Abstract
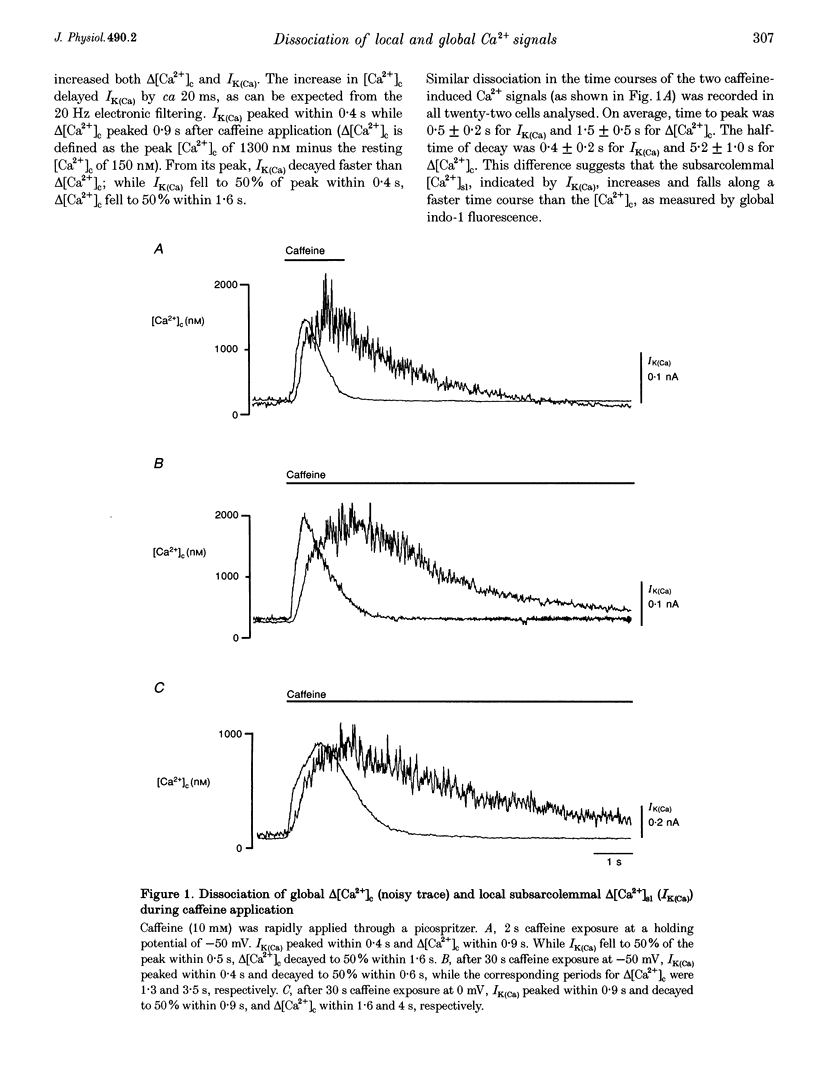
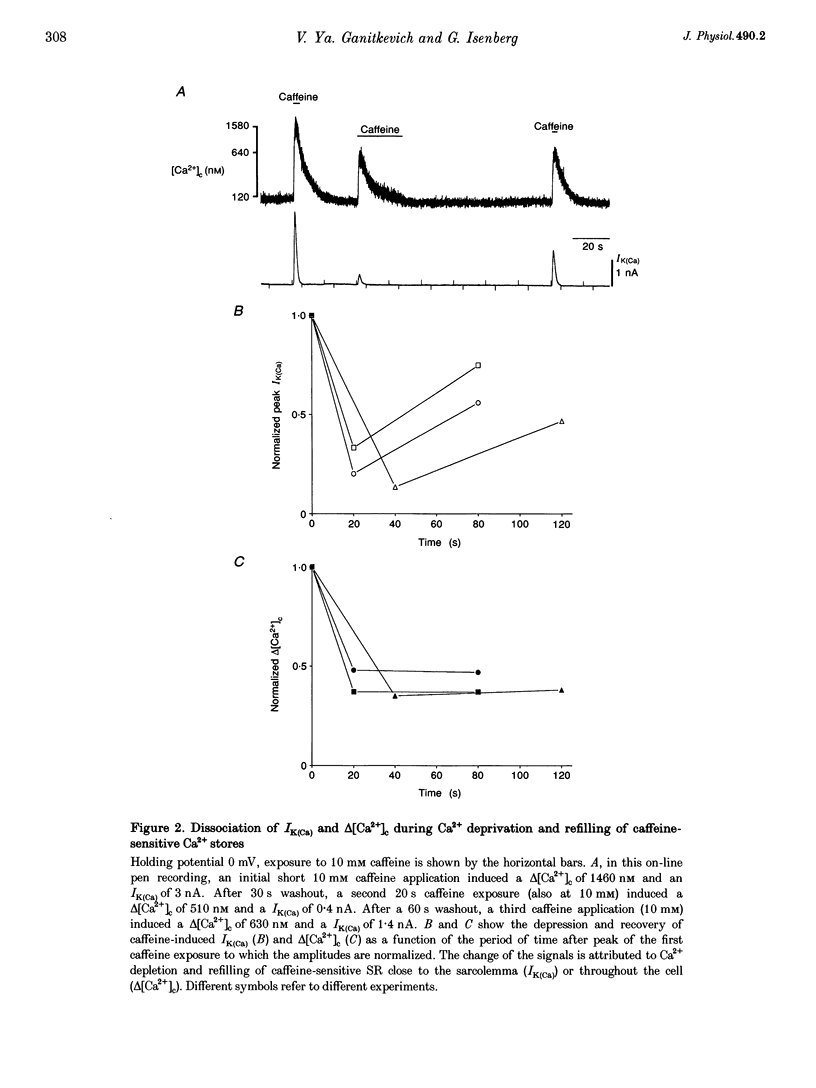
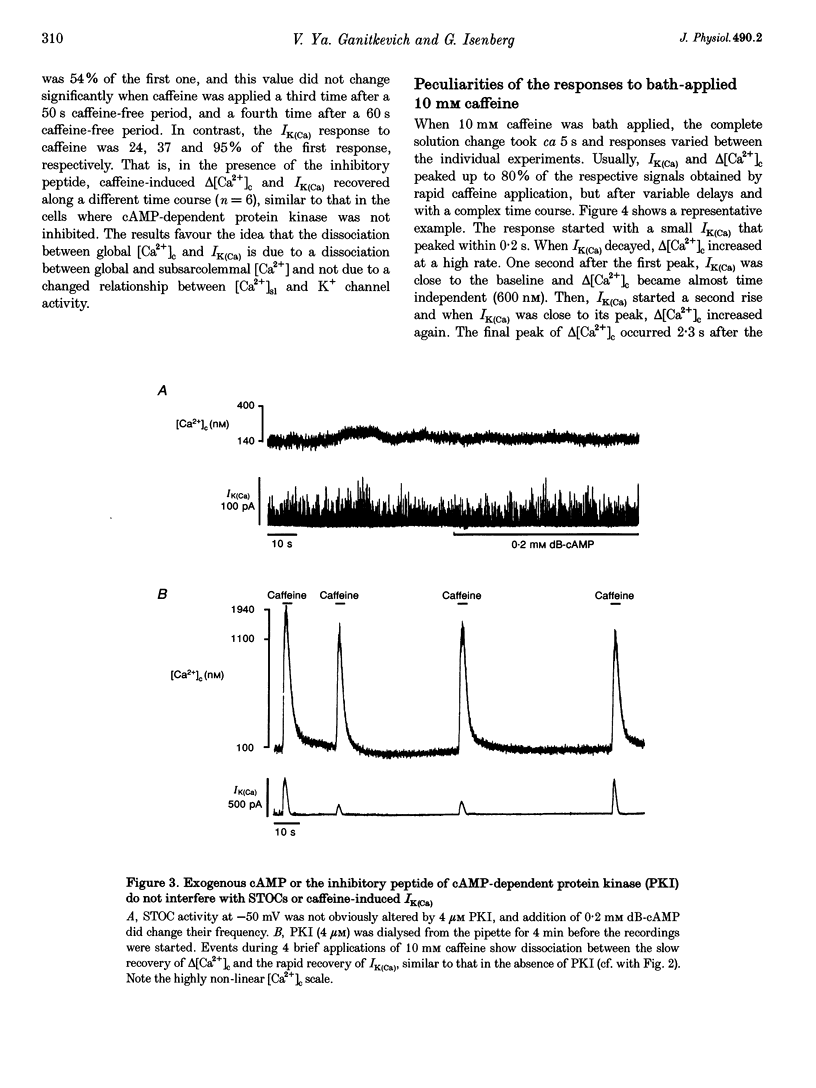
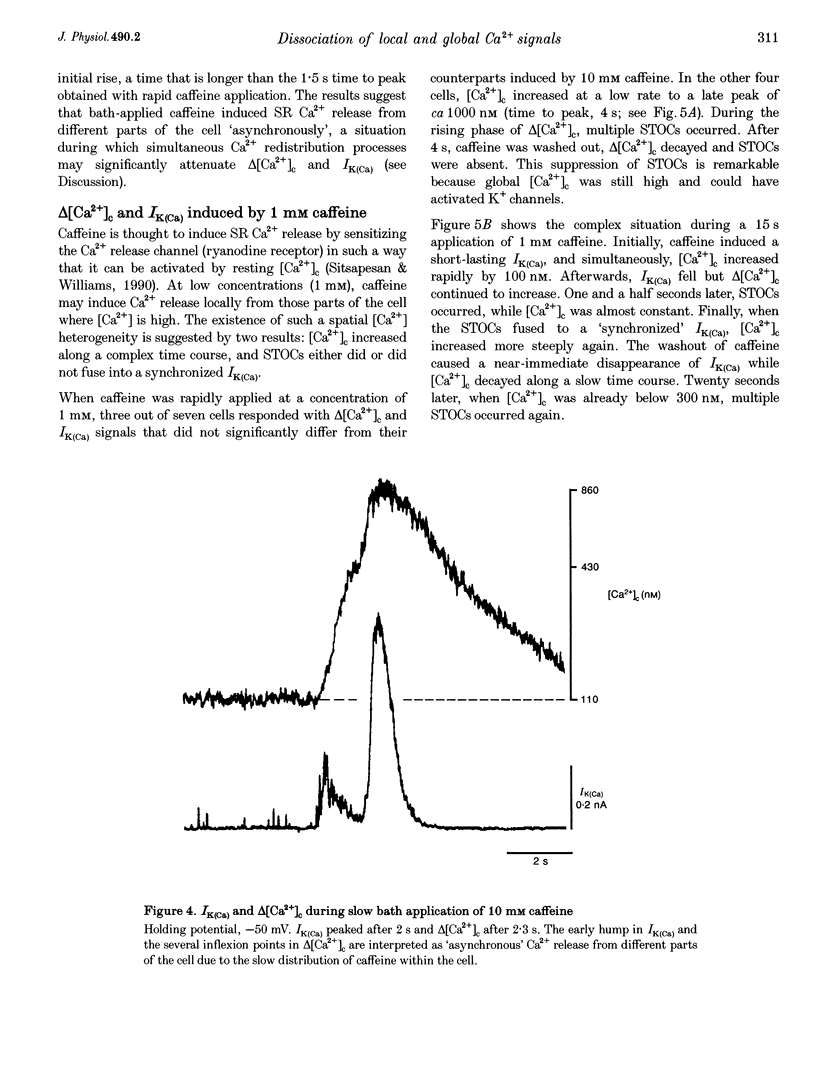
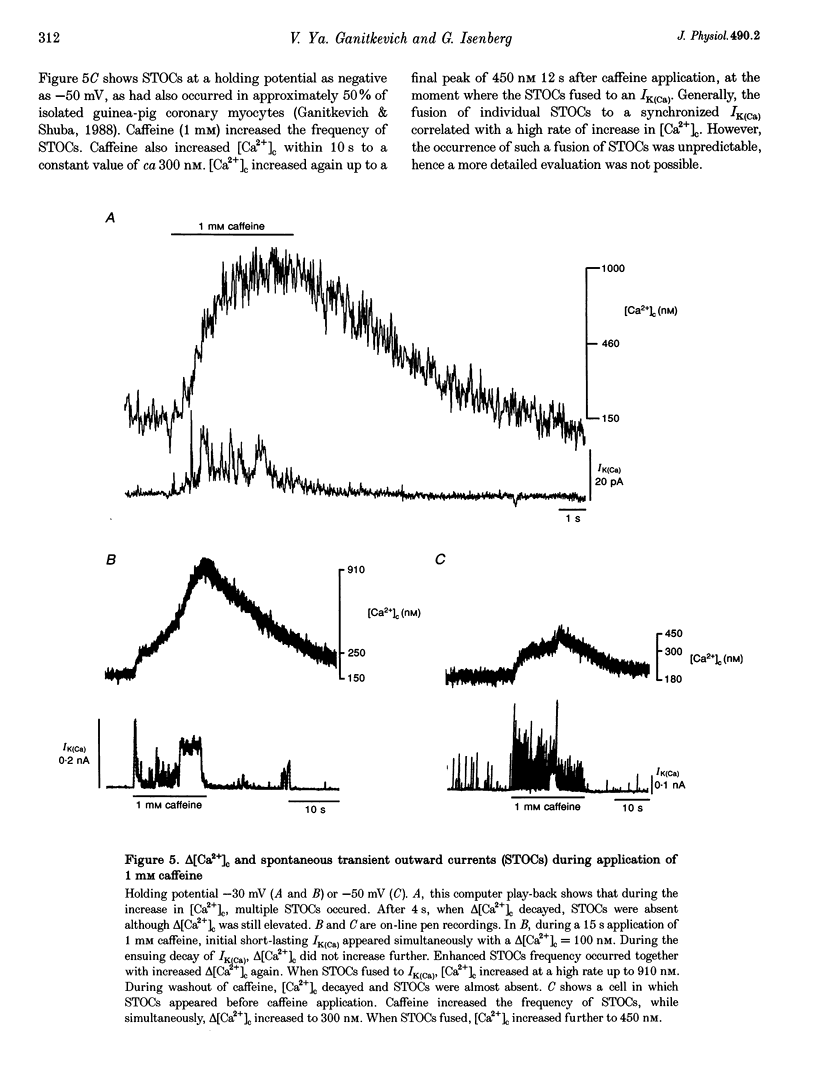
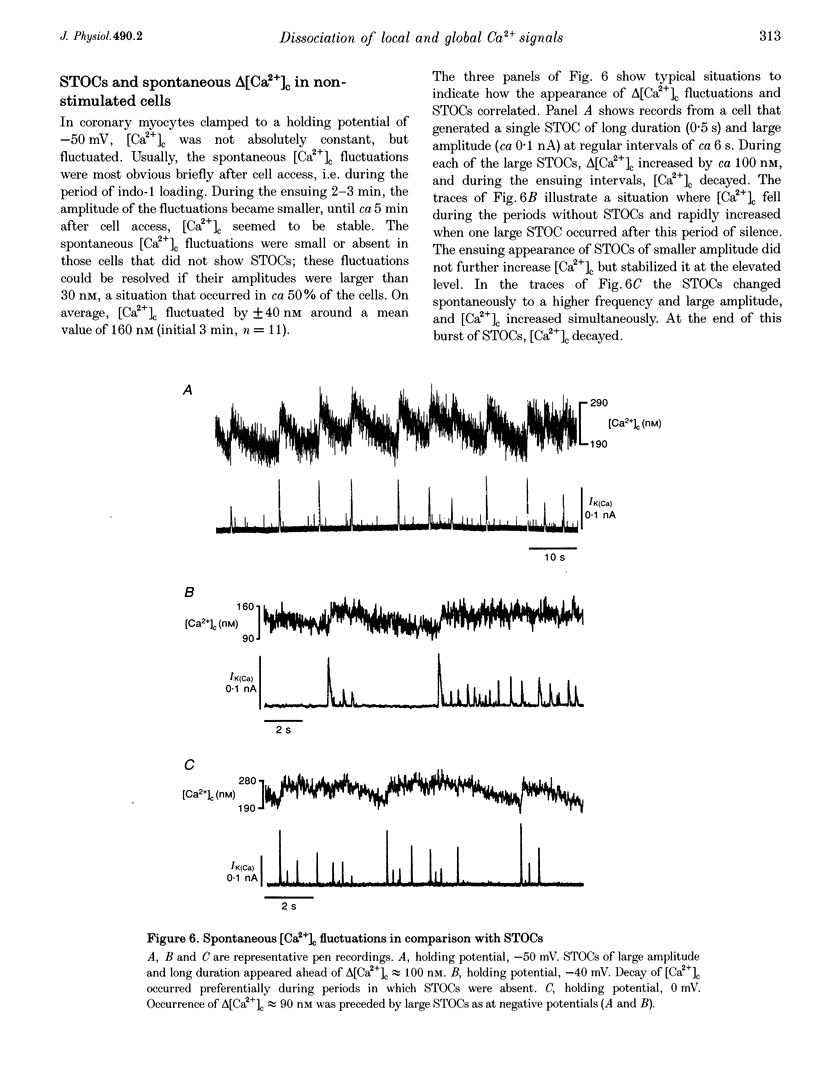
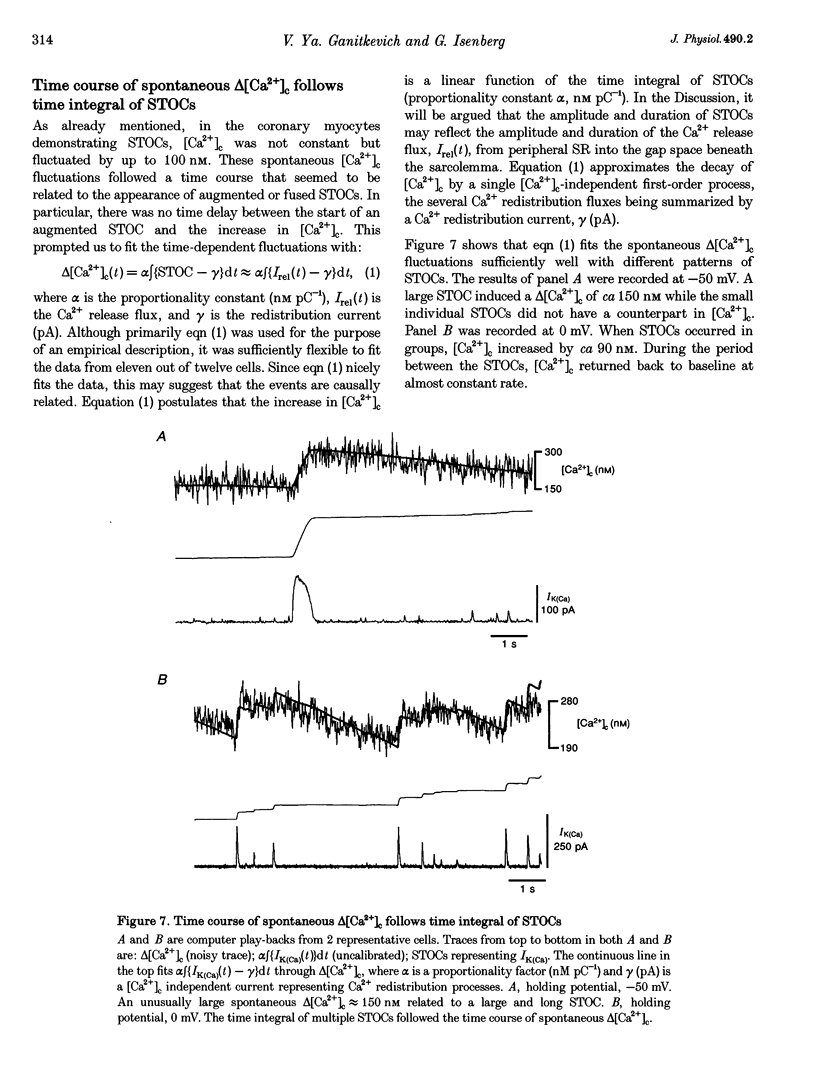
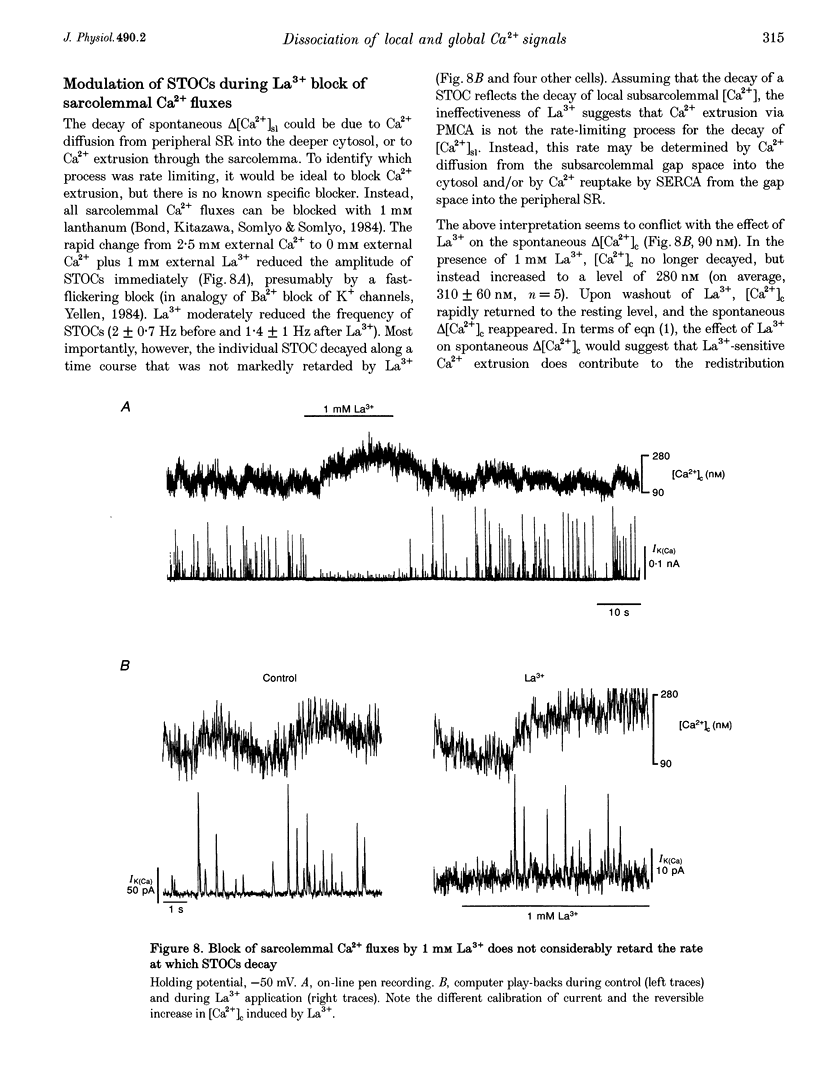
1. Changes in cytosolic Ca2+ concentration (delta[Ca2+]c) were measured by global indo-1 fluorescence and compared with changes in subsarcolemmal Ca2+ concentration (delta[Ca2+]sl) indicated by Ca(2+)-activated K+ currents (IK(Ca)). 2. At -50 mV holding potential, 10mM caffeine increased both IK(Ca) and [Ca2+]c without measurable delay. While IK(Ca) peaked within 0.3 +/- 0.16 s (mean +/- S.D.) and decayed to 50% within 0.4 +/- 0.2 s, delta[Ca2+]c peaked