Abstract
BACKGROUND
No effective treatment guarantees full recovery from osteoarthritis (OA), and few therapies have disadvantages.
AIM
To determine if bone marrow mesenchymal stem cells (BMMSCs) and hyaluronic acid (HA) treat ankle OA in Wistar rats.
METHODS
BMMSCs were characterized using flow cytometry with detection of surface markers [cluster of differentiation 90 (CD90), CD105, CD34, and CD45]. Fifty male Wistar rats were divided into five groups of 10 rats each: Group I, saline into the right tibiotarsal joint for 2 days; Group II, monosodium iodate (MIA) into the same joint; Groups III, MIA + BMMSCs; Group IV, MIA + HA; and Group V, MIA + BMMSCs + HA. BMMSCs (1 × 106 cells/rat), HA (75 µg/rat), and BMMSCs (1 × 106 cells/rat) alongside HA (75 µg/rat) were injected intra-articularly into the tibiotarsal joint of the right hind leg at the end of weeks 2, 3, and 4 after the MIA injection.
RESULTS
The elevated right hind leg circumference values in the paw and arthritis clinical score of osteoarthritic rats were significantly ameliorated at weeks 4, 5, and 6. Lipid peroxide significantly increased in the serum of osteoarthritic rats, whereas reduced serum glutathione and glutathione transferase levels were decreased. BMMSCs and HA significantly improved OA. The significantly elevated ankle matrix metalloproteinase 13 (MMP-13) mRNA and transforming growth factor beta 1 (TGF-β1) protein expression, and tumor necrosis factor alpha (TNF-α) and interleukin-17 (IL-17) serum levels in osteoarthritic rats were significantly downregulated by BMMSCs and HA. The effects of BMMSCs and HA on serum TNF-α and IL-17 were more potent than their combination. The lowered serum IL-4 levels in osteoarthritic rats were significantly upregulated by BMMSCs and HA. Additionally, BMMSCs and HA caused a steady decrease in joint injury and cartilage degradation.
CONCLUSION
BMMSCs and/or HA have anti-arthritic effects mediated by antioxidant and anti-inflammatory effects on MIA-induced OA. MMP-13 and TGF-β1 expression improves BMMSCs and/or HA effects on OA in Wistar rats.
Keywords: Osteoarthritis, Anti-inflammatory, Antioxidant, Bone marrow mesenchymal stem cells, Hyaluronic acid
Core Tip: We determined the effectiveness of bone marrow mesenchymal stem cells (BMMSCs) and/or hyaluronic acid (HA) in treating ankle osteoarthritis (OA). BMMSCs were characterized by flow cytometry. Lipid peroxidation, matrix metalloproteinase 13 (MMP-13) mRNA, transforming growth factor beta 1 (TGF-β1) protein expression, and tumor necrosis factor alpha and interleukin-17 serum levels significantly increased in osteoarthritic rats, while antioxidant serum glutathione and glutathione transferase levels decreased. Treatment of osteoarthritic rats with BMMSCs and HA improved these changes. BMMSCs and/or HA have anti-arthritic effects mediated by increased antioxidant and anti-inflammatory effects and suppression of MMP-13 and TGF-β1 expression in Wistar rats with OA.
INTRODUCTION
Osteoarthritis (OA), the most common joint disease, is a chronic degenerative disease that affects the cartilage, synovial membranes, and bones of the joints. It is characterized by articular cartilage deterioration, synovitis, joint effusion, and new periarticular bone formation, all of which result in pain, swelling joint stiffness, and joint dysfunction[1,2]. There are numerous risk factors for OA, including aging, hereditary factors, and trauma; however, aging is the most common risk factor for the disease[3]. Furthermore, OA is more common among middle-aged and aging people, obese patients, sports enthusiasts, and expert athletes. Ankle OA is common in degenerative ankle OA in the elderly, but can also be seen in ankle OA secondary to injury resulting in ankle fracture. Existing conventional therapies for OA, which are mostly directed toward the management of clinical symptoms, chiefly incorporate the use of usual analgesics, nonsteroidal anti-inflammatory drugs, and hyaluronic acid (HA) lubricants, in addition to surgical interventions, such as microfracture and drilling. Moreover, the combination of Chinese and Western inclusive therapy, a characteristic physiotherapy of traditional Chinese medicine, coupled with traditional Chinese medicine. However, all of these treatments fail to guarantee a complete cure for OA.
