ABSTRACT
The diversity of biotic and abiotic sounds that fill underwater ecosystems has become polluted by anthropogenic noise in recent decades. Yet, there is still great uncertainty surrounding how different acoustic stimuli influence marine and freshwater (i.e., aquatic) communities. Despite capabilities to detect and produce sounds, aquatic invertebrates are among the most understudied taxa within the field of soundscape ecology. We conducted a meta‐analysis to understand how sounds from various sources influence the behavior and physiology of aquatic invertebrates. We extracted 835 data points from 46 studies conducted in 15 countries. The resulting data included 50 species, a range of experimental conditions, and four sound categories: anthropogenic, environmental, synthetic, and music. We used meta‐analytic multivariate mixed‐effect models to determine how each sound category influenced aquatic invertebrates and if responses were homogeneous across taxa. Our analyses illustrate that anthropogenic noise and synthetic sounds have detrimental impacts on aquatic invertebrate behavior and physiology, and that environmental sounds have slightly beneficial effects on their behavior. Defence responses were the most impacted behaviors, while the most prominent physiological responses were related to biochemistry, genetics, and morphology. Additionally, arthropods and molluscs exhibited the most pronounced physiological responses to anthropogenic and synthetic noise. These findings support the conclusion that many invertebrate species are sensitive to changes in aquatic soundscapes, which can cause adverse or favorable consequences to individuals and populations, dependent on the sound source. This quantitative synthesis highlights the necessity of including marine and freshwater invertebrates in acoustic exposure studies, aquatic ecosystem assessments, and emerging noise pollution policies.
Keywords: acoustic enrichment, aquatic invertebrates, arthropods, fitness consequences, global change, molluscs, noise pollution, quantitative synthesis, soundscapes
We analyzed 46 studies to determine how sounds from various sources (i.e., human‐made noise, environmental sounds, computer‐generated or synthetic sounds, and music) influence aquatic invertebrates. Results show that human and synthetic sounds harm invertebrate behavior and physiology, and environmental (e.g., natural reef) sounds can be beneficial. These findings suggest that noise pollution can impact many aquatic invertebrate species, especially arthropods (e.g., crabs and lobsters) and molluscs (e.g., mussels and squid), and highlight the importance of considering invertebrates in noise policies and aquatic ecosystem conservation efforts.

1. Introduction
Sound is a critical sensory modality for invertebrates in marine, brackish, and freshwater environments (Kunc, McLaughlin, and Schmidt 2016; Solé et al. 2023). Underwater sound sources include biological sounds, abiotic natural sounds, and sounds generated by human activities, cumulatively creating soundscapes (Pijanowski et al. 2011). Invertebrates detect components of the soundscape through a variety of mechanisms to receive information about their surroundings (Solé et al. 2023). While underwater sound is composed of both sound pressure and particle motion, many invertebrate species perceive sounds mainly through particle motion (i.e., oscillating particles in the medium), using various internal and epidermal sensory organs dependent on taxa (André et al. 2016; Popper, Salmon, and Horch 2001; Solé et al. 2023). Particle motion is especially useful for directional hearing and short‐distance communication (Hawkins and Popper 2017); however, it remains an understudied aspect of acoustic ecology (Nedelec et al. 2016). Numerous invertebrate species, including crustaceans, cephalopods, and annelids, also use sounds for communication, such as in defensive or aggressive behaviors (Bouwma and Herrnkind 2009; Goto, Hirabayashi, and Palmer 2019; Guerra et al. 2007). The hearing and production of underwater sounds, their associated ecological functions, and the invertebrates themselves, however, may be disrupted by anthropogenic noise pollution (i.e., human‐made sounds that negatively affect the health and well‐being of organisms; Solé et al. 2023).
Global underwater soundscapes have become increasingly inundated by expanding ranges and intensities of anthropogenic noise, through activities such as shipping, resource exploration (e.g., seismic surveys), and infrastructure development (e.g., pile driving; Duarte et al. 2021; Kunc, McLaughlin, and Schmidt 2016; Slabbekoorn et al. 2010). Low‐frequency ambient sound levels in open ocean environments have increased around 3 dB per decade between the 1950s and early 2000s (Frisk 2012; McDonald, Hildebrand, and Wiggins 2006). Anthropogenic sounds dominate these lower frequencies, overlapping with the hearing and acoustic communication ranges of many animals (Duarte et al. 2021; Merchant et al. 2020). Anthropogenic noise has become so pervasive that it has been recognized by the World Health Organization as a major global pollutant and hazardous contributor to environmental change (World Health Organization 2011). Since most human‐made sounds contain little to no beneficial information, they are generally considered to be noise pollution (Kunc, McLaughlin, and Schmidt 2016; Pijanowski et al. 2011). Many aquatic (i.e., marine and freshwater) animals have adaptations that increase their abilities to detect and produce sounds, including marine mammals, fishes, and some aquatic invertebrates (Kasumyan 2008; Morley, Jones, and Radford 2013; Tyack 2008); however, these adaptations also make them very susceptible to the effects of noise pollution (Cox et al. 2018; Duarte et al. 2021). Studies investigating the potential impacts of noise pollution exposure on aquatic animals have found that it can cause both behavioral and physiological changes, such as movement from preferred habitats, impaired ability to detect predators or prey, modified stress levels, reduced fertility, permanent injury or death, and many others (Duarte et al. 2021; Kunc, McLaughlin, and Schmidt 2016).
While noise pollution is a critical concern in marine and freshwater ecosystems around the globe, other sources of underwater sounds are also of interest (Cox et al. 2018; Duarte et al. 2021). Synthetic sounds (e.g., linear sweeps, tones, and white noise) are used in aquatic environments and laboratory tank experiments to expose animals to specific frequency ranges and amplitudes of sounds, often mimicking anthropogenic noise sources (Hubert et al. 2022; Vazzana et al. 2020; Yağcılar and Yardımcı 2021). These simulated sounds also have unique applications, including inhibition of barnacle settlement and reduced survival of invasive snails (Branscomb and Rittschof 1984; Solé et al. 2021). Aquatic animals are also exposed to a variety of environmental sounds, including biotic reef sounds (e.g., from fishes and crustaceans) and freshwater fish sounds (Looby et al. 2022; Rountree, Juanes, and Bolgan 2020; Vermeij et al. 2010), as well as species‐specific acoustic cues (Hughes, Mann, and Kimbro 2014). Additionally, acoustic enrichment (e.g., classical music) can promote aquatic animal welfare and growth but has yet to be thoroughly studied for fishes and aquatic invertebrates (Arechavala‐Lopez et al. 2022; Cox et al. 2018; Ren et al. 2021). While the impacts of most sound sources on marine mammals and fishes are relatively well‐studied, an increasing amount of research is being conducted to investigate impacts on aquatic invertebrates (Carroll et al. 2017; Duarte et al. 2021; Wale, Briers, and Diele 2021).
To assess the importance of sounds in aquatic ecosystems, it is critical to consider invertebrate taxa, as many are highly valued economically and ecologically, including species that are important food resources, form the base of marine food webs, create habitat, and recycle nutrients (Anderson et al. 2011; Kunc, McLaughlin, and Schmidt 2016). There are over 170,000 known species of multi‐cellular marine invertebrates, and they are increasingly being documented detecting and responding to acoustic cues (Weilgart 2018). Research on the effects of sounds has been conducted on a diversity of aquatic invertebrate taxa, including corals, jellyfishes, bivalves, cuttlefish, gastropod molluscs, squid, crabs, lobsters, shrimp, sea urchins, and various zooplankton (Lecchini et al. 2018; Solé et al. 2023; Tu et al. 2021). A variety of behavioral and physiological responses to sounds have also been recorded, ranging from impacts on acoustic emissions and recruitment, to oxygen levels and survival (Filiciotto et al. 2018; Lillis, Bohnenstiehl, and Eggleston 2015; Ren et al. 2021; Wale et al. 2019).
We conducted a meta‐analysis to quantify the impacts of sounds on aquatic invertebrate behavior and physiology. Through our analysis, we reveal the main sound sources that invertebrates have been experimentally exposed to. We also examine how invertebrates respond to these sound sources, including bioacoustic behaviors, biochemical metrics, defence, development, foraging ability, genetics, morphology, movement, recruitment, and survival. Finally, we assess the extent to which responses to each sound source are homogeneous across the taxonomic groups represented in our study to identify taxa that may be more susceptible to acoustic stimuli. Taxa included arthropods (e.g., barnacles, copepods, and decapod crustaceans), bryozoans, cnidarians (i.e., corals and jellyfishes), echinoderms (i.e., sea urchins), and molluscs (e.g., bivalves, cuttlefish, sea slugs, snails, and squid). While syntheses of the effects of sounds on aquatic invertebrates have been conducted (e.g., Di Franco et al. 2020; Duarte et al. 2021; Solé et al. 2023), these studies are limited to review methodologies that do not quantify impacts to species, focus on limited sound sources or locations, or only briefly discuss invertebrates as part of wider taxonomic reviews. Meta‐analyses allow for transparent and generalizable conclusions to be reached through data synthesized from many studies and are an effective method for evaluating ecological trends (Del Re 2015). This research is the first quantitative, systematized review of how aquatic sounds influence invertebrate behavior and physiology, providing insight into the impacts of anthropogenic noise on understudied taxa, the importance of natural sounds for maintaining ecological processes, and the extent to which aquatic invertebrates will respond to experimentally manipulated or enriched soundscapes.
2. Materials and Methods
2.1. Literature Review
Web of Science was used to consolidate peer‐reviewed literature on how sounds influence aquatic invertebrate behavior and physiology. The specific search terms used were “noise or sound or acoustic*”, “marine or aquatic”, and “invertebrate* or benthic or arthropod* or cephalopod* or cnidaria* or crustacean* or echinoderm* or mollus*”, where asterisks indicate truncation wildcards and quotation marks enclose search phrases. The search initially considered studies published between 1900 and 2018 and returned 1095 potentially relevant peer‐reviewed studies. An additional 10 studies were identified through other sources such as previous knowledge and reference searching (i.e., thoroughly reviewing bibliographies of relevant reviews; Murchy et al. 2020). The search was updated in March 2022 using the same string, identifying 611 potentially relevant peer‐reviewed studies published from 2018 to 2022. To ensure sufficient coverage between the two searches, the year 2018 was included in both examinations. Following the second search, an additional 10 studies were identified from other sources. The titles and abstracts of the 1726 studies identified by both searches were reviewed to determine which papers addressed the effects of sound on aquatic invertebrate behavior or physiology (Figure 1). Articles that met these criteria (n = 73) were then further evaluated to identify those that met the following criteria: original research, listed sound source, the inclusion of an experimental control, reported mean values with either standard deviation or standard error, and reported sample sizes.