within 1.5 +/- 0.5 s and decayed to 50% within 5.2 +/- 1.0 s. The different time courses support the idea that [Ca2+]sl and [Ca2+]c deviate. 3. When 10 mM caffeine was applied 20 s after an initial 2 s caffeine application, IK(Ca) was suppressed to 22 +/- 5% and delta [Ca2+]c to 40 +/- 4%. During the following 1 min caffeine-free period, IK(Ca) recovered to 61 +/- 7% while delta [Ca2+]c remained at 40 +/- 3%. The differences between IK(Ca) and delta[Ca2+]c suggest that Ca2+ deprivation and Ca2+ refilling is faster in peripheral than in central sarcoplasmic reticulum (SR). 4. During the loading period of indo-1, a spontaneous delta[Ca2+]c of 30-80 nM appeared both at -50 mV and at more positive potentials. The amplitude of spontaneous delta[Ca2+]c increased with the amplitude, the frequency or the fusion of spontaneous transient outward currents (STOCs). 5. Block of sarcolemmal Ca2+ fluxes by 1 mM La3+ increased [Ca2+]c by 250 +/- 100 nM and suppressed the spontaneous delta[Ca2+]c. However, La3+ did not significantly retard the rate of decay of STOCs which may therefore be limited by Ca2+ diffusion into the cytosol and not by Ca2+ extrusion. 6. The dissociation of IK(Ca) (or STOCs) and delta[Ca2+]c may indicate a Ca2+ concentration gradient during Ca2+ release directed from the sarcolemma towards the centre of the cell, which later reverses direction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Cannell M., van Breemen C. The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol. 1992 Apr;70(4):509–514. doi: 10.1139/y92-066. [DOI] [PubMed] [Google Scholar]