To date, only a few effective treatments are available for OA, highlighting the need to develop novel and effective remedies. Cell therapy is an interesting therapeutic choice for various types of arthritis, as it might have the potential to prevent further damage to cartilage[4,5]. Recently, numerous studies on the effects of intra-articular mesenchymal stem cells (MSCs) in experimental and clinical arthritis have shown that stem cells are effective in curing cartilaginous defects and have suppressive effects on OA[6,7]. MSCs can differentiate into various mesenchymal tissues including cartilage, bone, muscle, and tendons. MSCs can be easily isolated from the bone marrow and further proliferated in culture, preserving their ability to differentiate in response. MSCs possess self-renewal ability, anti-inflammatory properties, and regenerative properties; therefore, MSCs may have the potential to regenerate damaged tissues and could be used as a treatment for OA.
MSCs are the ideal cell type for repairing damaged joints in different arthritic disorders. In animal models of arthritis, intraperitoneal or intravenous MSC injections have resulted in several curative effects, ranging from significant amelioration to no effect; thus, the results remain inconclusive. This may be explained by the differences in the route of administration. Intra-articular administration of MSCs directly to the affected tissues may be more advantageous than any other route of administration[7]. Among different MSCs, bone marrow-derived MSCs (BMMSCs) have been shown to efficiently reduce the development of OA in several animal models[6].
Intra-articular HA has been proposed as a treatment for ameliorating the mechanical functions of joints. It exhibits analgesic, anti-inflammatory, mechanical, and chondroprotective properties[8]. HA treatment can restore articular viscosity by substituting dysfunctional synovial fluid, which is formed due to changes associated with early inflammatory responses[9].
Therefore, we assessed the effectiveness of intra-articular BMMSCs, HA, and the combination of HA and BMMSCs in repairing damaged joints, diminishing joint swelling, and ameliorating inflammation of the osteoarthritic ankle induced by monosodium iodate (MIA) in a rat model.
MATERIALS AND METHODS
Chemicals
MIA was obtained from Sigma-Aldrich (St Louis, MO, United States). HA, orthovisc® (15 mg/mL) of high molecular weight ranging from 1000 to 2900 kDa was obtained from Anika Therapeutics, Inc. (Bedford, MA, United States). Dulbecco’s modified Eagle medium (DMEM), penicillin/streptomycin, trypsin/EDTA, and fetal bovine serum (FBS) were purchased from Lonza (Verviers, Belgium). Sodium hydrogen carbonate was purchased from Loba Chemie (Maharashtra, India). Culture flasks and other consumables were purchased from Greiner Bio-One (Frickenhausen, Germany). All other reagents and chemicals used were of high purity grade.
Preparation of complete culture medium
To prepare the complete culture medium, penicillin/streptomycin solution, sodium hydrogen carbonate, and FBS were added to 89% DMEM, based on the methods employed by Sun et al[10].
Isolation and culture of BMMSCs
The procedure used for isolating and culturing BMMSCs was based on the technique described by Ahmed et al[11].
Cell viability analysis
The trypan blue dye exclusion test was used to assess the viability of isolated and cultured cells. This technique is based on the theory that the surviving cells do not take up the dye, whereas dead cells do. After washing them twice in phosphate-buffered saline (PBS), the cells were resuspended in DMEM, and the viability percentage was assessed by adding equal volumes of 0.2% trypan blue (100 µL) and cell suspension (100 µL). The viability of the isolated cells was found to be 97%-98%.