FIGURE 1.

Evaluating the changing acoustic conditions that aquatic invertebrates are exposed to using a meta‐analytical approach. (a) A representative marine ecosystem depicting the invertebrate taxa and main sound sources (i.e., anthropogenic noise, environmental sounds, synthetic sounds, and music) included in the analysis and (b) a PRISMA diagram of each phase of the meta‐analysis literature review.
In total, 46 studies from 15 countries met the search criteria (Figure 1; Table S1). The studies spanned the years 1982 to 2022, included four main types of sound exposures (i.e., anthropogenic, environmental, music, and synthetic), and investigated behavioral and physiological responses of 50 species from seven taxonomic groups (Figure 2). The mean, standard deviation, and sample size were extracted from the treatment and control groups of each relevant comparison within a selected study, and studies commonly included multiple relevant comparisons. Data were obtained from tables and text wherever possible, and the extraction software GraphClick (Arizona‐Software 2008) and WebPlotDigitizer (Rohatgi 2022) were used to collect data from figures when necessary. A total of 835 data points (i.e., each mean response of a species to sound exposure within a reference) were collected from the 46 studies.
FIGURE 2.
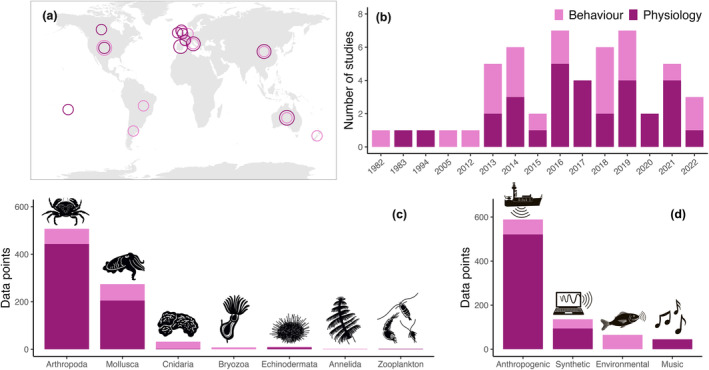
The spatiotemporal coverage and data composition of the 46 studies included in the meta‐analysis. Behavioral data are light pink, and physiological data are dark pink—in the five cases where single studies included both behavioral and physiological data, they were plotted as separate points. (a) The 15 countries where the research was conducted. Circle size is proportional to the density of studies (the number of studies in each country as follows: Argentina 1, Australia 7, Brazil 1, Canada 1, China 5, England 2, France 3, French Polynesia 2, Ireland 1, Italy 5, Netherlands 2, New Zealand 1, Spain 5, United Kingdom 3, and United States 7). (b) The number of studies published in each year (years with zero studies are not shown). (c) The number of data points corresponding to each invertebrate taxonomic group. (d) The number of data points included within each sound source category.
2.2. Effect Size (ES) Calculation
ES and variance are standardized measures of the magnitude of the relationship between two variables and the associated precision of the ES estimate, respectively.
ES and variance were calculated for each response variable in RStudio using the metafor package (R Core Team 2023; Viechtbauer 2010). The standardized mean difference ES (Cohen's d), which compares the mean of a numerical response variable between two groups based on their sample sizes and standard deviations, was calculated using Equation (1) (Del Re 2015).
| (1) |
Ȳ 1 and Ȳ 2 are the mean values of the treatment and control group, respectively; sd pooled is the pooled standard deviation of both group means, computed as in Equation (2) (Del Re 2015).
| (2) |
The sample sizes are indicated by n 1 and n 2 and standard deviations by s 1 and s 2. The variance of Cohen's d is given by Equation (3).
| (3) |
The positive bias in Cohen's d was automatically corrected within the function, yielding Hedges' g (Viechtbauer 2010). The difference between the mean responses is weighted by the sd pooled . The conversion of Cohen's d to Hedges' g was computed using the correction factor given by Equation 4 (Del Re 2015).
| (4) |
The degrees of freedom (df) are the group size minus one. The correction factor J was used to compute unbiased estimators g and V g as in Equations 5 and 6, respectively.
| (5) |
| (6) |
Accounting for the diversity of response variables is a critical step in an ecological meta‐analysis. The directionality of each response was determined to ensure that negative and positive ESs represented negative and positive responses, respectively. For example, we ensured that an increase in the survival rate would result in a positive ES since it is a beneficial response, while an increase in damaged hair cells would result in a negative ES since it is a detrimental response. Directionality was accounted for by multiplying the ES by a correction factor of 1 if the response direction was accurate and by a negative 1 if it was inaccurate, thereby ensuring that the various responses could be evaluated along the same axis (Cox et al. 2018; Vesterinen et al. 2014).
2.3. Statistical Analyses
Data were separated into behavioral and physiological responses and were further divided into the four sound source categories: anthropogenic noise, environmental sounds, music, and synthetic sounds (Table S2). Within each sound source, behavioral response variables were categorized as bioacoustics, defence (i.e., anti‐predator and aggressive behaviors), foraging, movement, or recruitment (i.e., larval settlement); physiological response variables were categorized as biochemistry, defence (i.e., color changes), development, genetics, morphology, or survival (Table S3).
All analyses were conducted in RStudio (R Core Team 2023). Data were organized and plotted using dplyr and ggplot2 packages (Wickham 2016; Wickham et al. 2022). Zero values present in the data were accounted for by approximating a small constant, 10−7 (Viechtbauer 2010). To determine the summary effect (i.e., weighted average of individual study ES) and confidence intervals (CIs) of each sound category, random‐effects models with a restricted maximum‐likelihood estimator were used to create forest plots using metafor and MAd packages (Figures S1 and S2; Del Re and Hoyt 2014; Viechtbauer 2010). Individual ESs were aggregated by study when generating forest plots to reduce bias in the estimates, using Borenstein's method within the MAd package (Del Re 2015; Del Re and Hoyt 2014). This model was selected to account for the varied methodologies and characteristics of the studies included in the meta‐analysis, such as different invertebrate taxa, recorded sound pressure levels, experimental conditions (e.g., field and tanks), life stage of organisms (e.g., larvae, juvenile, and adult), and other factors. Since publication bias (i.e., the discrepancy between published studies and all studies on a topic) is a concern when conducting a meta‐analysis, funnel plots of the random‐effects models for each sound source category were created and visually examined for asymmetry and heterogeneity (Figures S3 and S4; Del Re and Hoyt 2014; Viechtbauer 2010).
Meta‐analytic multivariate mixed‐effect models for each sound source category were used to determine how the ESs of behavioral and physiological responses differed from a “zero” line of no effect, using the metafor package (Viechtbauer 2010). To allow the models to run, behavioral or physiological response variables with less than three data points were excluded, which occurred only once (Table S4). Histograms of the ES for each category were plotted and examined for normality with Shapiro–Wilk tests. The ESs were transformed into a normal shape using the Box–Cox method in the MASS package, and the transformed histograms were visually examined. Reduced models were constructed with response variables as a fixed effect and study as a random effect, while full models included response variables as a fixed effect and data point ID nested within study as a random effect (Table S4). The full models were consistently the best‐fitted models after comparison through analysis of variance (ANOVA) tests and examination of the Akaike information criterion (AIC) values. When the sound category of interest contained a single study with multiple response types (i.e., physiological responses to music), the model included response as a fixed effect and data point ID as a random effect (Table S4). The resulting model estimates and 95% CIs were then plotted to visualize the effect of each sound source on the response variables, and 95% CIs that did not overlap with the zero‐line indicated trends that were non‐random (Table S5; Wickham 2016; Wilke 2020). The p‐value (α = 0.05) and z‐value were reported for all model estimates, and both statistically significant and non‐significant trends were explored.
The influence of each sound source on the taxonomic groups was determined following the same modelling process, with the only difference being the inclusion of phyla (e.g., Arthropoda and Mollusca) instead of behavioral or physiological response category (e.g., foraging and biochemistry). Briefly, meta‐analytic multivariate mixed‐effect models for each sound source category were used to determine if the behavioral and physiological responses of each taxonomic group differed from a “zero” line of no effect, using the metafor package (Viechtbauer 2010). Reduced models were constructed with the taxonomic group as a fixed effect and study as a random effect, while full models included the taxonomic group as a fixed effect and data point ID nested within study as a random effect (Table S6). Six taxa with less than three data points were excluded from the models (Table S6). Model outputs were plotted to visualize the differences between the taxonomic groups (Table S7).
The validity of all models was assessed using several statistical tests. Model residuals were examined for normality using histograms and studentized quantile‐quantile (Q‐Q) plots, and variance components were visualized using profile likelihood plots (Figure S5a–c; Viechtbauer 2010). Influential case diagnostics using Cook's distances, DFBETAS values, and hat values ensured that outliers did not cause considerable changes to the fitted models (Figure S5d). Cook's distance estimates the influence of data points by excluding them from model fitting, DFBETAS evaluates the change in the standard deviation of the predicted values with and without each data point, and hat values determine the extent to which a predictor value differs from the other predictor values (i.e., leverage). Comparatively large Cook's distances, DFBETAS values above 2 divided by the square root of the sample size, and hat values approaching or exceeding 1 were examined as potential outliers (Viechtbauer 2010; Viechtbauer and Cheung 2010). This process identified three data points across three models that required examination and ultimately warranted removal. Excluding these data points had minimal influence on the insights drawn from these models, but improved model fit in all cases. The ability of all models to generate data that overlaps the data used to create the model was examined using the “ranef” function in the metafor package (Figure S5e; Viechtbauer 2010; Viechtbauer and Cheung 2010). The distribution of these predictions was compared to the raw data visually and using Student's t‐tests. The data and code that support this meta‐analysis are openly available in Borealis (Davies et al. 2024).
