Abstract
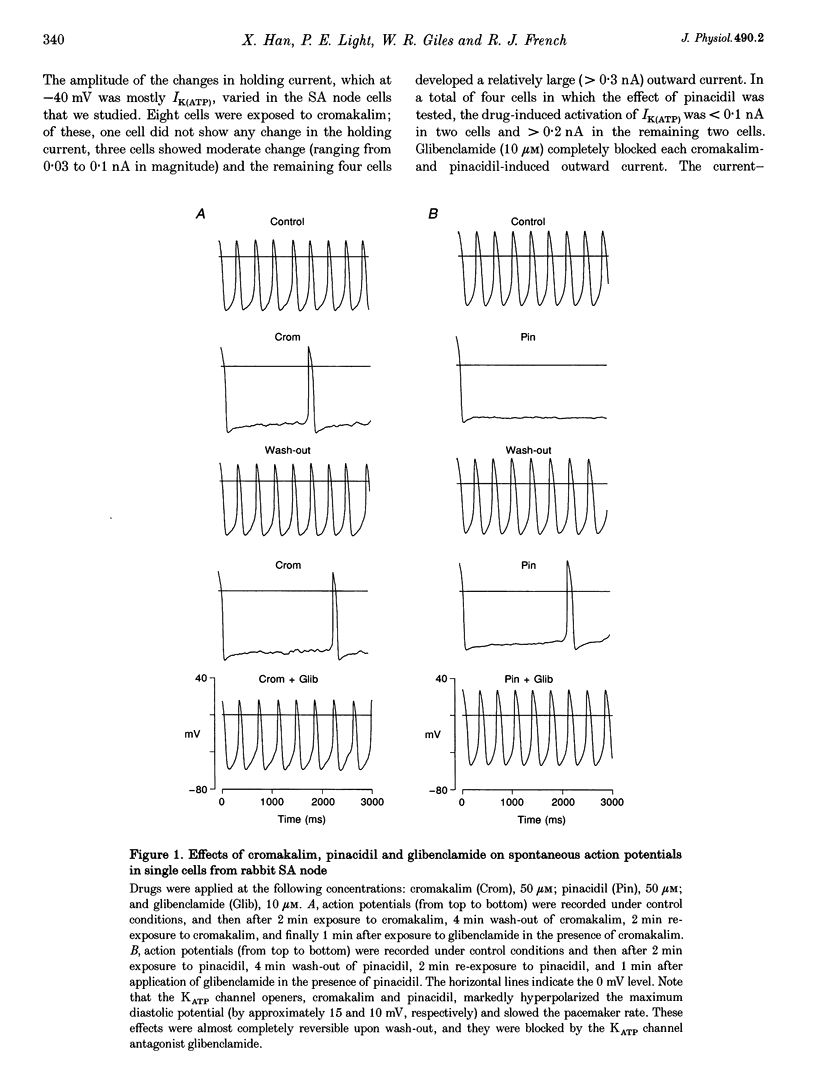
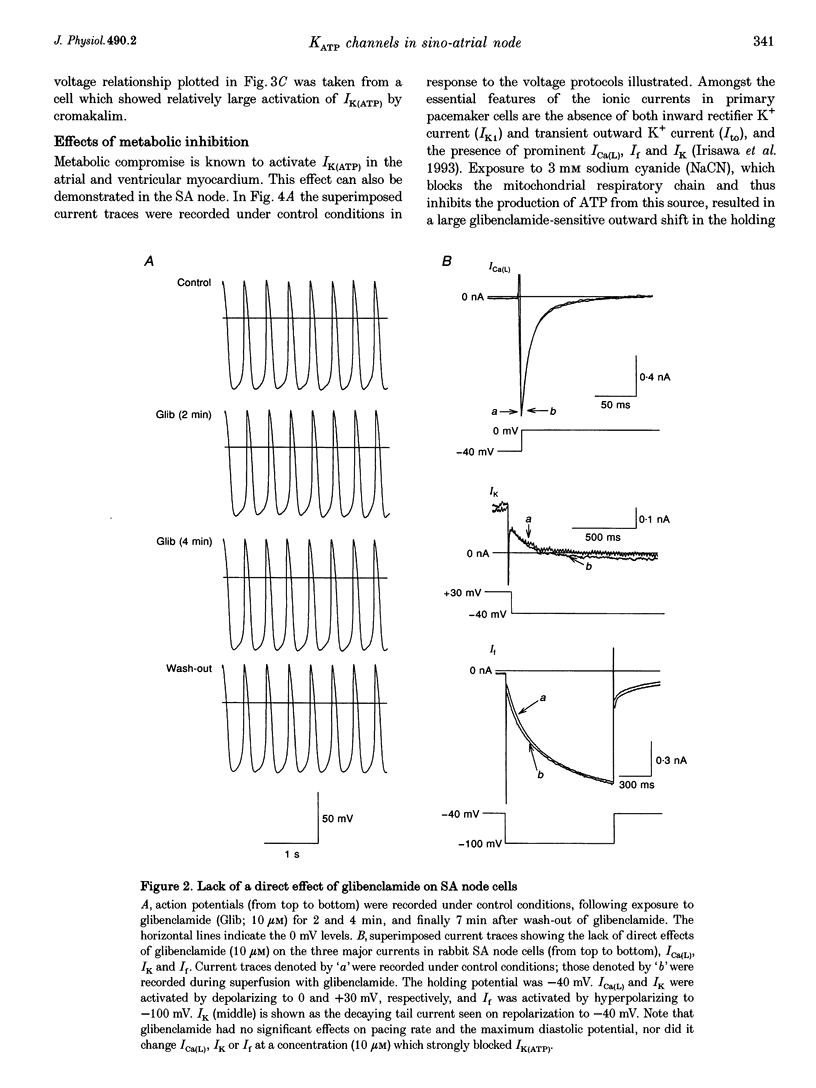
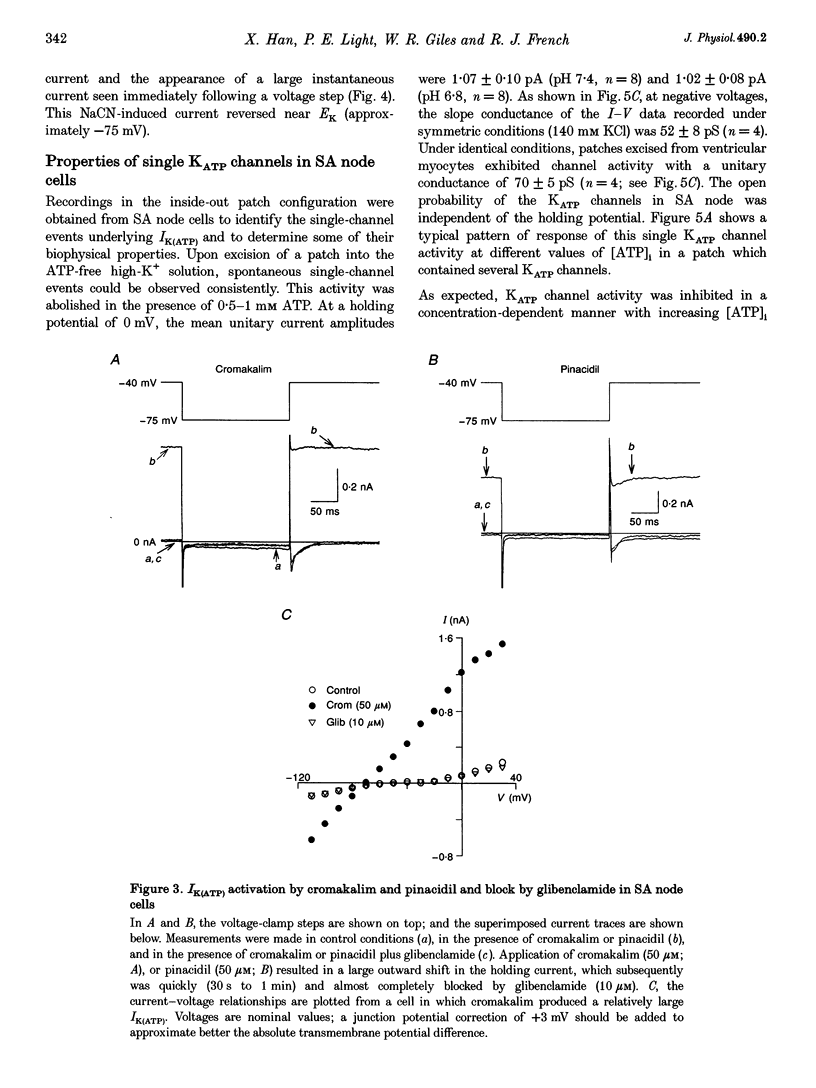
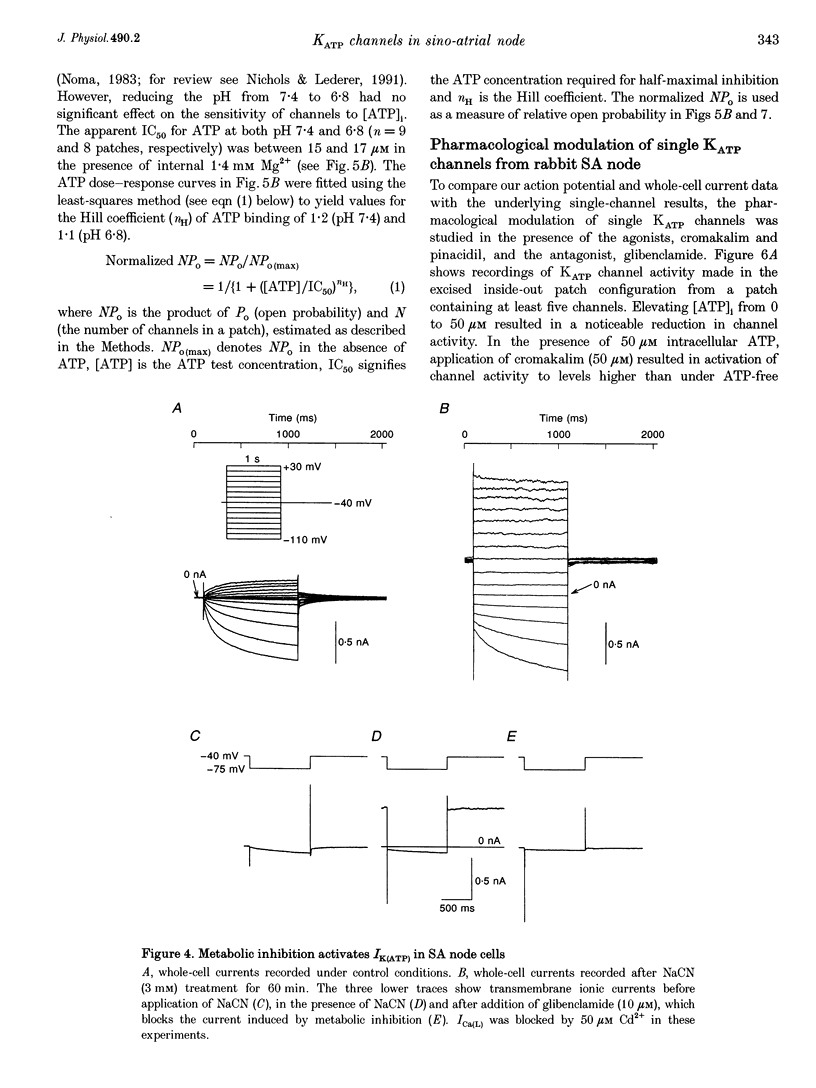
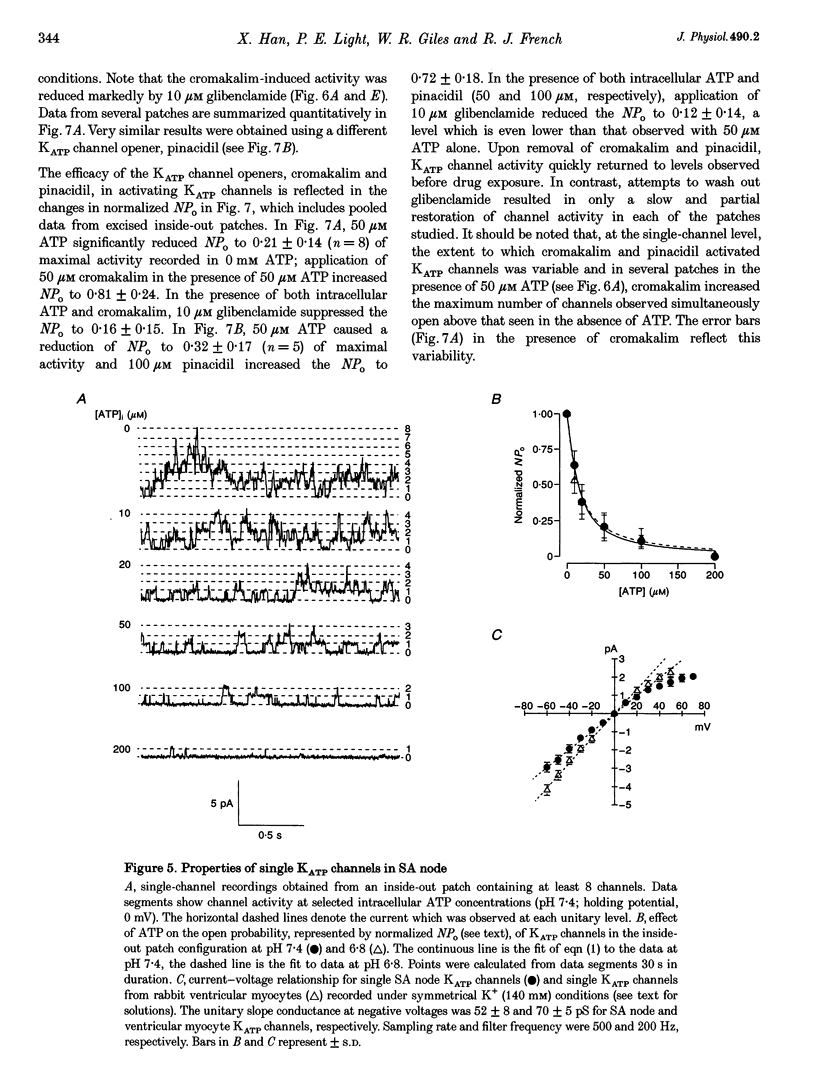
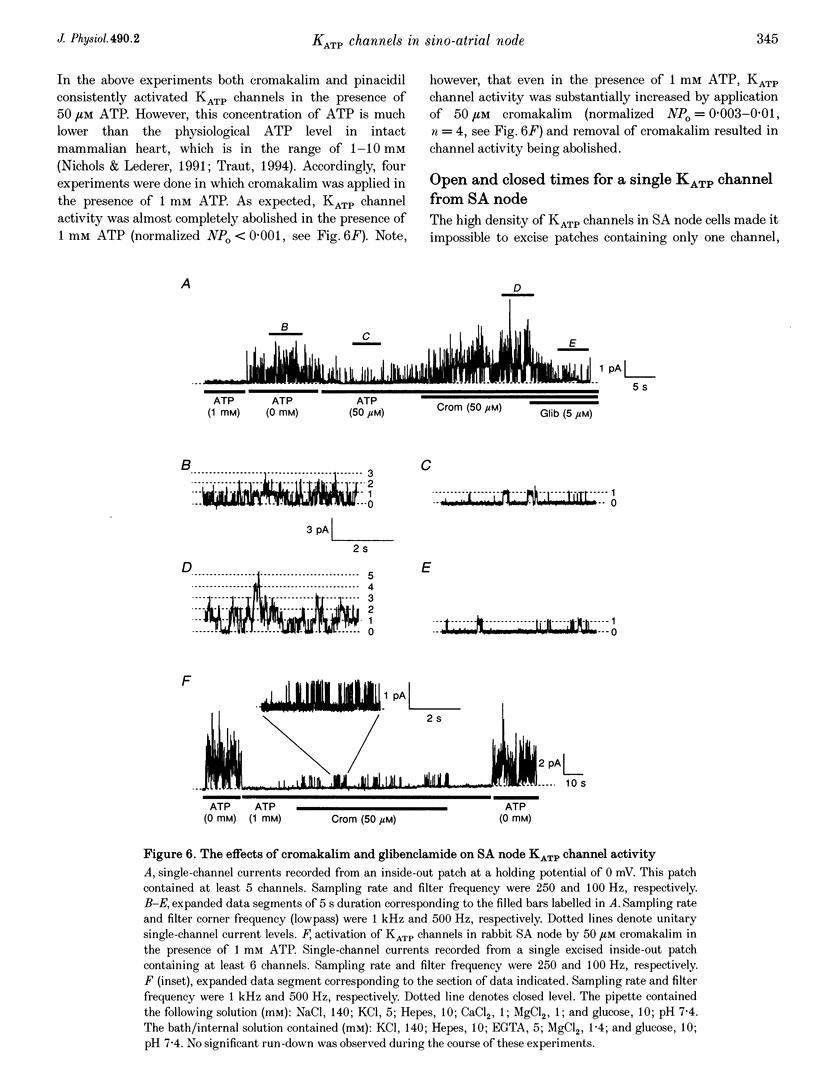
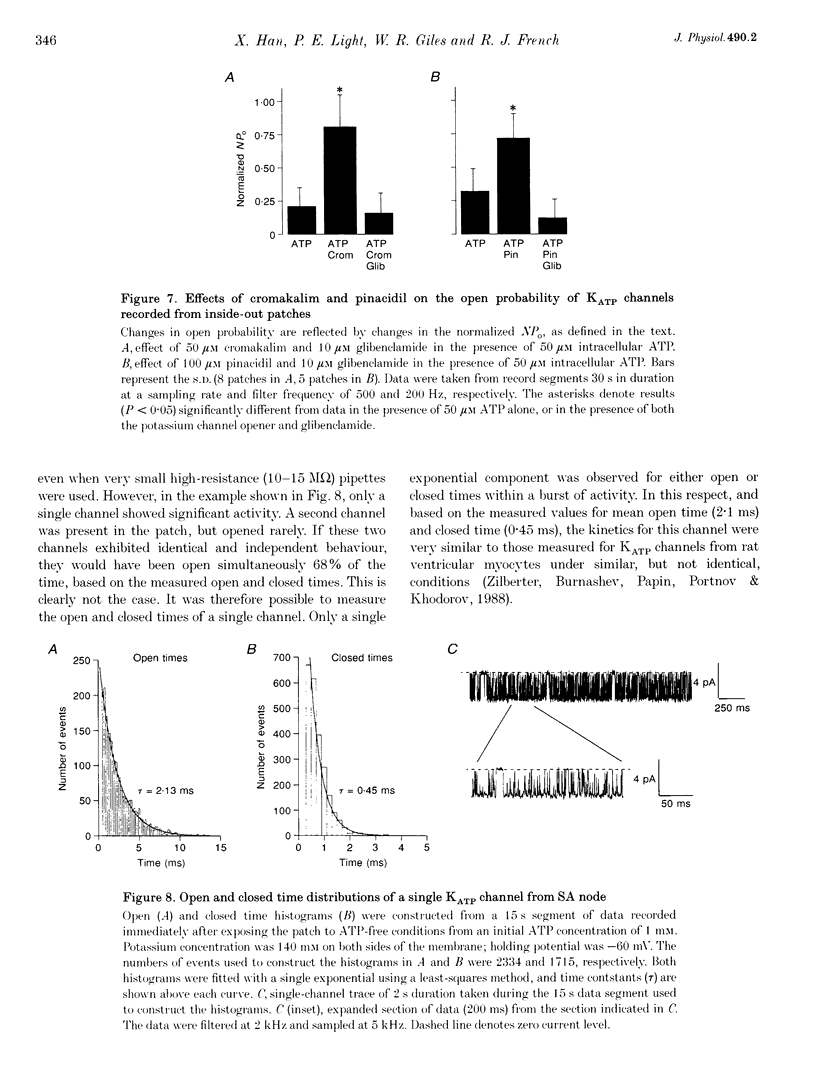
1. Single myocytes were isolated from rabbit sino-atrial (SA) node by enzymatic dissociation. Spontaneous pacemaker activity, whole-cell and single-channel currents were recorded under conditions known to modulate ATP-sensitive K+ (KATP) channels. 2. The KATP channel openers, cromakalim and pinacidil, slowed or abolished the pacemaker activity, and caused hyperpolarization of the maximum diastolic potential (MDP). Glibenclamide, a KATP channel blocker, reversed these effects. Cromakalim- and pinacidil-activated currents reversed near the potassium equilibrium potential, EK. Glibenclamide had no effect on the L-type calcium current, ICa(L), the hyperpolarization-activated inward current, If, or the delayed rectifier potassium current, IK. 3. Sodium cyanide, which inhibits mitochondrial ATP production, induced a macroscopic current that reversed near EK and was blocked by glibenclamide. 