Labeling of BMMSCs
Labeling of BMMSCs was accomplished according to the technique by Aboul-Fotouh et al[12] by incubating BMMSCs with ferumoxides solution (25 µg Fe/mL, Feridex; Berlex Laboratories, Montville, NJ, United States) in a culture medium for 24 h with adding 375 ng/mL of polylysine 1 h pre-incubation. Labeling was histologically detected using Prussian’s blue (Pb) staining. After incubation, the Feridex-labeled BMMSCs were washed in PBS, treated with trypsin, re-washed, and re-suspended in 0.01 mol/L PBS at a cell density of 1 × 106 cells/mL.
Morphological examination of BMMSCs
BMMSCs were observed under the Biobase inverted microscope to confirm the characteristics of adherent spindle-shaped BMMSCs with their in vitro growth characteristics according to Ghanayem et al[13]. Also, BMMSCs were identified by their adherence to the plastic surfaces of tissue culture flasks when maintained under standard culture conditions.
The BMMSCs suspended in DMEM with viability > 95% were rapidly injected into the ankle joint of osteoarthritic rats at a dose of 1 × 106 cells/rat.
Detection of surface markers (cluster of differentiation 90, cluster of differentiation 105, cluster of differentiation 34 and cluster of differentiation 45) by flow cytometry
Flow cytometry was applied for immunophenotyping of BM-MSCs through detection of cell surface markers [cluster of differentiation 90 (CD90), CD105, CD34, and CD45]. Briefly, the non-adherent hematopoietic cells were washed off, and the adherent MSCs were characterized by fluorescence-activated cell sorting using fluorescein isothiocyanate (FITC) and phycoerythrin (PE) with antibodies against CD90, CD105, CD34, and CD45. Both FITC and PE were obtained from Beckman Coulter Inc. (NE15106; Brea, CA, United States).
Experimental animals and housing
Adult male Wistar rats (n = 50) weighing 100-130 g and aged 8-10 weeks were used. The animals were obtained from VACSERA, Helwan, Egypt. For 1 week, the animals were retained under monitoring and strict care to rule out any communicable diseases. In the animal house of the Department of Zoology, Faculty of Science, Beni-Suef University (Beni Suef, Egypt), the animals were kept in cages with good-aerated stainless-steel coverings at a 10-12 h/day natural daily lighting cycle and a temperature of 20-25 °C, and were given a balanced diet and water ad libitum.
MIA-induced OA
After 1 week of adaptation, OA was experimentally induced in rats by injecting MIA in a dose level of 2 mg/50 μL of isotonic sterile saline using a 21-gauge needle into the tibiotarsal joint of the right hind limb on 2 consecutive days, as previously demonstrated by Ragab et al[5]. Before the MIA injection, the animals were anesthetized using intramuscular injection of ketamine (70 mg/kg body weight) and xylazine (7 mg/kg body weight).
