Abstract
1. We used the whole-cell configuration of the patch clamp technique to examine the different macroscopic Cl- currents present in single rat parotid acinar cells. 2. Cell swelling produced by negative osmotic pressure (hypotonic bath solutions) induced a large outwardly rectifying Cl- current with little or no time and voltage dependence. In contrast, an increase in intracellular [Ca2+] induced by ionomycin activated Cl- currents with very different properties. Ca(2+)-activated Cl- currents showed outward rectification, relatively slow activation kinetics and marked voltage dependence. These results are consistent with the existence of two different outwardly rectifying Cl- channels in rat parotid cells. 3. In conditions designed to eliminate the activation of these two Cl- currents, a third type of current was observed. This third current was activated in a time-dependent manner by hyperpolarized potentials and was about equally permeant to Cl-, I- and Br-. 4. The properties of the hyperpolarization-activated current were similar to those of the cloned ClC-2 channel. Polymerase chain reaction-based methods and ribonuclease protection analyses indicated the presence in parotid gland of mRNA homologous to ClC-2. 5. Individual parotid acinar cells expressed all three types of Cl- channels. Each type of channel may contribute to Cl- efflux in distinct stages of the secretion process depending on the intracellular [Ca2+], cell volume and membrane potential.
Full text
PDF


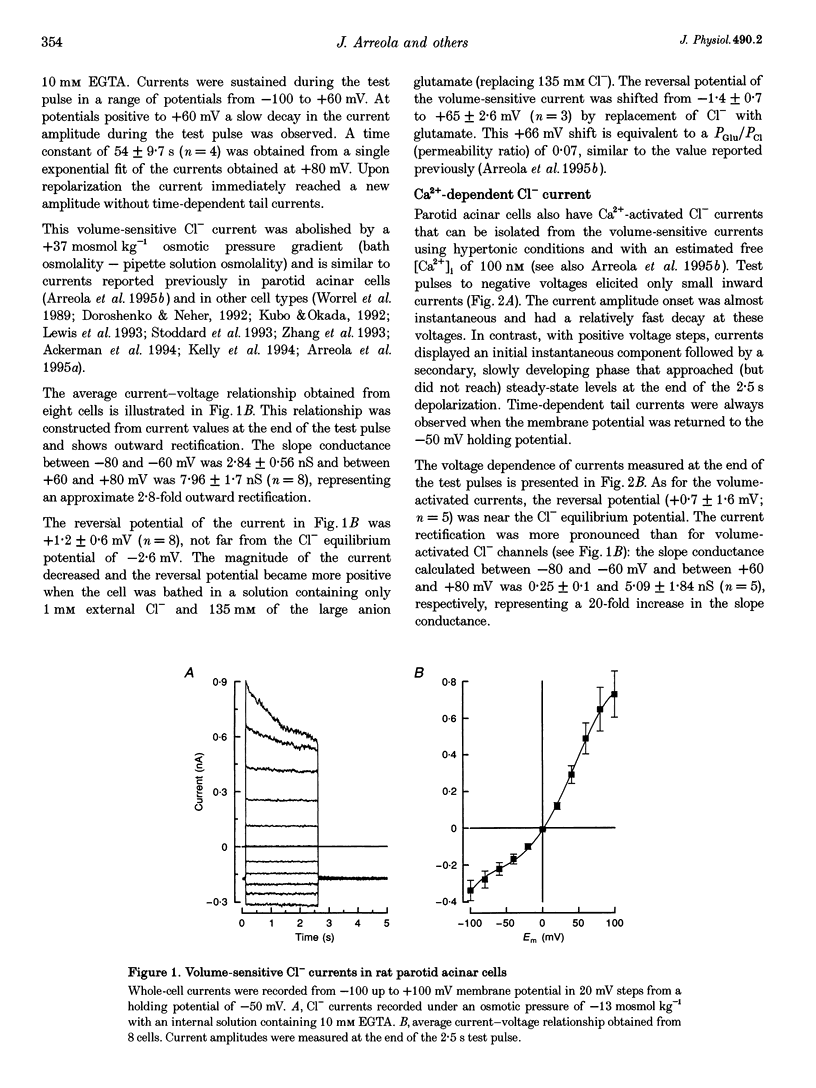
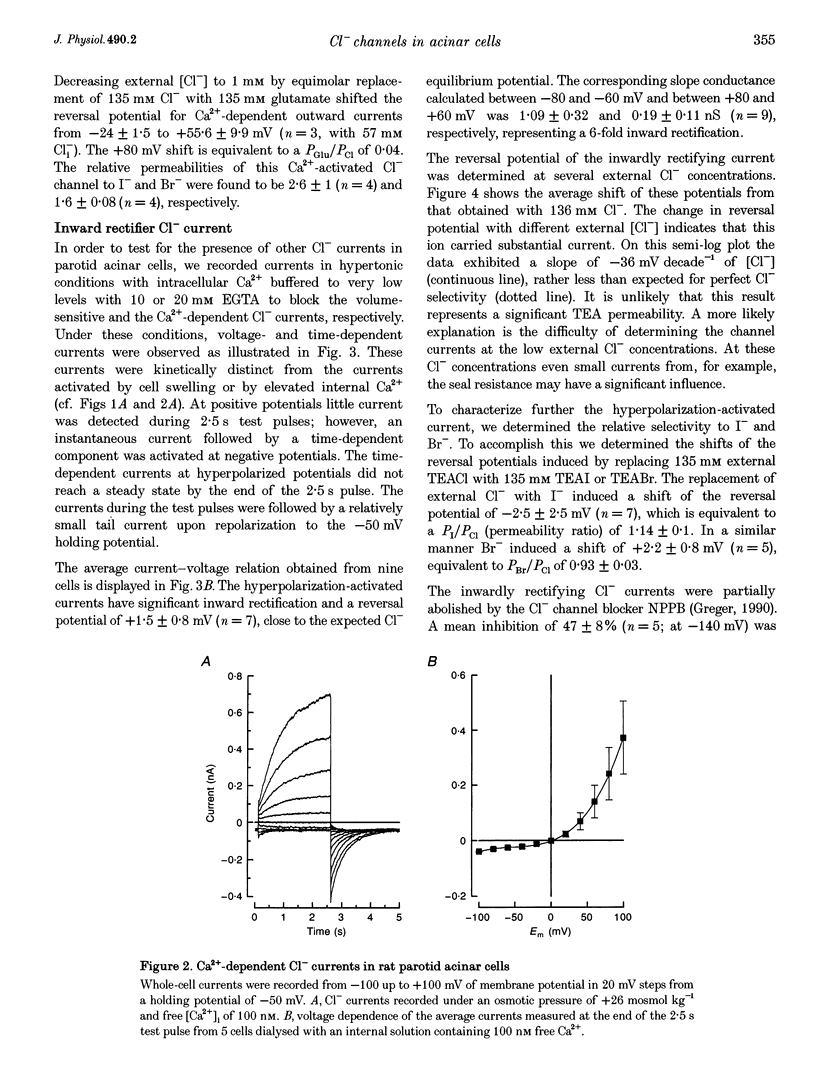
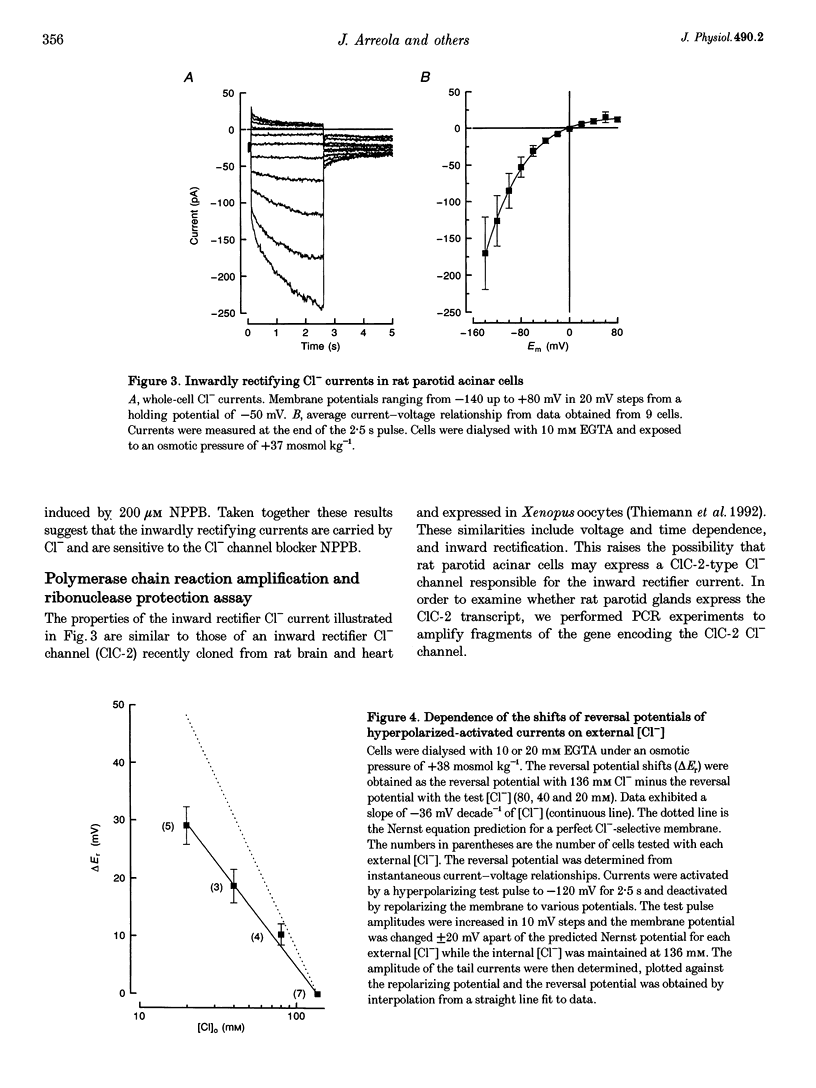
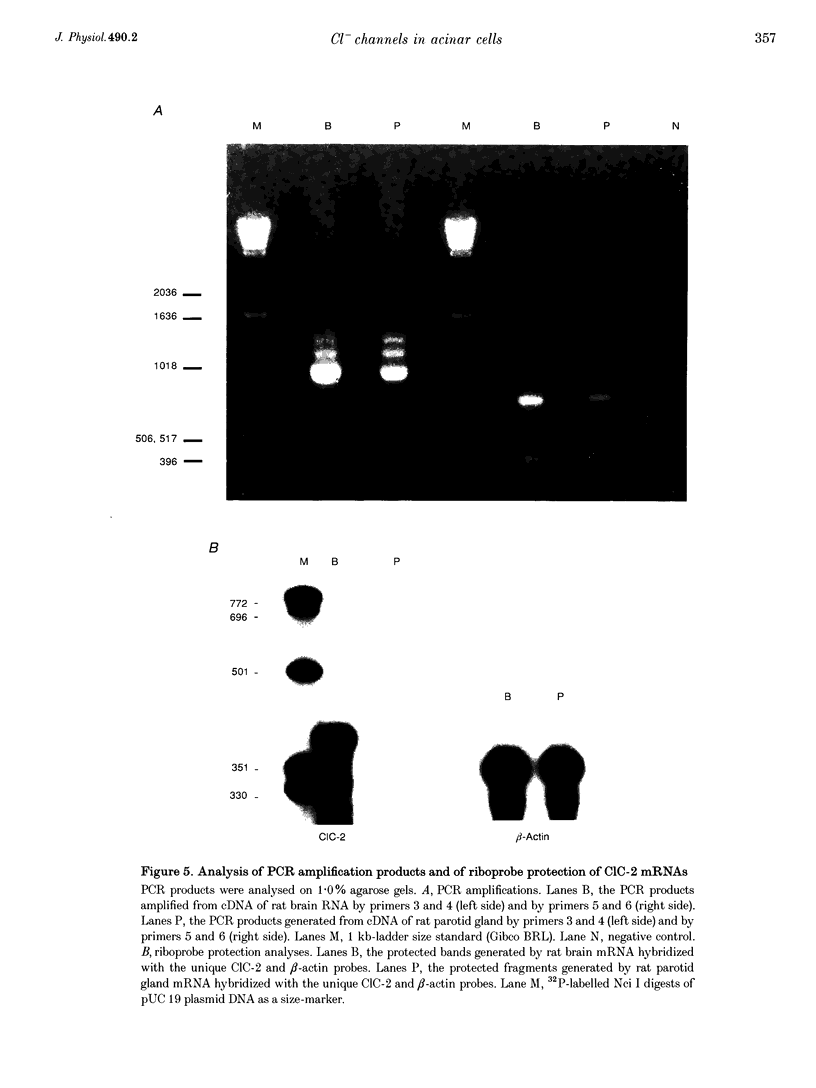
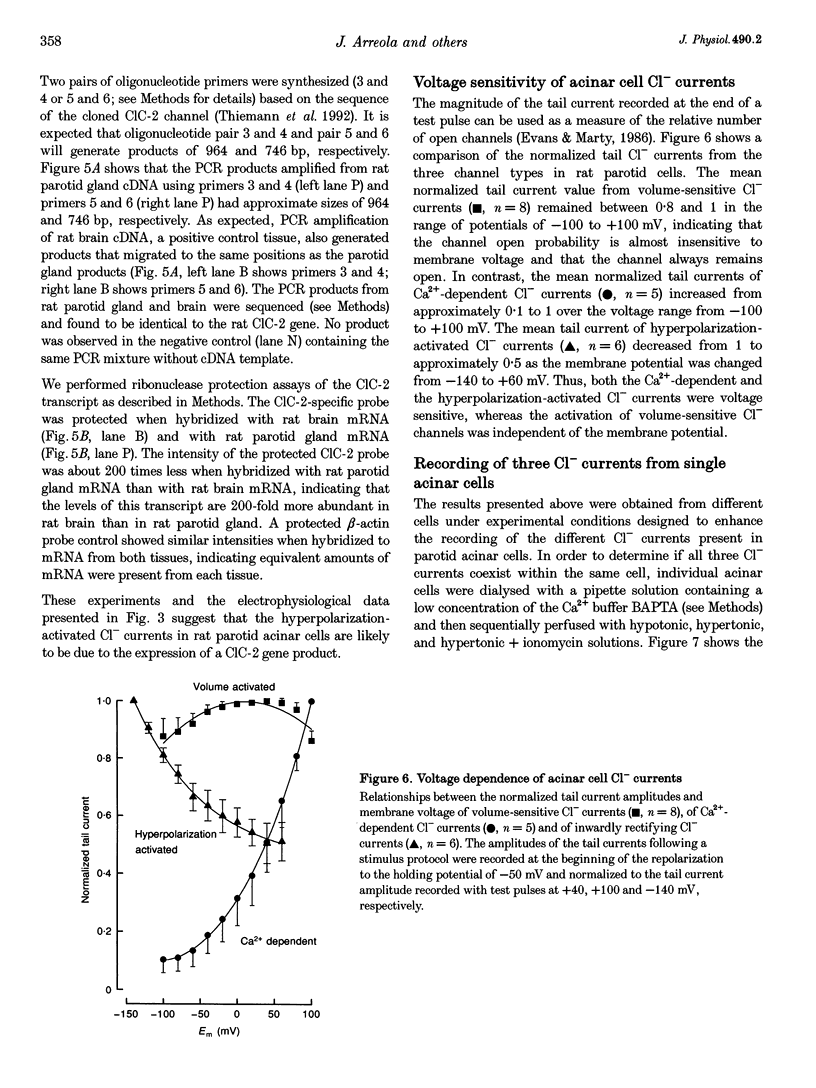
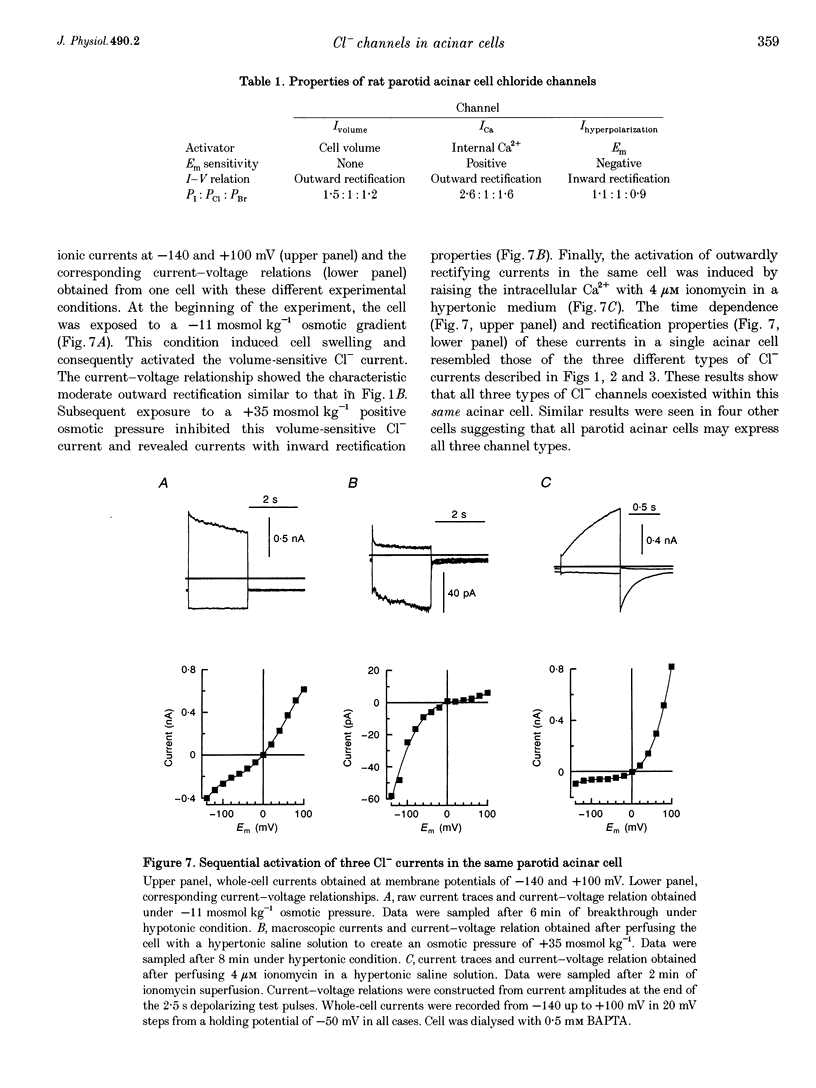



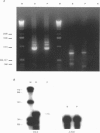