3. Results
3.1. Summary Effects
Forest plots showed that both behavioral and physiological responses varied strongly based on the sound source category (Figures 3, S1 and S2). Anthropogenic noise had a slight negative effect on invertebrate behavior and a significantly negative effect on physiology (Figure 3). Studies addressing behavioral responses had an overall ES of −0.37 with 95% CI of −0.98 and 0.24 (Figure S1a), while studies of physiological responses had an overall ES of −1.31 with 95% CI of −2.00 and −0.61 (Figure S2a). Environmental sounds had a variable, but overall, slightly positive effect on behavioral responses, with an ES of 0.10 (CI –0.00, 0.20; Figure 3, S1b), and we did not identify any studies that investigated the effects of environmental sounds on invertebrate physiology. No studies addressed the impact of music on invertebrate behavior. The single study that examined the effect of music on invertebrate physiology had a slight positive effect of 0.80 (CI –0.26, 1.87; Figures 3 and S2b). Synthetic sounds had a significantly negative effect on both invertebrate behavior and physiology (Figure 3)—behavioral responses had an overall ES of −0.57 (CI –1.06, −0.00; Figure S1c), and physiological responses had a much more negative ES of −3.82 (CI –5.78, −1.87; Figure S2c).
FIGURE 3.
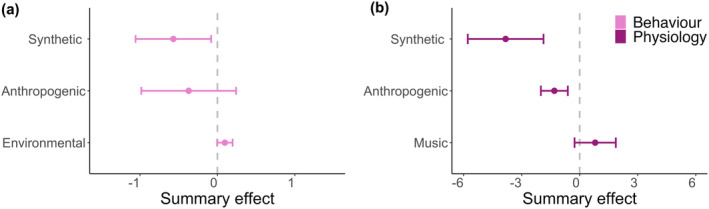
Summary effects and 95% confidence intervals (CIs), derived from forest plots that examined each study within the meta‐analysis (Table S1 and Figures S1 and S2), illustrating the influence of each broader sound category on aquatic invertebrate (a) behavior (light pink) and (b) physiology (dark pink). The vertical dotted line indicates an effect size of zero and non‐overlapping 95% CIs are significant.
Additionally, funnel plots did not indicate that there were major effects of publication bias within any of the behavioral response categories (Figure S3). Funnel plots examining physiological responses to anthropogenic noise and synthetic sounds indicated minor asymmetry and increased variation associated with these studies (Figure S4).
3.2. Effects of Sounds on Behavioral Responses
Anthropogenic noise had both detrimental (i.e., negative) and beneficial (i.e., positive) effects on behaviors (Figure 4a, Table S5a). Defence behaviors, including hiding time, raised arms, righting time, bivalve gape, and sheltering, were significantly and negatively affected (estimate = −0.60, p = 0.007, z = −2.72). Movement‐related behaviors, such as activity level, chemical cue attraction, and time swimming were slightly negatively impacted (estimate = −0.44, p = 0.051, z = −1.95). In contrast, anthropogenic noise marginally impacted foraging (estimate = 0.08, p = 0.841, z = 0.20) and slightly positively impacted recruitment (estimate = 0.50, p = 0.192, z = 1.30).
FIGURE 4.
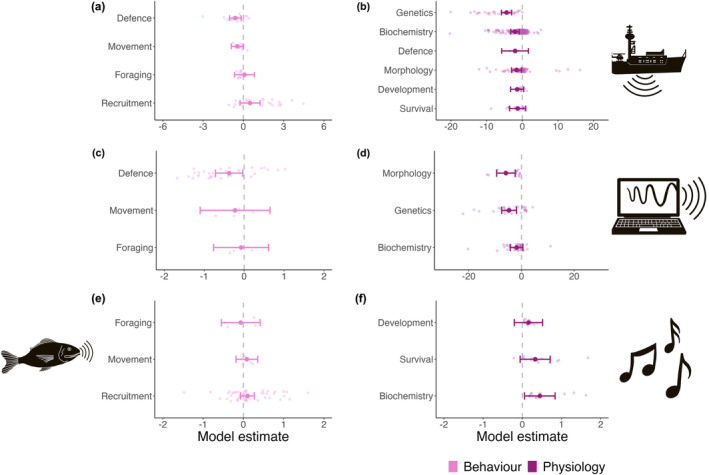
Model estimates and 95% confidence intervals (CIs) of the behavioral (light pink) and physiological (dark pink) responses of aquatic invertebrates to sounds, derived from meta‐analytic multivariate mixed‐effect models which evaluated responses to (a) and (b) anthropogenic noise, (c) and (d) synthetic sounds, (e) environmental sounds, and (f) music. Points illustrate the transformed effect size data that were used in each model. The vertical dotted line indicates an effect size of zero and non‐overlapping 95% CIs are significant.
Synthetic sounds were detrimental to all the response categories examined (Figure 4c, Table S5a). Defence behaviors were significantly negatively impacted by the presence of synthetic sounds, including digging depth, bivalve gape, fights, and tail flips (estimate = −0.37, p = 0.032, z = −2.15). Movement responses (i.e., encounters and number moving) were slightly negatively affected (estimate = −0.23, p = 0.613, z = −0.51), and foraging behaviors (i.e., the number of individuals foraging or near a food source) were marginally impacted (estimate = −0.07, p = 0.831, z = −0.21).
Environmental sounds had variable effects on the response categories examined (Figure 4e, Table S5a). Foraging behaviors, measured by consumption, were marginally negatively impacted (estimate = −0.07, p = 0.789, z = −0.27). In contrast, movement (i.e., activity level) and recruitment behaviors were slightly positively impacted in the presence of environmental sounds (estimate = 0.09, p = 0.529, z = 0.63; estimate = 0.10, p = 0.236, z = 1.19).
3.3. Effects of Sounds on Physiological Responses
Anthropogenic noise detrimentally impacted various physiological responses (Figure 4b, Table S5b). Genetic responses, including biomarkers of DNA health and gene expression, were significantly negatively impacted by anthropogenic noise (estimate = −4.43, p < 0.0001, z = −5.99). Biochemical metrics were also significantly negatively affected overall, such as adenosine triphosphate (ATP) content, heat shock proteins, respiration rate, and enzyme activity (estimate = −2.06, p = 0.001, z = −3.38). Non‐behavioral defence responses, including color changes in crabs and cuttlefish, were slightly negatively impacted (estimate = −2.04, p = 0.287, z = −1.06). Invertebrate morphology was significantly negatively affected by anthropogenic noise, including reductions in body condition and byssal thread size and strength as well as increased hair cell damage and shell size (estimate = −1.70, p = 0.022, z = −2.30). A slight negative effect was detected for developmental parameters, such as developmental success, development time, and percent metamorphosis (estimate = −1.63, p = 0.023, z = −2.28). Finally, survival rates were also slightly negatively impacted by anthropogenic noise (estimate = −1.31, p = 0.256, z = −1.14).
Examinations of how invertebrate physiology was influenced by synthetic noise and music exhibited varied responses (Table S5b). Synthetic sounds had significantly negative impacts on morphology in the form of increased hair cell damage (estimate = −5.91, p = 0.001, z = −3.30) and genetics (i.e., gene expression; estimate = −4.72, p = 0.001, z = −3.29; Figure 4f). Biochemical parameters, such as enzyme activity, heat shock proteins, hemocyte count, and respiration rate, were slightly negatively affected (estimate = −1.87, p = 0.128, z = −1.52). One study on one species examined the effects of classical music on three response types. Development time and survival rate slightly increased (estimate = 0.16, p = 0.395, z = 0.85; estimate = 0.33, p = 0.094, z = 1.68), while biochemical metrics (i.e., respiration rate) were significantly improved in music‐enhanced conditions (estimate = 0.45, p = 0.025, z = 2.25; Figure 4g).
3.4. Effects of Sounds on Taxa
The responses of different taxonomic groups to sound exposure varied according to the sound source and whether responses were behavioral or physiological (Table S7). The significantly negative physiological responses of arthropods and molluscs to anthropogenic noise (estimate = −2.00, p = 0.005, z = −2.79; estimate = −2.08, p = 0.021, z = −2.31; Figure 5b) and synthetic sounds (estimate = −6.02, p = 0.024, z = −2.26; estimate = −4.96, p < 0.0001, z = −5.35; Figure 5d) were the most pronounced. To a lesser extent, this trend was mirrored by the significantly negative behavioral responses of molluscs to synthetic sounds (estimate = 0.48, p = 0.030, z = −2.17). Behavioral responses of all other taxa included in this analysis were not significantly impacted (Figure 5a,c,e). Additionally, responses within each analysis (e.g., behavioral responses to anthropogenic noise) were relatively similar across taxa (Figure 5).
FIGURE 5.
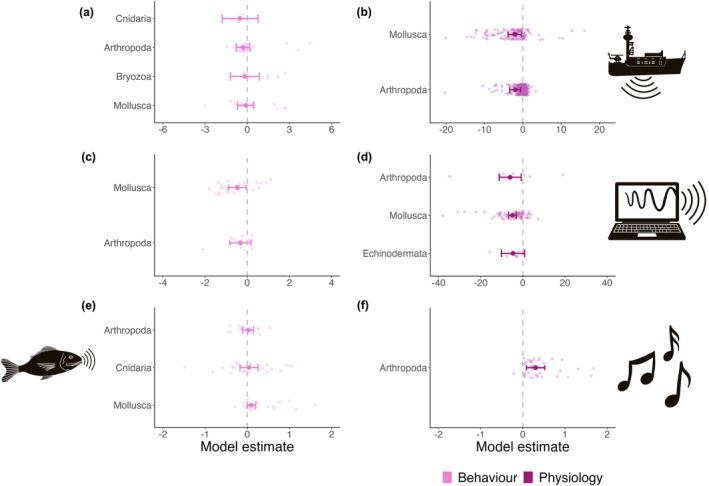
Model estimates and 95% confidence intervals (CIs) of the behavioral (light pink) and physiological (dark pink) responses of aquatic invertebrate taxa to sounds, derived from meta‐analytic multivariate mixed‐effect models which evaluated responses to (a) and (b) anthropogenic noise, (c) and (d) synthetic sounds, (e) environmental sounds, and (f) music. Points illustrate the transformed effect size data that were used in each model. The vertical dotted line indicates an effect size of zero and non‐overlapping 95% CIs are significant. Annelida and zooplankton were excluded from these models due to insufficient data.