Experimental design and animal grouping
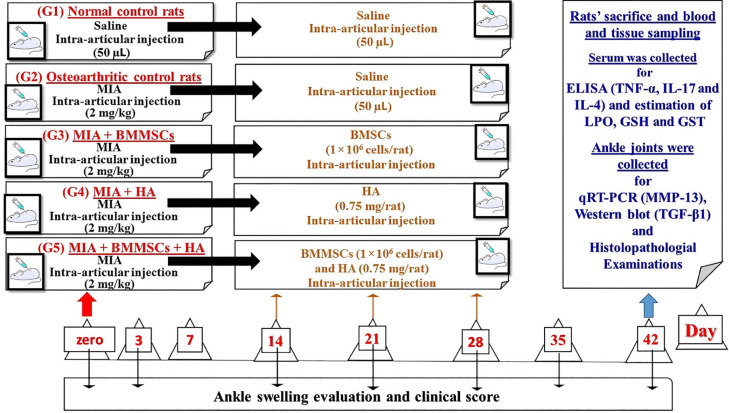
After receiving the MIA injections, the rats were randomly allotted to five groups of 10 rats each, as shown in Figure 1. Group I (normal control) consisted of normal rats, which received 50 µL of sterile 0.9% NaCl by injection at the ends of weeks 0, 2, 3, and 4 into the ankle joint of each rat's right hind leg. Rats in Group II (OA control) received MIA via intra-articular injection into the right tibiotarsal joint over 2 days. At the end of weeks 2, 3, and 4 following MIA injection, the rats in this group additionally received 50 µL of isotonic sterile saline intra-articular injections into the tibiotarsal joint of the right hind leg. Group III (MIA + BMMSCs) consisted of osteoarthritic rats that were intra-articularly injected into the tibiotarsal joint of the right hind leg at a dose of 1 × 106 cells/rat at the end of weeks 2, 3, and 4 after MIA injection. Group IV (MIA + HA) comprised osteoarthritic rats that were intra-articularly treated with 50 μL of 15 mg/mL HA [orthovisc® (15 mg/mL)] by injection into the tibiotarsal joint of the right hind leg at the end of weeks 2, 3, and 4 after MIA injection. Group V (MIA + BMMSCs + HA) consisted of osteoarthritic rats that were intra-articularly treated with BMMSCs and 50 μL of HA by injection into the tibiotarsal joint of the right hind leg at the end of weeks 2, 3, and 4 after the MIA injection.
Figure 1.
Design, setting of the study and animal grouping. BMMSCs: Bone marrow mesenchymal stem cells; GSH: Glutathione; GST: Glutathione S-transferase; HA: Hyaluronic acid; IL-17: Interleukin-17; LPO: Lipid peroxidation; MIA: Monosodium iodate; MMP-13: Matrix metalloproteinase 13; ROS: Reactive oxygen species; TGF-β1: Transforming growth factor beta 1; TNF-α: Tumor necrosis factor alpha.
Blood samples were taken from each rat's jugular vein after the experiment under diethyl ether inhalation anesthesia, placed in gel with a clot activator, and centrifuged at 3000 rpm for 15 min. Quickly removed and stored at 20 °C until needed, the clear, non-hemolyzed supernatant sera were used. Each animal had its right ankle joint quickly removed. Phosphate-buffered formalin was used to fix three samples from each group for histopathological analysis. The rest of the ankles of each group were kept at -20 °C until they were used in molecular and Western blot analyses.
Measurement of leg circumference: Using a thread that was wrapped around the leg at the area of the ankle, the circumference of the right hind leg at the region of the ankle was measured as a gauge of the rate of paw edema and swelling in various groups. The thread's length was then determined using a ruler. From zero days to the conclusion of the experiment following MIA, the measurements were anticipated once a week. To demonstrate the gross morphology, a high-resolution camera was also used to take pictures of the right legs.
Assessment of clinical signs of inflammation and scoring of arthritis: The rats were observed for clinical signs of arthritis every week (from day zero to the end of the experiment) following the injection of MIA. Swelling in at least one joint or paw was taken into consideration when MIA-induced arthritis was suspected to have occurred. According to the methodology employed in a prior paper[14], the degree of arthritis was graded in each paw as follows: 0 = normal; 0.5 = slight redness of ankle joint or digits; 1 = mild but distinct redness and ankle swelling; 2 = moderate redness and ankle swelling; 3 = severe redness and entire paw swelling, including digits; more than two joints involved; 4 = maximally inflamed limb with involvement of more than one joint.
Determination of serum cytokine levels: Special enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN, United States) were used to measure the serum levels of tumor necrosis factor alpha (TNF-α), interleukin-17 (IL-17), and IL-4.
Determination of serum oxidative stress and antioxidant defense system biomarkers: Based on the methods described by Yilgor et al[15], serum glutathione (GSH) levels, lipid peroxidation (LPO) product (malondialdehyde), and GSH-S-transferase (GST) activities were determined.