4. In excised, inside-out patches from SA node cells, single KATP channels showed a slope conductance of 52 +/- 8 pS (mean +/- S.D.) when measurements were made at negative voltages in symmetric, 140 mM K+. Channels from ventricular myocytes showed a somewhat larger slope conductance (70 +/- 5 pS). 5. Raising the intracellular ATP concentration caused a concentration-dependent reduction in the open probability of the KATP channels (IC50, 16 microM; Hill coefficient, approximately 1; at both pH 7.4 and 6.8). 6. In excised inside-out patches, cromakalim or pinacidil induced significant increases in KATP channel activity in the presence of 50 microM or 1 mM intracellular ATP. This channel activity was blocked by glibenclamide. 7. Our results suggest that sino-atrial node cells express a distinct isoform of KATP channel which may play an important role in pharmacological and pathophysiological modulation of pacemaker activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990 Oct;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D. The pharmacology of ATP-sensitive K+ channels in the heart. Pflugers Arch. 1989;414 (Suppl 1):S93–S98. doi: 10.1007/BF00582255. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988 Jul;412(1-2):37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier P., Bertrand J. P., Guiraudou P. Effects of SR 44866, a potassium channel opener, on action potentials of rabbit, guinea pig, and human heart fibers. J Cardiovasc Pharmacol. 1991 May;17(5):692–700. doi: 10.1097/00005344-199105000-00002. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotanda K., Satoh K., Taira N. Is the cardiovascular profile of BRL 34915 characteristic of potassium channel activators? J Cardiovasc Pharmacol. 1988 Aug;12(2):239–246. doi: 10.1097/00005344-198808000-00015. [DOI] [PubMed] [Google Scholar]
- Grover G. J., McCullough J. R., Henry D. E., Conder M. L., Sleph P. G. Anti-ischemic effects of the potassium channel activators pinacidil and cromakalim and the reversal of these effects with the potassium channel blocker glyburide. J Pharmacol Exp Ther. 1989 Oct;251(1):98–104. [PubMed] [Google Scholar]
