 (2011): 231–238, 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24. Shi M. H., Xing Y. F., Zhang Z. L., Huang J. A., and Chen Y. J., “Effect of Soluble PD‐L1 Released by Lung Cancer Cells in Regulating the Function of T Lymphocytes,” Zhonghua Zhong Liu za Zhi [Chinese Journal of Oncology] 35 (2013): 85–88, 10.3760/cma.j.issn.0253-3766.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25. Shigemori T., Toiyama Y., Okugawa Y., et al., “Soluble PD‐L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD‐L1 Expression,” Annals of Surgical Oncology 26 (2019): 876–883, 10.1245/s10434-018-07112-x. [DOI] [PubMed] [Google Scholar]
- 26. Kruger S., Legenstein M. L., Rösgen V., et al., “Serum Levels of Soluble Programmed Death Protein 1 (sPD‐1) and Soluble Programmed Death Ligand 1 (sPD‐L1) in Advanced Pancreatic Cancer,” Oncoimmunology 6 (2017): e1310358, 10.1080/2162402X.2017.1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aghajani M. J., Roberts T. L., Yang T., et al., “Elevated Levels of Soluble PD‐L1 Are Associated With Reduced Recurrence in Papillary Thyroid Cancer,” Endocrine Connections 8 (2019): 1040–1051, 10.1530/EC-19-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo W., Wang P., Li N., et al., “Prognostic Value of PD‐L1 in Esophageal Squamous Cell Carcinoma: A Meta‐Analysis,” Oncotarget 9 (2017): 13920–13933, 10.18632/oncotarget.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