Quantitative reverse transcriptase-polymerase chain reaction assay for matrix metalloproteinase 13 mRNA of the ankle joint: RNA extraction kit that enables purification of high-quality RNA from tissues (ankle joint articular tissues) was purchased from (Qiagen, Germantown, MD, United States). The method was carried out according to the company’s instructions. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) amplification and analysis were performed using Applied Biosystems StepOne™ software version 3.1 (Foster City, CA, United States). The qRT-PCR assay with the primer sets was optimized at the annealing temperature. The primer sequences for matrix metalloproteinase 13 (MMP-13) were as follows: Forward: 5′-CCCTGGAATTGGCGACAAAG-3′; Reverse: 5′GCATGACTCTCACAATGCGATTAC-3′.
Western blot analysis for detection of ankle transforming growth factor beta 1: Western blot analysis was performed as previously described[16]. Ankle samples preserved at -20 °C were used to study the effect of BMMSCs and/or HA on the expression level of transforming growth factor beta 1 (TGF-β1). Briefly, ankles were homogenized in radioimmunoprecipitation assay (RIPA) buffer supplemented with proteinase inhibitor and centrifuged, after which the protein concentration was estimated in the clear homogenate supernatant by Bradford reagent. Thirty milligrams of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrotransferred to nitrocellulose membranes, and blocked in 5% skimmed milk dissolved in a mixture of Tris-buffered saline Tween 20 (TBST). Then the loaded membranes were incubated with 1ry antibodies against TGF-β1. After washing with TBST, the membranes were probed with the corresponding 2ry antibodies and developed using an improved chemiluminescence assay kit (Bio-Rad Laboratories, Hercules, CA, United States).
Western blot analysis, ELISA, and qRT-PCR were performed at the Laboratory of Biochemistry Department, Kasr Al Ainy Faculty of Medicine, Cairo University (Giza, Egypt).
Histopathological investigation
After sacrifice (6 weeks), the right ankle joints of rats in the different groups were dissected out and fixed in 10% phosphate-buffered formalin. Then the decalcification process was carried out by using a 10% formic acid solution, which was replaced twice weekly for 2 weeks, and the endpoint of decalcification was detected physically with a surgical blade. After complete decalcification, the samples were washed with PBS, dehydrated in a graded ethyl alcohol series, and embedded in paraffin wax. Sagittal sections measuring 5 µm in thickness by sludge microtome. The tissue sections were collected on glass slides and stained with hematoxylin and eosin (H&E). The prepared H&E-stained sections were examined to detect histological lesions. After histological examinations, sections were graded according to the system illustrated by Allam et al[17] for pannus formation (synovial hypertrophy), inflammation represented by inflammatory cell infiltration, cartilage erosion, and bone damage. For each histological lesion, four grades were characterized: 0 = no lesion, 1 = mild, 2 = moderate, 3 = severe.
Histochemical investigation
Pb staining technique for the detection of iron oxide labeled therapeutic MSCs was used according to the method of Ellis[18]. Labeled therapeutic MSCs in joint tissues were marked by a blue staining color.
Statistical analysis
Statistical analysis was carried out by SPSS (version 25; IBM Co., Armonk, NY, United States). Data are presented as the mean ± standard error of the mean, and statistical comparisons were analyzed by Duncan's test post hoc. P < 0.05 was considered statistically significant.