4. Discussion
This study comprises the first quantitative global review addressing the effects of sounds on aquatic invertebrates. We synthesized 46 peer‐reviewed studies across 15 countries and quantified impacts on invertebrate behavior and physiology. The studies consisted exclusively of marine taxa apart from two freshwater species (Pomacea maculata and Procambarus clarkii ); however, many of the taxonomic groups studied are represented in both habitats (e.g., arthropods and crustaceans). Sound exposure occurred in highly varied field and laboratory environments, and models summarized trends across these studies from the 825 extracted data points. Our meta‐analysis found that anthropogenic noise and synthetic sounds had overall negative effects on aquatic invertebrate behavior and physiology, and environmental sounds had slightly positive effects on behavior. Defence behaviors, genetics, biochemistry, and morphology were the most impacted response types. Additionally, the greatest impacts were on arthropods and molluscs exposed to anthropogenic noise and synthetic sounds.
Exposure to anthropogenic noise negatively affected invertebrate behavior and physiology. Behavioral responses including defence and movement were negatively impacted, similar to the negative behavioral effects of noise pollution on other taxa including fishes and marine mammals (Cox et al. 2018; Duarte et al. 2021; Southall et al. 2019). However, in some cases, invertebrate responses to anthropogenic noise were inconsistent. Effects of ambient tank noise and shipping on foraging responses were marginal, possibly due to limited data and higher levels of uncertainty interpreting these behaviors. Shipping noise had a positive effect on recruitment of various larval invertebrate species (e.g., barnacles and bivalves) due to increased settlement (Jolivet et al. 2016; Stanley, Wilkens, and Jeffs 2014), while settlement of coral larvae decreased (Lecchini et al. 2018). This trend supports the findings that larval invertebrates use sounds to identify suitable habitats (Simpson et al. 2011, 2005; Vermeij et al. 2010) but suggests that the acoustic stimuli used are not limited to natural sounds (and increased recruitment to unsuitable habitats may not be beneficial in the long‐term). Notably, behavioral impacts were not always consistent between similar taxa. For example, increased valve gape was considered a stress response in mussels (Wale et al. 2019), but the same behavior was found to improve the growth rate of oysters (Charifi et al. 2018).
We observed an array of physiological responses to anthropogenic noise related to or independent of behavioral responses. Invertebrate genetics and morphology were significantly impacted, causing damage and downregulation of various genes, hair cell damage, and reduced body size and strength (e.g., Day et al. 2019; Zhao et al. 2021). Despite these trends, size and condition were not always impacted in the months following noise exposure, possibly due to the added dynamic of in situ ecological pressures (Przeslawski et al. 2018). Biochemical parameters, most often stress bioindicators extracted from invertebrate hemolymph, were also significantly impacted (Solé et al. 2023). However, certain biochemical parameters such as hepatopancreas index might have more limited applications, and elevated glucose has only recently been supported as a stress response to acoustic exposure (Fitzgibbon et al. 2017; Simon et al. 2015; Solé et al. 2023). While the effects were less pronounced likely due to limited data, physiological defence responses were also detrimentally impacted (e.g., Carter, Tregenza, and Stevens 2020). Additionally, anthropogenic noise increased development failure and accelerated metamorphosis, negatively impacting larval shell growth and body mass in mussels (Jolivet et al. 2016). Conversely, no significant differences in development were found in crab larvae when exposed to seismic survey noise (Pearson et al. 1994), suggesting that some taxa may be more resistant. Finally, larvae and zooplankton survival were less impacted than other response categories (McCauley et al. 2017; Pearson et al. 1994), but more studies are needed to sufficiently evaluate invertebrate mortality. Overall, physiological responses to noise were generally more pronounced than behavioral, which could indicate that altered physiology compounded on behavioral changes (e.g., decreased foraging success leading to elevated stress levels), or that physiological effects are associated with more severe sound exposures (Hawkins and Popper 2017; Kunc, McLaughlin, and Schmidt 2016).
Synthetic sound exposure, such as linear sweeps, negatively influenced invertebrate behavior and physiology. In addition to broad impacts, studies incorporating synthetic sounds can effectively test how aquatic invertebrates respond to specific sound frequencies and modulations (e.g., Hubert et al. 2022). Defence, movement, and foraging behaviors were all impacted, but the largest effects were on defence. Estimates of movement and foraging responses may have been less pronounced than defence behaviors as these were the most data‐limited response categories (i.e., a single study for each category). These behavioral changes could be induced through several mechanisms, including masking of relevant acoustic cues or disturbance leading to decreased risk‐taking (Hubert et al. 2022, 2018). There were also negative effects on invertebrate physiological responses including morphology, genetics, and biochemistry. Morphological responses to synthetic sounds were quantified through damaged hair cells following exposure to tones (Solé et al. 2021, 2018, 2017, 2016), allowing for the relationship between sound exposure and hearing impairment to be examined directly. Significant effects on genetics were also detected through changes in gene expression, including increases in genes linked to stress responses (Peng et al. 2016; Tu et al. 2021). Further, examples of biochemical responses included effects on enzyme activity in sea slugs and sea urchins (Tu et al. 2021; Vazzana et al. 2020).
Our examination of environmental sounds provided insight into the varied invertebrate behavioral responses to animal and reef sounds, and we did not identify any research on physiological responses. One study showed decreased foraging success of crabs in the wild exposed to predatory fish sounds (Hughes, Mann, and Kimbro 2014). In contrast, environmental sounds had positive effects on larval recruitment, which supports further exploration of acoustic enrichment techniques (Gordon et al. 2019; Simpson et al. 2005). The single examination of movement found that the activity levels of various larvae slightly increased in response to reef sounds (Stocks et al. 2012). Further research incorporating animal bioacoustics and physiological responses to environmental sounds would help substantiate these findings.
Similar to acoustic exposure studies on fishes, limited research has been conducted on the effects of music on aquatic invertebrate physiology (Cox et al. 2018; Ren et al. 2021). Music was shown to positively influence invertebrate development, survival, and biochemistry, but these insights were drawn from a single study (Ren et al. 2021). While environmental enrichment is increasingly being considered in fish aquaculture settings and music welfare research is beginning to develop (Arechavala‐Lopez et al. 2022; Barcellos et al. 2018; Kriengwatana, Mott, and ten Cate 2022), studies on invertebrates remain limited (e.g., Pereira 2015). Expanding the field of acoustic enrichment research could nonetheless have substantial applications for improving invertebrate aquaculture and welfare.
In addition to examining the responses of aquatic invertebrates to sound exposure, we quantified the effects of each sound category on invertebrate taxa. Behavioral and physiological responses were typically consistent within each sound source analysis (e.g., behavioral responses to anthropogenic noise), and arthropods and molluscs were most likely to have pronounced physiological responses to anthropogenic and synthetic sounds. The responses to synthetic sounds may have been most detrimental since these sounds do not occur naturally in aquatic ecosystems and can have sound characteristics less constrained by natural forces. In general, the behavioral responses of taxa were less notable, though synthetic sounds did elicit significantly negative responses in molluscs. Decapod crustaceans and cephalopods especially have several physiological adaptations that could increase their hearing sensitivity (e.g., acceleration detection) and therefore adverse responses to noise, which should be incorporated into aquatic ecosystem assessments (Solé et al. 2023). Additionally, the studies we identified were heavily biased towards arthropods and molluscs in countries outside of equatorial regions. Therefore, more acoustic exposure research on a variety of invertebrate taxa from different regions is critical as some groups are particularly understudied (e.g., annelids and echinoderms), and research on many ecologically and commercially important invertebrates is still absent (Solé et al. 2023).
While the responses of cetaceans, pinnipeds, and fishes to sound exposure have been more extensively studied than aquatic invertebrates, key differences between these groups highlight the importance of continued research efforts on invertebrates (Duarte et al. 2021). Aquatic invertebrate phyla, body plans, and ontogenetic stages are notably more diverse than those of fishes and marine mammals, so sound exposure impacts are likely to be less uniform and generalizations should be made with caution (Aguilar de Soto 2016). While there are some similarities in sound detection anatomy among these groups, invertebrate taxa have receptor systems consisting of unique combinations of internal statocysts and epidermal flow detectors (Duarte et al. 2021; Solé et al. 2023). Unlike fishes, aquatic invertebrates do not have accessory hearing structures (e.g., swim bladder connections) that allow them to sense the pressure component of sound, so are likely only sensitive to particle motion (Solé et al. 2023). Sound exposure studies on aquatic invertebrates should therefore measure particle motion in addition to sound pressure levels and frequency (Nedelec et al. 2016). Further, particle motion travels both through the water as well as the epibenthos so some aquatic invertebrates (e.g., hermit crabs) are also impacted by epibenthic vibrations (Roberts and Elliott 2017; Roberts and Laidre 2019).
The diverse responses of aquatic invertebrate taxa to sounds suggests that species will experience both behavioral and physiological impacts while navigating rapidly changing underwater soundscapes (Duarte et al. 2021). These adjustments have the potential to affect individual fitness (e.g., growth rate) as well as populations (Hawkins and Popper 2017)—for example, noise exposure can cause species emigration, which may impact population structures, create unbalanced predator–prey dynamics, or lead to reduced recruitment through impaired larval development (Peng, Zhao, and Liu 2015). Reduced populations could also negatively affect the productivity of fisheries, although no significantly negative effects of noise on invertebrate catch rates have yet been reported (Solé et al. 2023). Additionally, sound exposure can change social interactions in a variety of ways including altered signal characteristics and communication, mating behaviors, and group cohesion (Fisher et al. 2021). For invertebrate species that are dominant in underwater soundscapes, such as snapping shrimp, any changes to their health and behaviors could lead to acoustically mediated feedback loops further exacerbating the extent of environmental changes (Gordon et al. 2018; Spiga 2016). More holistic assessments of underwater soundscapes and how invertebrates interact with them will provide greater insight into the larger ecosystem implications of anthropogenic noise and other global changes.
Meta‐analyses are an effective technique for reaching transparent conclusions and evaluating ecological trends but nonetheless have limitations (Del Re 2015). Some of the challenges in our meta‐analysis included the widely differing behavioral and physiological responses among diverse invertebrate phyla (and uncertainty of the response directionality in some cases), varied methodologies of the selected studies (e.g., laboratory and field environments), and an inability to include relevant data due to its published format (e.g., mean or variability not available) or lack of peer‐review (e.g., government reports). There are also notable difficulties with conducting laboratory bioacoustic studies, including the sound field within tanks not matching those of natural aquatic environments and the complex relationship between sound pressure and particle motion (Dinh and Radford 2021; Hawkins and Popper 2017; Nedelec et al. 2016). Additionally, there was some indication of publication bias for physiological responses to anthropogenic noise and synthetic sounds, which suggests that significant results may have been published more frequently in these categories. We recommend cautious interpretations of these data as the ESs and model estimates could be inflated, and we emphasize the importance of publishing all results to avoid bias in subsequent meta‐analyses. Future work should consider these issues as well as continue to assess the impacts of various sound sources and acoustic enrichment on a diversity of aquatic invertebrates, especially understudied and ecologically important groups, early life stages, freshwater species, and sound‐producing taxa (e.g., sea urchins; Coquereau et al. 2016; Rountree, Juanes, and Bolgan 2020; Solé et al. 2023), in addition to ensuring that these data are accessible to future meta‐analyses.