- Etter E. F., Kuhn M. A., Fay F. S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J Biol Chem. 1994 Apr 1;269(13):10141–10149. [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Ca2+ entry through Na(+)-Ca2+ exchange can trigger Ca2+ release from Ca2+ stores in Na(+)-loaded guinea-pig coronary myocytes. J Physiol. 1993 Aug;468:225–243. doi: 10.1113/jphysiol.1993.sp019768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Efficacy of peak Ca2+ currents (ICa) as trigger of sarcoplasmic reticulum Ca2+ release in myocytes from the guinea-pig coronary artery. J Physiol. 1995 Apr 15;484(Pt 2):287–306. doi: 10.1113/jphysiol.1995.sp020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993 Oct;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G. J. Calcium signaling in restricted diffusion spaces. Biophys J. 1994 Jul;67(1):262–272. doi: 10.1016/S0006-3495(94)80477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G., Fay F. S. Ca2+ movement in smooth muscle cells studied with one- and two-dimensional diffusion models. Biophys J. 1991 Nov;60(5):1088–1100. doi: 10.1016/S0006-3495(91)82145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Grégoire G., Pacaud P. Photoreleased inositol 1,4,5-trisphosphate-induced response in single smooth muscle cells of rat portal vein. J Physiol. 1994 Aug 15;479(Pt 1):41–52. doi: 10.1113/jphysiol.1994.sp020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt F., Isenberg G. Gating of maxi K+ channels studied by Ca2+ concentration jumps in excised inside-out multi-channel patches (myocytes from guinea pig urinary bladder). J Gen Physiol. 