- Hamada E., Takikawa R., Ito H., Iguchi M., Terano A., Sugimoto T., Kurachi Y. Glibenclamide specifically blocks ATP-sensitive K+ channel current in atrial myocytes of guinea pig heart. Jpn J Pharmacol. 1990 Dec;54(4):473–477. doi: 10.1254/jjp.54.473. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Han J., So I., Kim E. Y., Earm Y. E. ATP-sensitive potassium channels are modulated by intracellular lactate in rabbit ventricular myocytes. Pflugers Arch. 1993 Dec;425(5-6):546–548. doi: 10.1007/BF00374883. [DOI] [PubMed] [Google Scholar]
- Han X., Habuchi Y., Giles W. R. Relaxin increases heart rate by modulating calcium current in cardiac pacemaker cells. Circ Res. 1994 Mar;74(3):537–541. doi: 10.1161/01.res.74.3.537. [DOI] [PubMed] [Google Scholar]
- Han X., Shimoni Y., Giles W. R. An obligatory role for nitric oxide in autonomic control of mammalian heart rate. J Physiol. 1994 Apr 15;476(2):309–314. doi: 10.1113/jphysiol.1994.sp020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Single-channel properties and regulation of pinacidil/glibenclamide-sensitive K+ channels in follicular cells from Xenopus oocyte. Pflugers Arch. 1993 Jul;424(2):113–121. doi: 10.1007/BF00374601. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano T., Kakei M., Nakashima H., Yoshinaga M., Matsuoka T., Tanaka H. ATP-regulated K+ channels are modulated by intracellular H+ in guinea-pig ventricular cells. J Physiol. 1993 Apr;463:747–766. doi: 10.1113/jphysiol.1993.sp019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G., Gordon E. A., Wickman K., Velimirović B., Krapivinsky L., Clapham D. E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995 Mar 9;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G., Smith G. L. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989 Jun;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Li G. R., Feng J., Shrier A., Nattel S. Contribution of ATP-sensitive potassium channels to the electrophysiological effects of adenosine in guinea-pig atrial cells. J Physiol. 1995 May 1;484(Pt 3):629–642. doi: 10.1113/jphysiol.1995.sp020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light P. E., Allen B. G., Walsh M. P., French R. J. Regulation of adenosine triphosphate-sensitive potassium channels from rabbit ventricular myocytes by protein kinase C and type 2A protein phosphatase. Biochemistry. 1995 May 30;34(21):7252–7257. doi: 10.1021/bi00021a041. [DOI] [PubMed] [Google Scholar]
- McCullough J. R., Normandin D. E., Conder M. L., Sleph P. G., Dzwonczyk S., Grover G. J. Specific block of the anti-ischemic actions of cromakalim by sodium 5-hydroxydecanoate. Circ Res. 1991 Oct;69(4):949–958. doi: 10.1161/01.res.69.4.949. [DOI] [PubMed] [Google Scholar]
- McLarnon J. G., Hamman B. N., Tibbits G. F. Temperature dependence of unitary properties of an ATP-dependent potassium channel in cardiac myocytes. Biophys J. 1993 Nov;65(5):2013–2020. doi: 10.1016/S0006-3495(93)81243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. Effects of nicorandil on the membrane currents of rabbit sino-atrial node cells. Jpn J Pharmacol. 1984 Apr;34(4):411–415. doi: 10.1254/jjp.34.411. [DOI] [PubMed] [Google Scholar]
- Shaw D. B., Linker N. J., Heaver P. A., Evans R. Chronic sinoatrial disorder (sick sinus syndrome): a possible result of cardiac ischaemia. Br Heart J. 1987 Dec;58(6):598–607. doi: 10.1136/hrt.58.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. The G. L. Brown Lecture. Potassium channels, metabolism and muscle. Exp Physiol. 1992 Jan;77(1):1–25. doi: 10.1113/expphysiol.1992.sp003564. [DOI] [PubMed] [Google Scholar]
- Taira N. Similarity and dissimilarity in the mode and mechanism of action between nicorandil and classical nitrates: an overview. J Cardiovasc Pharmacol. 1987;10 (Suppl 8):S1–S9. [PubMed] [Google Scholar]
- Traut T. W. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994 Nov 9;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Venkatesh N., Lamp S. T. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992 Feb;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A. A., Janse M. J. Electrophysiological effects of ATP sensitive potassium channel modulation: implications for arrhythmogenesis. Cardiovasc Res. 1994 Jan;28(1):16–24. doi: 10.1093/cvr/28.1.16. [DOI] [PubMed] [Google Scholar]
- Zilberter Y., Burnashev N., Papin A., Portnov V., Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Arch. 1988 May;411(5):584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]