RESULTS
Characterization of BMMSCs
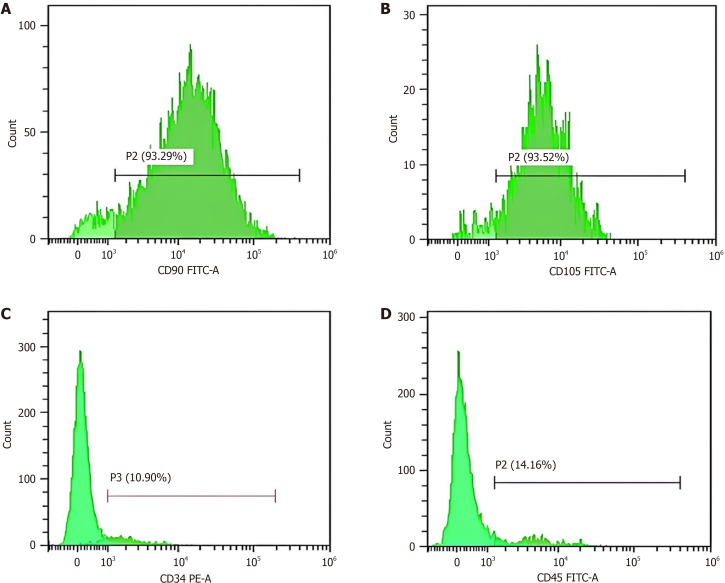
To characterize the isolated BMMSCs, flow cytometry was performed to detect the surface markers CD73, CD105, CD45, and CD34. The purified adherent cells at the end of the incubation period exhibited high expression of specific biomarkers of BMMSCs including CD90 (93.29%) and CD105 (93.52%), and weak expression of CD34 (10.90%) and CD45 (14.16%), which are specific biomarkers for hematopoietic stem cells (Figure 2).
Figure 2.
Immunophenotyping of bone marrow mesenchymal stem cells by detection of surface markers including cluster of differentiation 90, cluster of differentiation 105, cluster of differentiation 34, and cluster of differentiation 45 by flow cytometry. The cells are cluster of differentiation 90 (CD90)- and CD105-positive (> 90%) while the expression of CD34 and CD45 is weak (< 15%). A: CD90; B: CD105; C: CD34; D: CD45. FITC: Fluorescein isothiocyanate; PE: Phycoerythrin.
Effects of BMMSCs and HA on morphological signs of the right leg of osteoarthritic rats
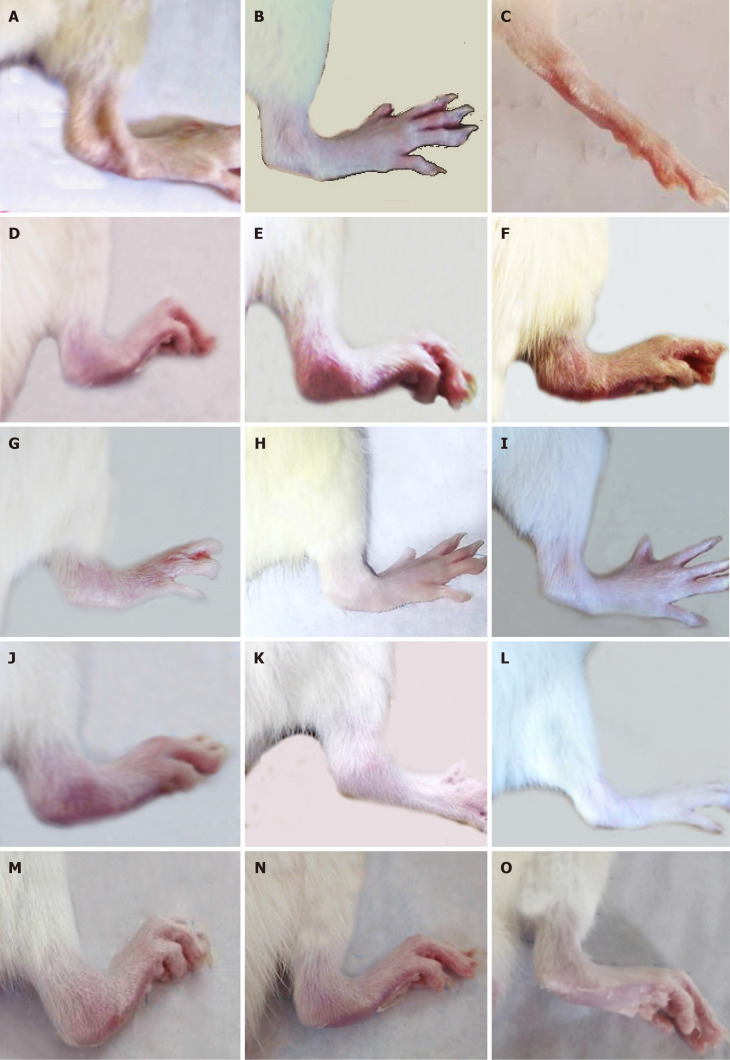
Figure 3 shows the gross changes in the right legs of normal, osteoarthritic, and osteoarthritic-treated rats. The right hind leg ankles exhibited marked swelling and redness in the 4th, 5th, and 6th weeks of MIA injection (Figure 3D-F) compared with those of normal rats (Figure 3A-C). These deteriorated signs were more pronounced as the period extended from the 4th to 6th weeks.