Our study illustrates that rapidly changing underwater acoustic conditions, including increased noise pollution and altered soundscapes (Duarte et al. 2021; Gordon et al. 2018), can significantly impact aquatic invertebrates at various life stages. While the literature on the effects of sounds on aquatic invertebrates is growing, our study is the first to quantitatively analyze and categorize responses to a variety of sound types across a range of invertebrate species. Since increasingly prevalent underwater noise poses a global threat to aquatic ecosystems and their inhabitants, we also highlight the necessity to develop management strategies and policies surrounding acoustic exposure that consider the wide array of marine and freshwater invertebrates that could be impacted.
Author Contributions
Hailey L. Davies: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing – original draft, writing – review and editing. Kieran D. Cox: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing – original draft, writing – review and editing. Kelsie A. Murchy: data curation, writing – original draft, writing – review and editing. Hailey M. Shafer: data curation, visualization, writing – original draft, writing – review and editing. Audrey Looby: validation, writing – original draft, writing – review and editing. Francis Juanes: conceptualization, funding acquisition, investigation, project administration, supervision, writing – review and editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1.
Acknowledgments
We thank Dr. Dana Haggarty, Katie Innes, Katrina Nikolich, and Gauthier Cervello for their support of this research. This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Canadian Healthy Oceans Network (CHONe II). H.L.D. was supported by the NSERC Vanier Canada Graduate Scholarship. K.D.C. was supported by the Hakai Institute, the Liber Ero Fellowship, and the NSERC Postdoctoral Fellowship. K.A.M. was supported by Fisheries and Oceans Canada's Ocean and Freshwater Science Contribution Program and the University ofVictoria. H.M.S was supported by the University of Victoria. A.L. was supported by a graduate fellowship from the School of Forest, Fisheries, and Geomatic Sciences at the University of Florida. F.J. was supported by the Liber Ero Foundation and the NSERC Discovery Grant.
Funding: This work was supported by Natural Sciences and Engineering Research Council of Canada; Hakai Institute; Liber Ero Foundation; Canadian Healthy Oceans Network (CHONe); University of Florida; University of Victoria; Fisheries and Oceans Canada.
Contributor Information
Hailey L. Davies, Email: hldavies@uvic.ca.
Kieran D. Cox, Email: kcox@uvic.ca.
Data Availability Statement
The data and code that support the findings of this study are openly available in Borealis at https://doi.org/10.5683/SP3/EV6QQH.
References
References
- Aguilar de Soto, N. 2016. “Peer‐Reviewed Studies on the Effects of Anthropogenic Noise on Marine Invertebrates: From Scallop Larvae to Giant Squid.” In The Effects of Noise on Aquatic Life II, edited by Popper A. N. and Hawkins A., 17–26. NewYork, NY: Springer. 10.1007/978-1-4939-2981-8_54. [DOI] [PubMed] [Google Scholar]
- Anderson, S. C. , Mills Flemming J., Watson R., and Lotze H. K.. 2011. “Rapid Global Expansion of Invertebrate Fisheries: Trends, Drivers, and Ecosystem Effects.” PLoS One 6, no. 3: e0014735. 10.1371/journal.pone.0014735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André, M. , Kaifu K., Solé M., et al. 2016. “Contribution to the Understanding of Particle Motion Perception in Marine Invertebrates.” In The Effects of Noise on Aquatic Life II, edited by Popper A. N. and Hawkins A., 47–55. NewYork, NY: Springer. 10.1007/978-1-4939-2981-8. [DOI] [PubMed] [Google Scholar]
- Arechavala‐Lopez, P. , Cabrera‐Álvarez M. J., Maia C. M., and Saraiva J. L.. 2022. “Environmental Enrichment in Fish Aquaculture: A Review of Fundamental and Practical Aspects.” Reviews in Aquaculture 14, no. 2: 704–728. 10.1111/raq.12620. [DOI] [Google Scholar]
- Arizona‐Software . 2008. “GraphClick (Version 3.0).”
- Barcellos, H. H. A. , Koakoski G., Chaulet F., et al. 2018. “The Effects of Auditory Enrichment on Zebrafish Behavior and Physiology.” PeerJ 6: e5162. 10.7717/peerj.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwma, P. E. , and Herrnkind W. F.. 2009. “Sound Production in Caribbean Spiny Lobster Panulirus argus and Its Role in Escape During Predatory Attack by Octopus briareus .” New Zealand Journal of Marine and Freshwater Research 43, no. 1: 3–13. 10.1080/00288330909509977. [DOI] [Google Scholar]
- Branscomb, E. S. , and Rittschof D.. 1984. “An Investigation of Low Frequency Sound Waves as a Means of Inhibiting Barnacle Settlement.” Journal of Experimental Marine Biology and Ecology 79: 149–154. 10.1016/0022-0981(84)90215-6. [DOI] [Google Scholar]
- Carroll, A. G. , Przeslawski R., Duncan A., Gunning M., and Bruce B.. 2017. “A Critical Review of the Potential Impacts of Marine Seismic Surveys on Fish & Invertebrates.” Marine Pollution Bulletin 114: 9–24. 10.1016/j.marpolbul.2016.11.038. [DOI] [PubMed] [Google Scholar]
- Carter, E. E. , Tregenza T., and Stevens M.. 2020. “Ship Noise Inhibits Colour Change, Camouflage, and Anti‐Predator Behaviour in Shore Crabs.” Current Biology 30: 191–214. 10.1016/j.cub.2019.12.032. [DOI] [PubMed] [Google Scholar]
- Charifi, M. , Miserazzi A., Sow M., et al. 2018. “Noise Pollution Limits Metal Bioaccumulation and Growth Rate in a Filter Feeder, the Pacific Oyster Magallana gigas .” PLoS One 13, no. 4: e0194174. 10.1371/journal.pone.0194174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquereau, L. , Grall J., Chauvaud L., et al. 2016. “Sound Production and Associated Behaviours of Benthic Invertebrates From a Coastal Habitat in the North‐East Atlantic.” Marine Biology 163: 1–13. 10.1007/s00227-016-2902-2. [DOI] [Google Scholar]
- Cox, K. , Brennan L. P., Gerwing T. G., Dudas S. E., and Juanes F.. 2018. “Sound the Alarm: A Meta‐Analysis on the Effect of Aquatic Noise on Fish Behavior and Physiology.” Global Change Biology 24, no. 7: 3105–3116. 10.1111/gcb.14106. [DOI] [PubMed] [Google Scholar]
- Davies, H. L. , Cox K. D., Murchy K. A., Shafer H. M., Looby A., and Juanes F.. 2024. “ Impacts of Sounds on Aquatic Invertebrates Dataset [Dataset].” Borealis. 10.5683/SP3/EV6QQH. [DOI] [Google Scholar]
- Day, R. D. , McCauley R. D., Fitzgibbon Q. P., Hartmann K., and Semmens J. M.. 2019. “Seismic Air Guns Damage Rock Lobster Mechanosensory Organs and Impair Righting Reflex.” Proceedings of the Royal Society B: Biological Sciences 286, no. 1907: 1–10. 10.1098/rspb.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re, A. C. 2015. “A Practical Tutorial on Conducting Meta‐Analysis in R.” Quantitative Methods for Psychology 11, no. 1: 37–50. 10.20982/tqmp.11.1.p037. [DOI] [Google Scholar]
- Del Re, A. C. , and Hoyt W. T.. 2014. “MAd: Meta‐Analysis With Mean Differences (R Package Version 0.8‐2).”