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S., Uchida S., Ito H., Hata M., Hiroe M., Marumo F., Sasaki S. Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J Biol Chem. 1994 Jul 1;269(26):17677–17683. [PubMed] [Google Scholar]
- Arreola J., Melvin J. E., Begenisich T. Volume-activated chloride channels in rat parotid acinar cells. J Physiol. 1995 May 1;484(Pt 3):677–687. doi: 10.1113/jphysiol.1995.sp020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doroshenko P., Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol. 1992 Apr;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. The relation of elevation of cytosolic free calcium to activation of membrane conductance in rat parotid acinar cells. Proc R Soc Lond B Biol Sci. 1989 Jun 22;237(1286):99–107. doi: 10.1098/rspb.1989.0039. [DOI] [PubMed] [Google Scholar]
- Greger R. Chloride channel blockers. Methods Enzymol. 1990;191:793–810. doi: 10.1016/0076-6879(90)91048-b. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Cook D. I. A Ca(2+)-activated Cl- current in sheep parotid secretory cells. J Membr Biol. 1993 Sep;135(3):261–271. doi: 10.1007/BF00211098. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Dixon S. J., Sims S. M. Outwardly rectifying chloride current in rabbit osteoclasts is activated by hyposmotic stimulation. J Physiol. 1994 Mar 15;475(3):377–389. doi: 10.1113/jphysiol.1994.sp020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J Physiol. 1992 Oct;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Ross P. E., Cahalan M. D. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993 Jun;101(6):801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahari T., Murakami M., Yoshida H., Miyamoto M., Sohma Y., Imai Y. Decrease in rat submandibular acinar cell volume during ACh stimulation. Am J Physiol. 1990 Jun;258(6 Pt 1):G878–G886. doi: 10.1152/ajpgi.1990.258.6.G878. [DOI] [PubMed] [Google Scholar]
- Nauntofte B., Dissing S. K+ transport and membrane potentials in isolated rat parotid acini. Am J Physiol. 1988 Oct;255(4 Pt 1):C508–C518. doi: 10.1152/ajpcell.1988.255.4.C508. [DOI] [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Viana F., De Greef C., Raeymaekers L., Eggermont J., Droogmans G. Volume-activated Cl- currents in different mammalian non-excitable cell types. Pflugers Arch. 1994 Oct;428(3-4):364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992 Mar;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Stoddard J. S., Steinbach J. H., Simchowitz L. Whole cell Cl- currents in human neutrophils induced by cell swelling. Am J Physiol. 1993 Jul;265(1 Pt 1):C156–C165. doi: 10.1152/ajpcell.1993.265.1.C156. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rasmusson R. L., Hall S. K., Lieberman M. A chloride current associated with swelling of cultured chick heart cells. J Physiol. 1993 Dec;472:801–820. doi: 10.1113/jphysiol.1993.sp019974. [DOI] [PMC free article] [PubMed] [Google Scholar]