Figure 3.
Right hind leg pictures of the tested groups. A-C: Right hind legs normal rats at the 4th week (A), 5th week (B), and 6th week (C); D-F: Osteoarthritic rats in the 4th week (D), 5th week (E), and 6th week (F) showed swelling and redness as external signs of inflammation; G-O: Osteoarthritic rats treated with bone marrow mesenchymal stem cells (BMMSCs) (G-I), hyaluronic acid (HA), (J-L) and BMMSCs + HA (M-O) exhibited marked improvements in these morphological signs (in the 4th, 5th, and 6th weeks, respectively).
The treatment of MID-induced osteoarthritic Wistar rats with BMMSCs, HA, and BMMSCs plus HA resulted in a remarkable amelioration of these morphological signs as shown in Figure 3G-O in the 4th, 5th and 6th weeks, respectively.
Effect of BMMSCs and HA on right hind leg paw circumference in osteoarthritic rats
The change in right hind leg paw circumference in MIA-administered rats through 6 weeks after MIA administration and as a result of BMMSC and/or HA treatment is shown in Table 1. The osteoarthritic control animals exhibited a significant (P < 0.05) increase in the hind leg paw edema; the recorded percentage increases were 95%, 68%, 65%, 64%, 61%, and 50% at the 1st, 2nd in the 3rd, 4th, 5th, and 6th weeks, respectively, in relation to the normal control group. Treatment of the osteoarthritic rats with BMMSCs, HA, and BMMSCs + HA produced a significant decline in the elevated level of paw edema recording percentage changes of -17%, -22%, and -17%, respectively, at the end of the experiment in comparison with the osteoarthritic control animals.
Table 1.
Effect of bone marrow mesenchymal stem cell and/ or hyaluronic acid treatments on right hind leg circumference at paw region (cm) of osteoarthritic rats
|
Period/group
|
Zero day
|
%
|
3rd day
|
%
|
2nd week
|
%
|
3rd week
|
%
|
4th week
|
%
|
5th week
|
%
|
6th week
|
%
|
| Normal | 1.50 ± 0.03a | - | 1.53 ± 0.03b | - | 1.68 ± 0.05b | - | 1.68 ± 0.05b | - | 1.71 ± 0.05d | - | 1.70 ± 0.04d | - | 1.80 ± 0.04c | - |
| Osteoarthritic control | 1.58 ± 0.05a | 5 | 2.98 ± 0.05a | 95 | 2.82 ± 0.05a | 68 | 2.77 ± 0.054a | 65 | 2.80 ± 0.03a | 64 | 2.74 ± 0.03a | 61 | 2.70 ± 0.03a | 50 |
| Osteoarthritic + BMMSCs | 1.67 ± 0.06a | 6 | 2.89 ± 0.06a | -3 | 2.79 ± 0.04a | -1 | 2.69 ± 0.035a | -3 | 2.67 ± 0.04c | -5 | 2.28 ± 0.04b | -6 | 2.23 ± 0.07b | -17 |
| Osteoarthritic + HA | 1.64 ± 0.06a | 4 | 2.92 ± 0.04a | -2 | 2.90 ± 0.06a | 3 | 2.74 ± 0.06a | -1 | 2.55 ± 0.04b | -9 | 2.45 ± 0.05c | -11 | 2.10 ± 0.04b | -22 |
| Osteoarthritic + BMMSCs + HA | 1.63 ± 0.06a | 3 | 2.91 ± 0.04a | -2 | 2.89 ± 0.05a | 2 | 2.76 ± 0.052a | 0 | 2.600 ± 0.05b,c | -7 | 2.50 ± 0.03b,c | -9 | 2.25 ± 0.05b | -17 |
P < 0.05, in the same column, values with b, c, and d are significantly different;
P < 0.05, in the same column, values with a, c, and d are significantly different;
P < 0.05, in the same column, values with a, b, and d are significantly different;
P < 0.05, in the same column, values with a, b, and c are significantly different.