- Di Franco, E. , Pierson P., Di Iorio L., et al. 2020. “Effects of Marine Noise Pollution on Mediterranean Fishes and Invertebrates: A Review.” Marine Pollution Bulletin 159: 1–13. 10.1016/j.marpolbul.2020.111450. [DOI] [PubMed] [Google Scholar]
- Dinh, J. P. , and Radford C.. 2021. “Acoustic Particle Motion Detection in the Snapping Shrimp ( Alpheus richardsoni ).” Journal of Comparative Physiology A 207, no. 5: 641–655. 10.1007/s00359-021-01503-4. [DOI] [PubMed] [Google Scholar]
- Duarte, C. M. , Chapuis L., Collin S. P., et al. 2021. “The Soundscape of the Anthropocene Ocean.” Science 371, no. 6529: 1–10. 10.1126/science.aba4658. [DOI] [PubMed] [Google Scholar]
- Filiciotto, F. , Sal Moyano M. P., de Vincenzi G., et al. 2018. “Are Semi‐Terrestrial Crabs Threatened by Human Noise? Assessment of Behavioural and Biochemical Responses of Neohelice granulata (Brachyura, Varunidae) in Tank.” Marine Pollution Bulletin 137: 24–34. 10.1016/j.marpolbul.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Fisher, D. N. , Kilgour R. J., Siracusa E. R., et al. 2021. “Anticipated Effects of Abiotic Environmental Change on Intraspecific Social Interactions.” Biological Reviews 96, no. 6: 2661–2693. 10.1111/brv.12772. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon, Q. P. , Day R. D., McCauley R. D., Simon C. J., and Semmens J. M.. 2017. “The Impact of Seismic Air Gun Exposure on the Haemolymph Physiology and Nutritional Condition of Spiny Lobster, Jasus edwardsii .” Marine Pollution Bulletin 125: 146–156. 10.1016/j.marpolbul.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Frisk, G. V. 2012. “Noiseonomics: The Relationship Between Ambient Noise Levels in the Sea and Global Economic Trends.” Scientific Reports 2: 1–4. 10.1038/srep00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, T. A. C. , Harding H. R., Wong K. E., et al. 2018. “Habitat Degradation Negatively Affects Auditory Settlement Behavior of Coral Reef Fishes.” Proceedings of the National Academy of Sciences of the United States of America 115, no. 20: 5193–5198. 10.1073/pnas.1719291115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, T. A. C. , Radford A. N., Davidson I. K., et al. 2019. “Acoustic Enrichment Can Enhance Fish Community Development on Degraded Coral Reef Habitat.” Nature Communications 10: 1–7. 10.1038/s41467-019-13186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, R. , Hirabayashi I., and Palmer A. R.. 2019. “Remarkably Loud Snaps During Mouth‐Fighting by a Sponge‐Dwelling Worm.” Current Biology 29, no. 13: R617–R618. 10.1016/j.cub.2019.05.047. [DOI] [PubMed] [Google Scholar]
- Guerra, A. , Martinell X., González A. F., Vecchione M., and Martinell J.. 2007. “A New Noise Detected in the Ocean.” Journal of the Marine Biological Association of the United Kingdom 87, no. 5: 1255–1256. 10.1017/S0025315407058225. [DOI] [Google Scholar]
- Hawkins, A. D. , and Popper A. N.. 2017. “A Sound Approach to Assessing the Impact of Underwater Noise on Marine Fishes and Invertebrates.” ICES Journal of Marine Science 74, no. 3: 635–651. 10.1093/icesjms/fsw205. [DOI] [Google Scholar]
- Hubert, J. , Booms E., Witbaard R., and Slabbekoorn H.. 2022. “Responsiveness and Habituation to Repeated Sound Exposures and Pulse Trains in Blue Mussels.” Journal of Experimental Marine Biology and Ecology 547: 151668. 10.1016/j.jembe.2021.151668. [DOI] [Google Scholar]
- Hubert, J. , Campbell J., van der Beek J. G., et al. 2018. “Effects of Broadband Sound Exposure on the Interaction Between Foraging Crab and Shrimp – A Field Study.” Environmental Pollution 243: 1923–1929. 10.1016/j.envpol.2018.09.076. [DOI] [PubMed] [Google Scholar]
- Hughes, A. , Mann D. A., and Kimbro D. L.. 2014. “Predatory Fish Sounds Can Alter Crab Foraging Behaviour and Influence Bivalve Abundance.” Proceedings of the Royal Society B: Biological Sciences 281, no. 1788: 20140715. 10.1098/rspb.2014.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet, A. , Tremblay R., Olivier F., et al. 2016. “Validation of Trophic and Anthropic Underwater Noise as Settlement Trigger in Blue Mussels.” Scientific Reports 6: 33829. 10.1038/srep33829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumyan, A. O. 2008. “Sounds and Sound Production in Fishes.” Journal of Ichthyology 48, no. 11: 981–1030. 10.1134/S0032945208110039. [DOI] [Google Scholar]
- Kriengwatana, B. P. , Mott R., and ten Cate C.. 2022. “Music for Animal Welfare: A Critical Review & Conceptual Framework.” Applied Animal Behaviour Science 251: 105641. 10.1016/j.applanim.2022.105641. [DOI] [Google Scholar]
- Kunc, H. P. , McLaughlin K. E., and Schmidt R.. 2016. “Aquatic Noise Pollution: Implications for Individuals, Populations, and Ecosystems.” Proceedings of the Royal Society B: Biological Sciences 283, no. 1836: 20160839. 10.1098/rspb.2016.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecchini, D. , Bertucci F., Gache C., et al. 2018. “Boat Noise Prevents Soundscape‐Based Habitat Selection by Coral Planulae.” Scientific Reports 8: 9283. 10.1038/s41598-018-27674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis, A. , Bohnenstiehl D. W. R., and Eggleston D. B.. 2015. “Soundscape Manipulation Enhances Larval Recruitment of a Reef‐Building Mollusk.” PeerJ 3: e999. 10.7717/peerj.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looby, A. , Cox K., Bravo S., et al. 2022. “A Quantitative Inventory of Global Soniferous Fish Diversity.” Reviews in Fish Biology and Fisheries 32: 1–15. 10.1007/s11160-022-09702-1. [DOI] [Google Scholar]
- McCauley, R. D. , Day R. D., Swadling K. M., Fitzgibbon Q. P., Watson R. A., and Semmens J. M.. 2017. “Widely Used Marine Seismic Survey Air Gun Operations Negatively Impact Zooplankton.” Nature Ecology & Evolution 1, no. 7: 195. 10.1038/s41559-017-0195. [DOI] [PubMed] [Google Scholar]
- McDonald, M. A. , Hildebrand J. A., and Wiggins S. M.. 2006. “Increases in Deep Ocean Ambient Noise in the Northeast Pacific West of San Nicolas Island, California.” Journal of the Acoustical Society of America 120, no. 2: 711–718. 10.1121/1.2216565. [DOI] [PubMed] [Google Scholar]
- Merchant, N. D. , Andersson M. H., Box T., et al. 2020. “Impulsive Noise Pollution in the Northeast Atlantic: Reported Activity During 2015–2017.” Marine Pollution Bulletin 152: 110951. 10.1016/j.marpolbul.2020.110951. [DOI] [PubMed] [Google Scholar]
- Morley, E. L. , Jones G., and Radford A. N.. 2013. “The Importance of Invertebrates When Considering the Impacts of Anthropogenic Noise.” Proceedings of the Royal Society B: Biological Sciences 281, no. 1776: 20132683. 10.1098/rspb.2013.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchy, K. A. , Davies H., Shafer H., Cox K., Nikolich K., and Juanes F.. 2020. “Impacts of Noise on the Behavior and Physiology of Marine Invertebrates: A Meta‐Analysis.” Proceedings of Meetings on Acoustics 37: 40002. 10.1121/2.0001217. [DOI] [Google Scholar]
- Nedelec, S. L. , Campbell J., Radford A. N., Simpson S. D., and Merchant N. D.. 2016. “Particle Motion: The Missing Link in Underwater Acoustic Ecology.” Methods in Ecology and Evolution 7, no. 7: 836–842. 10.1111/2041-210X.12544. [DOI] [Google Scholar]
- Pearson, W. H. , Skalski J. R., Sulkin S. D., and Malme C. I.. 1994. “Effects of Seismic Energy Releases on the Survival and Development of Zoeal Larvae of Dungeness Crab ( Cancer magister ).” Marine Environmental Research 38, no. 2: 93–113. 10.1016/0141-1136(94)90018-3. [DOI] [Google Scholar]
- Peng, C. , Zhao X., and Liu G.. 2015. “Noise in the Sea and Its Impacts on Marine Organisms.” International Journal of Environmental Research and Public Health 12, no. 10: 12304–12323. 10.3390/ijerph121012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. , Zhao X., Liu S., et al. 2016. “Effects of Anthropogenic Sound on Digging Behavior, Metabolism, Ca2+/Mg2+ ATPase Activity, and Metabolism‐Related Gene Expression of the Bivalve Sinonovacula constricta .” Scientific Reports 6: 1–12. 10.1038/srep24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, C. 2015. “Music Enhances Cognitive‐Related Behaviour in Snails ( Achatina fulica ).” Journal of Entomology and Zoology Studies 3, no. 4: 379–386. [Google Scholar]
- Pijanowski, B. C. , Villanueva‐Rivera L. J., Dumyahn S. L., et al. 2011. “Soundscape Ecology: The Science of Sound in the Landscape.” Bioscience 61, no. 3: 203–216. 10.1525/bio.2011.61.3.6. [DOI] [Google Scholar]
- Popper, A. N. , Salmon M., and Horch K. W.. 2001. “Acoustic Detection and Communication by Decapod Crustaceans.” Journal of Comparative Physiology A 187: 83–89. 10.1007/s003590100184. [DOI] [PubMed] [Google Scholar]
- Przeslawski, R. , Huang Z., Anderson J., et al. 2018. “Multiple Field‐Based Methods to Assess the Potential Impacts of Seismic Surveys on Scallops.” Marine Pollution Bulletin 129: 750–761. 10.1016/j.marpolbul.2017.10.066. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2023. “R: A Language and Environment for Statistical Computing.” R Foundation for Statistical Computing. https://www.R‐project.org/.
- Ren, Z. , Wang J., Wang C., Mu C., Ye Y., and Shi C.. 2021. “Music Stimulus Has a Positive Effect on Survival and Development of the Larvae in Swimming Crab Portunus trituberculatus .” Journal of Oceanology and Limnology 40, no. 3: 1277–1285. 10.1007/s00343-021-1060-7. [DOI] [Google Scholar]
- Roberts, L. , and Elliott M.. 2017. “Good or Bad Vibrations? Impacts of Anthropogenic Vibration on the Marine Epibenthos.” Science of the Total Environment 595: 255–268. 10.1016/j.scitotenv.2017.03.117. [DOI] [PubMed] [Google Scholar]
- Roberts, L. , and Laidre M. E.. 2019. “Finding a Home in the Noise: Cross‐Modal Impact of Anthropogenic Vibration on Animal Search Behaviour.” Biology Open 8: bio041988. 10.1242/bio.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi, A. 2022. “WebPlotDigitizer: Version 4.6.” https://automeris.io/WebPlotDigitizer.