1992 Jun;99(6):841–862. doi: 10.1085/jgp.99.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon G. F., Mignery G. A., Somlyo A. V. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J Muscle Res Cell Motil. 1994 Dec;15(6):682–700. doi: 10.1007/BF00121075. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis D. A., Clark C. L., O'Brien K. J. Lanthanum can be transported by the sodium-calcium exchange pathway and directly triggers catecholamine release from bovine chromaffin cells. Cell Calcium. 1994 Nov;16(5):377–390. doi: 10.1016/0143-4160(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Schubert R., Hescheler J., Nelson M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Wellner M. C., Isenberg G. cAMP accelerates the decay of stretch-activated inward currents in guinea-pig urinary bladder myocytes. J Physiol. 1995 Jan 1;482(Pt 1):141–156. doi: 10.1113/jphysiol.1995.sp020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Voigt T., Isenberg G. Microheterogeneity of subsarcolemmal sodium gradients. Electron probe microanalysis in guinea-pig ventricular myocytes. J Physiol. 1993 Dec;472:33–44. doi: 10.1113/jphysiol.1993.sp019934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z. L., Kitamura K., Kuriyama H. Evidence for contribution of Ca2+ storage sites on unitary K+ channel currents in inside-out membrane of rabbit portal vein. Pflugers Arch. 1992 Jan;420(1):112–114. doi: 10.1007/BF00378651. [DOI] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J Gen Physiol. 1984 Aug;84(2):187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