- Rountree, R. A. , Juanes F., and Bolgan M.. 2020. “Temperate Freshwater Soundscapes: A Cacophony of Undescribed Biological Sounds Now Threatened by Anthropogenic Noise.” PLoS One 15, no. 3: e0221842. 10.1371/journal.pone.0221842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, C. J. , Fitzgibbon Q. P., Battison A., Carter C. G., and Battaglene S. C.. 2015. “Bioenergetics of Nutrient Reserves and Metabolism in Spiny Lobster Juveniles Sagmariasus verreauxi : Predicting Nutritional Condition From Hemolymph Biochemistry.” Physiological and Biochemical Zoology 88, no. 3: 266–283. 10.1086/681000. [DOI] [PubMed] [Google Scholar]
- Simpson, S. D. , Meekan M., Montgomery J., McCauley R., and Jeffs A.. 2005. “Homeward Sound.” Science 308, no. 5720: 221. 10.1126/science.1107406. [DOI] [PubMed] [Google Scholar]
- Simpson, S. D. , Radford A. N., Tickle E. J., Meekan M. G., and Jeffs A. G.. 2011. “Adaptive Avoidance of Reef Noise.” PLoS One 6, no. 2: e16625. 10.1371/journal.pone.0016625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn, H. , Bouton N., van Opzeeland I., Coers A., ten Cate C., and Popper A. N.. 2010. “A Noisy Spring: The Impact of Globally Rising Underwater Sound Levels on Fish.” Trends in Ecology & Evolution 25, no. 7: 419–427. 10.1016/j.tree.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Solé, M. , Fortuño J.‐M., van der Schaar M., and André M.. 2021. “An Acoustic Treatment to Mitigate the Effects of the Apple Snail on Agriculture and Natural Ecosystems.” Journal of Marine Science and Engineering 9: 969. 10.3390/jmse9090969. [DOI] [Google Scholar]
- Solé, M. , Kaifu K., Mooney T. A., et al. 2023. “Marine Invertebrates and Noise.” Frontiers in Marine Science 10: 1129057. 10.3389/fmars.2023.1129057. [DOI] [Google Scholar]
- Solé, M. , Lenoir M., Fortuño J. M., Durfort M., van der Schaar M., and André M.. 2016. “Evidence of Cnidarians Sensitivity to Sound After Exposure to Low Frequency Underwater Sources.” Scientific Reports 6: 1–16. 10.1038/srep37979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Lenoir M., José‐Manuel F., Van Der Schaar M., and André M.. 2018. “A Critical Period of Susceptibility to Sound in the Sensory Cells of Cephalopod Hatchlings.” Biology Open 7, no. 11: 1–13. 10.1242/bio.033860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Sigray P., Lenoir M., van der Schaar M., Lalander E., and André M.. 2017. “Offshore Exposure Experiments on Cuttlefish Indicate Received Sound Pressure and Particle Motion Levels Associated With Acoustic Trauma.” Scientific Reports 7: 45899. 10.1038/srep45899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall, E. B. L. , Finneran J. J., Reichmuth C., et al. 2019. “Marine Mammal Noise Exposure Criteria: Updated Scientific Recommendations for Residual Hearing Effects.” Aquatic Mammals 45, no. 2: 125–232. 10.1578/AM.45.2.2019.125. [DOI] [Google Scholar]
- Spiga, I. 2016. “Acoustic Response to Playback of Pile‐Driving Sounds by Snapping Shrimp.” Advances in Experimental Medicine and Biology 875: 1081–1088. 10.1007/978-1-4939-2981-8_134. [DOI] [PubMed] [Google Scholar]
- Stanley, J. A. , Wilkens S. L., and Jeffs A. G.. 2014. “Fouling in Your Own Nest: Vessel Noise Increases Biofouling.” Biofouling 30, no. 7: 837–844. 10.1080/08927014.2014.938062. [DOI] [PubMed] [Google Scholar]
- Stocks, J. R. , Broad A., Radford C., Minchinton T. E., and Davis A. R.. 2012. “Response of Marine Invertebrate Larvae to Natural and Anthropogenic Sound: A Pilot Study.” Open Journal of Marine Science 6: 57–61. [Google Scholar]
- Tu, Z. , Tang L., Zhang X., Jia J., and Shen H.. 2021. “Transcriptome Analysis of the Central Nervous System of Sea Slug (Onchidium reevesii) Exposed to Low‐Frequency Noise.” Frontiers in Marine Science 8: 807489. 10.3389/fmars.2021.807489. [DOI] [Google Scholar]
- Tyack, P. L. 2008. “Implications for Marine Mammals of Large‐Scale Changes in the Marine Acoustic Environment.” Journal of Mammalogy 89, no. 3: 549–558. [Google Scholar]
- Vazzana, M. , Mauro M., Ceraulo M., et al. 2020. “Underwater High Frequency Noise: Biological Responses in Sea Urchin Arbacia lixula (Linnaeus, 1758).” Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 248: 110730. 10.1016/j.cbpa.2020.110730. [DOI] [PubMed] [Google Scholar]
- Vermeij, M. J. A. , Marhaver K. L., Huijbers C. M., Nagelkerken I., and Simpson S. D.. 2010. “Coral Larvae Move Toward Reef Sounds.” PLoS One 5: e10660. 10.1371/journal.pone.0010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen, H. M. , Sena E. S., Egan K. J., et al. 2014. “Meta‐Analysis of Data From Animal Studies: A Practical Guide.” Journal of Neuroscience Methods 221: 92–102. 10.1016/j.jneumeth.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. 2010. “Conducting Meta‐Analysis in R With the Metafor Package.” Journal of Statistical Software 36, no. 3: 1–48. 10.1103/PhysRevB.91.121108. [DOI] [Google Scholar]
- Viechtbauer, W. , and Cheung M. W.‐L.. 2010. “Outlier and Influence Diagnostics for Meta‐Analysis.” Research Synthesis Methods 1: 112–125. 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- Wale, M. A. , Briers R. A., and Diele K.. 2021. “Marine Invertebrate Anthropogenic Noise Research‐Trends in Methods and Future Directions.” Marine Pollution Bulletin 173: 112958. 10.1016/j.marpolbul.2021.112958. [DOI] [PubMed] [Google Scholar]
- Wale, M. A. , Briers R. A., Hartl M. G. J., Bryson D., and Diele K.. 2019. “From DNA to Ecological Performance: Effects of Anthropogenic Noise on a Reef‐Building Mussel.” Science of the Total Environment 689: 126–132. 10.1016/j.scitotenv.2019.06.380. [DOI] [PubMed] [Google Scholar]
- Weilgart, L. S. . 2018. “The Impact of Ocean Noise Pollution on Marine Biodiversity.” OceanCare 39. https://www.oceancare.org/wp‐content/uploads/2022/05/Underwater‐Noise‐Pollution_Impact‐on‐fish‐and‐invertebrates_Report_OceanCare_EN_36p_2018.pdf. [Google Scholar]
- Wickham, H. 2016. “ggplot2: Elegant Graphics for Data Analysis.”
- Wickham, H. , François R., Henry L., and Müller K.. 2022. “Dplyr: A Grammar of Data Manipulation (R Package Version 1.0.10).”
- Wilke, C. O. 2020. “Streamline Plot Theme and Plot Annotations for “ggplot2” (R Package Version 1.1.1).”
- World Health Organization . 2011. Burden of Disease From Environmental Noise: Quantification of Healthy Life Years Lost in Europe. Geneva, Switzerland: Regional Office for Europe. [Google Scholar]
- Yağcılar, Ç. , and Yardımcı M.. 2021. “Effects of 432 Hz and 440 Hz Sound Frequencies on the Heart Rate, Egg Number, and Survival Parameters in Water Flea ( Daphnia magna ).” Journal of Ecological Engineering 22: 119–125. 10.12911/22998993/134038. [DOI] [Google Scholar]
- Zhao, X. , Sun S., Shi W., et al. 2021. “Mussel Byssal Attachment Weakened by Anthropogenic Noise.” Frontiers in Marine Science 8: 821019. 10.3389/fmars.2021.821019. [DOI] [Google Scholar]
Data Sources
- Anderson, E. R. , Butler J., and Butler M. J.. 2021. “Response of Fish and Invertebrate Larvae to Backreef Sounds at Varying Distances: Implications for Habitat Restoration.” Frontiers in Marine Science 8, no. 697: 1–13. 10.3389/fmars.2021.663887.35685121 [DOI] [Google Scholar]
- Andriguetto‐Filho, M. , Ostrensky A., Pie M. R., Silva A., and Boeger W. A.. 2005. “Evaluating the Impact of Seismic Prospecting on Artisanal Shrimp Fisheries.” Continental Shelf Research 25, no. 12: 1720–1727. 10.1016/j.csr.2005.05.003. [DOI] [Google Scholar]
- Carter, E. E. , Tregenza T., and Stevens M.. 2020b. “Ship Noise Inhibits Colour Change, Camouflage, and Anti‐Predator Behaviour in Shore Crabs.” Current Biology 30: 191–214. 10.1016/j.cub.2020.11.014. [DOI] [PubMed] [Google Scholar]
- Celi, M. , Filiciotto F., Parrinello D., et al. 2013. “Physiological and Agonistic Behavioural Response of Procambarus clarkii to an Acoustic Stimulus.” Journal of Experimental Biology 216, no. 4: 709–718. 10.1242/jeb.078865. [DOI] [PubMed] [Google Scholar]
- Celi, M. , Filiciotto F., Vazzana M., et al. 2015. “Shipping Noise Affecting Immune Responses of European Spiny Lobster ( Palinurus elephas ).” Canadian Journal of Zoology 93, no. 2: 113–121. 10.1139/cjz-2014-0219. [DOI] [Google Scholar]
- Charifi, M. , Miserazzi A., Sow M., et al. 2018b. “Noise Pollution Limits Metal Bioaccumulation and Growth Rate in a Filter Feeder, the Pacific Oyster Magallana gigas .” PLoS One 13, no. 2: e0194174. 10.1371/journal.pone.0194174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, R. D. , McCauley R. D., Fitzgibbon Q. P., Hartmann K., and Semmens J. M.. 2017. “Exposure to Seismic Air Gun Signals Causes Physiological Harm and Alters Behavior in the Scallop Pecten fumatus .” Proceedings of the National Academy of Sciences 114, no. 40: E8537–E8546. 10.1073/pnas.1700564114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, R. D. , McCauley R. D., Fitzgibbon Q. P., Hartmann K., and Semmens J. M.. 2019b. “Seismic Air Guns Damage Rock Lobster Mechanosensory Organs and Impair Righting Reflex.” Proceedings of the Royal Society B 286, no. 1907: 20191424. 10.1098/rspb.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, R. D. , McCauley R. D., Fitzgibbon Q. P., and Semmens J. M.. 2016. “Seismic Air Gun Exposure During Early‐Stage Embryonic Development Does Not Negatively Affect Spiny Lobster Jasus edwardsii Larvae (Decapoda: Palinuridae).” Scientific Reports 6: 22723. 10.1038/srep22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiciotto, F. , Sal Moyano M. P., de Vincenzi G., et al. 2018b. “Are Semi‐Terrestrial Crabs Threatened by Human Noise? Assessment of Behavioural and Biochemical Responses of Neohelice Granulata (Brachyura, Varunidae) in Tank.” Marine Pollution Bulletin 137: 24–34. 10.1016/j.marpolbul.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Filiciotto, F. , Vazzana M., Celi M., et al. 2016. “Underwater Noise From Boats: Measurement of Its Influence on the Behaviour and Biochemistry of the Common Prawn Palaemon serratus , Pennant 1777).” Journal of Experimental Marine Biology and Ecology 478: 24–33. 10.1016/j.jembe.2016.01.014. [DOI] [Google Scholar]
- Filiciotto, F. , Vazzana M., Celi M., et al. 2014. “Behavioural and Biochemical Stress Responses of Palinurus elephas After Exposure to Boat Noise Pollution in Tank.” Marine Pollution Bulletin 84: 104–114. 10.1016/j.marpolbul.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon, Q. P. , Day R. D., McCauley R. D., Simon C. J., and Semmens J. M.. 2017b. “The Impact of Seismic Air Gun Exposure on the Haemolymph Physiology and Nutritional Condition of Spiny Lobster, Jasus edwardsii .” Marine Pollution Bulletin 125: 146–156. 10.1016/j.marpolbul.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Hubert, J. , Booms E., Witbaard R., and Slabbekoorn H.. 2022b. “Responsiveness and Habituation to Repeated Sound Exposures and Pulse Trains in Blue Mussels.” Journal of Experimental Marine Biology and Ecology 547: 151668. 10.1016/J.JEMBE.2021.151668. [DOI] [Google Scholar]
- Hubert, J. , Campbell J., van der Beek J. G., et al. 2018b. “Effects of Broadband Sound Exposure on the Interaction Between Foraging Crab and Shrimp – A Field Study.” Environmental Pollution 243: 1923–1929. 10.1016/j.envpol.2018.09.076. [DOI] [PubMed] [Google Scholar]
- Hudson, D. M. , Krumholz J. S., Pochtar D. L., et al. 2022. “Potential Impacts From Simulated Vessel Noise and Sonar on Commercially Important Invertebrates.” PeerJ 10: e12841. 10.7717/peerj.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. , Mann D. A., and Kimbro D. L.. 2014b. “Predatory Fish Sounds Can Alter Crab Foraging Behaviour and Influence Bivalve Abundance.” Proceedings of the Royal Society B 281, no. 1788: 20140715. 10.1098/rspb.2014.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet, A. , Tremblay R., Olivier F., et al. 2016. “Validation of Trophic and Anthropic Underwater Noise as Settlement Trigger in Blue Mussels.” Scientific Reports 6: 33829. 10.1038/srep33829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunc, H. P. , Lyons G. N., Sigwart J. D., McLaughlin K. E., and Houghton J. D. R.. 2014. “Anthropogenic Noise Affects Behavior Across Sensory Modalities.” American Naturalist 184, no. 4: E93–E100. 10.1086/677545. [DOI] [PubMed] [Google Scholar]
- Lagardère, J. P. 1982. “Effects of Noise on Growth and Reproduction of Crangon crangon in Rearing Tanks.” Marine Biology 71: 177–185. [Google Scholar]
- Lecchini, D. , Bertucci F., Gache C., et al. 2018b. “Boat Noise Prevents Soundscape‐Based Habitat Selection by Coral Planulae.” Scientific Reports 8: 9283. 10.1038/s41598-018-27674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis, A. , Bohnenstiehl D. W. R., and Eggleston D. B.. 2015b. “Soundscape Manipulation Enhances Larval Recruitment of a Reef‐Building Mollusk.” PeerJ 3: e999. 10.7717/peerj.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis, A. , Eggleston D. B., and Bohnenstiehl D. R.. 2013. “Oyster Larvae Settle in Response to Habitat‐Associated Underwater Sounds.” PLoS One 8, no. 10: e79337. 10.1371/journal.pone.0079337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley, R. D. , Day R. D., Swadling K. M., Fitzgibbon Q. P., Watson R. A., and Semmens J. M.. 2017b. “Widely Used Marine Seismic Survey Air Gun Operations Negatively Impact Zooplankton.” Nature Ecology & Evolution 1, no. 7: 1095. 10.1038/s41559-017-0195. [DOI] [PubMed] [Google Scholar]
- Nedelec, S. L. , Radford A. N., Simpson S. D., Nedelec B., Lecchini D., and Mills S. C.. 2014. “Anthropogenic Noise Playback Impairs Embryonic Development and Increases Mortality in a Marine Invertebrate.” Scientific Reports 4: 5891. 10.1038/srep05891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, W. H. , Skalski J. R., Sulkin S. D., and Malme C. I.. 1994b. “Effects of Seismic Energy Releases on the Survival and Development of Zoeal Larvae of Dungeness Crab ( Cancer magister ).” Marine Environmental Research 38: 93–113. [Google Scholar]
- Peng, C. , Zhao X., Liu S., et al. 2016b. “Effects of Anthropogenic Sound on Digging Behavior, Metabolism, Ca2+/Mg2+ ATPase Activity, and Metabolism‐Related Gene Expression of the Bivalve Sinonovacula constricta .” Scientific Reports 6: 24266. 10.1038/srep24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przeslawski, R. , Huang Z., Anderson J., et al. 2018b. “Multiple Field‐Based Methods to Assess the Potential Impacts of Seismic Surveys on Scallops.” Marine Pollution Bulletin 129: 750–761. 10.1016/j.marpolbul.2017.10.066. [DOI] [PubMed] [Google Scholar]
- Regnault, M. , and Lagardère J.‐P.. 1983. “Effects of Ambient Noise on the Metabolic Level of Crangon crangon (Decapoda, Natantia).” Marine Ecology Progress Series 11: 71–78. 10.3354/meps011071. [DOI] [Google Scholar]
- Ren, Z. , Wang J., Wang C., Mu C., Ye Y., and Shi C.. 2021b. “Music Stimulus Has a Positive Effect on Survival and Development of the Larvae in Swimming Crab Portunus trituberculatus .” Journal of Oceanology and Limnology 40, no. 3: 1277–1285. 10.1007/s00343-021-1060-7. [DOI] [Google Scholar]
- Roberts, L. , and Laidre M. E.. 2019b. “Finding a Home in the Noise: Cross‐Modal Impact of Anthropogenic Vibration on Animal Search Behaviour.” Biology Open 8: bio041988. 10.1242/bio.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, W. , Han Y., Guan X., et al. 2019. “Anthropogenic Noise Aggravates the Toxicity of Cadmium on Some Physiological Characteristics of the Blood Clam Tegillarca granosa .” Frontiers in Physiology 10: 1–10. 10.3389/fphys.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Fortuño J.‐M., van der Schaar M., and André M.. 2021b. “An Acoustic Treatment to Mitigate the Effects of the Apple Snail on Agriculture and Natural Ecosystems.” Journal of Marine Science and Engineering 9: 969. 10.3390/jmse9090969. [DOI] [Google Scholar]
- Solé, M. , Lenoir M., Fortuño J. M., Durfort M., van der Schaar M., and André M.. 2016b. “Evidence of Cnidarians Sensitivity to Sound After Exposure to Low Frequency Underwater Sources.” Scientific Reports 6: 37979. 10.1038/srep37979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Lenoir M., José‐Manuel F., van der Schaar M., and André M.. 2018b. “A Critical Period of Susceptibility to Sound in the Sensory Cells of Cephalopod Hatchlings.” Biology Open 7: bio033860. 10.1242/bio.033860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Monge M., André M., and Quero C.. 2019. “A Proteomic Analysis of the Statocyst Endolymph in Common Cuttlefish ( Sepia officinalis ): An Assessment of Acoustic Trauma After Exposure to Sound.” Scientific Reports 9: 9340. 10.1038/s41598-019-45646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, M. , Sigray P., Lenoir M., van der Schaar M., Lalander E., and André M.. 2017b. “Offshore Exposure Experiments on Cuttlefish Indicate Received Sound Pressure and Particle Motion Levels Associated With Acoustic Trauma.” Scientific Reports 7: 45899. 10.1038/srep45899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, J. A. , Wilkens S. L., and Jeffs A. G.. 2014b. “Fouling in Your Own Nest: Vessel Noise Increases Biofouling.” Biofouling 30, no. 7: 837–844. 10.1080/08927014.2014.938062. [DOI] [PubMed] [Google Scholar]
- Stocks, J. R. , Broad A., Radford C., Minchinton T. E., and Davis A. R.. 2012b. “Response of Marine Invertebrate Larvae to Natural and Anthropogenic Sound: A Pilot Study.” Open Journal of Marine Biology 6: 57–61. [Google Scholar]
- Tidau, S. , and Briffa M.. 2019. “Distracted Decision Makers: Ship Noise and Predation Risk Change Shell Choice in Hermit Crabs.” Behavioral Ecology 30, no. 4: 1157–1167. 10.1093/beheco/arz045. [DOI] [Google Scholar]
- Tu, Z. , Tang L., Zhang X., Jia J., and Shen H.. 2021b. “Transcriptome Analysis of the Central Nervous System of Sea Slug (Onchidium reevesii) Exposed to Low‐Frequency Noise.” Frontiers in Marine Science 8: 807489. 10.3389/fmars.2021.807489. [DOI] [Google Scholar]
- Vazzana, M. , Mauro M., Ceraulo M., et al. 2020b. “Underwater High Frequency Noise: Biological Responses in Sea Urchin Arbacia lixula (Linnaeus, 1758).” Comparative Biochemistry and Physiology, Part A 242: 110650. 10.1016/j.cbpa.2020.110650. [DOI] [PubMed] [Google Scholar]
- Wale, M. A. , Briers R. A., Hartl M. G. J., Bryson D., and Diele K.. 2019b. “From DNA to Ecological Performance: Effects of Anthropogenic Noise on a Reef‐Building Mussel.” Science of the Total Environment 689: 126–132. 10.1016/j.scitotenv.2019.06.380. [DOI] [PubMed] [Google Scholar]
- Wale, M. A. , Simpson S. D., and Radford A. N.. 2013a. “Size‐Dependent Physiological Responses of Shore Crabs to Single and Repeated Playback of Ship Noise.” Biology Letters 9: 20121194. 10.1098/rsbl.2012.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wale, M. A. , Simpson S. D., and Radford A. N.. 2013b. “Noise Negatively Affects Foraging and Antipredator Behaviour in Shore Crabs.” Animal Behaviour 86: 111–118. 10.1016/j.anbehav.2013.05.001. [DOI] [Google Scholar]
- Zhao, X. , Sun S., Shi W., et al. 2021b. “Mussel Byssal Attachment Weakened by Anthropogenic Noise.” Frontiers in Marine Science 8: 821019. 10.3389/fmars.2021.821019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data and code that support the findings of this study are openly available in Borealis at https://doi.org/10.5683/SP3/EV6QQH.