Data are expressed as the mean ± standard error of the mean (SEM) of the mean (n = 6). Percentage changes were calculated by comparing osteoarthritic control with normal and osteoarthritic-treated groups with osteoarthritic control. BMMSCs: Bone marrow mesenchymal stem cells; HA: Hyaluronic acid.
Effects of BMMSCs and HA on arthritis clinical score of osteoarthritic rats
The change of clinical score of arthritis in MIA-administered rats through 6 weeks after MIA administration and as a result of BMMSCs, and/or HA treatment was shown in Table 2.
Table 2.
Effect of bone marrow mesenchymal stem cell and/ or hyaluronic acid treatments on clinical score of arthritis in osteoarthritic rats
|
Period croup
|
0 day
|
3rd day
|
%
|
2nd week
|
%
|
3rd week
|
%
|
4th week
|
%
|
5th week
|
%
|
6th week
|
%
|
| Normal | 0 | 0.20 ± 0.08b | - | 0.20 ± 0.08b | - | 0.20 ± 0.01b | - | 0.10 ± 0.07c | - | 0.10 ± 0.07c | - | 0.10 ± 0.05c | - |
| Osteoarthritic control | 0 | 3.80 ± 0.13a | 1800 | 2.80 ± 0.20a | 1300 | 2.60 ± 0.16a | 1200 | 3.20 ± 0.25a | 2400 | 2.60 ± 0.22a | 2300 | 2.10 ± 0.21a | 2000 |
| Osteoarthritic + BMMSCs | 0 | 3.60 ± 0.18a | -5 | 2.70 ± 0.21a | -4 | 2.40 ± 0.16a | -8 | 2.30 ± 0.25a | 4 | 2.10 ± 0.23b | -13 | 0.70 ± 0.19b | -67 |
| Osteoarthritic + HA | 0 | 3.70 ± 0.15a | -3 | 2.80 ± 0.20a | 0 | 2.50 ± 0.16a | -8 | 2.12 ± 0.13b | -16 | 1.70 ± 0.21b | -29 | 0.55 ± 0.14b | -74 |
| Osteoarthritic + BMMSCs + HA | 0 | 3.70 ± 0.15a | -3 | 2.70 ± 0.21a | -4 | 2.40 ± 0.16a | -8 | 2.50 ± 0.21a | 0 | 2.00 ± 0.21b | -17 | 0.70 ± 0.19b | -67 |
P < 0.05, in the same column, values with b, c, and d are significantly different;
P < 0.05, in the same column, values with a, c, and d are significantly different;
P < 0.05, in the same column, values with a, b, and d are significantly different;
P < 0.05, in the same column, values with a, b, and c are significantly different.
Data are expressed as the mean ± standard of the mean (SEM) of the mean (n = 6). Percentage changes were calculated by comparing osteoarthritic control with normal and osteoarthritic-treated groups with osteoarthritic control. BMMSCs: Bone marrow mesenchymal stem cells; HA: Hyaluronic acid.
The gross signs of arthritis started from the 1st day after MIA administration with slight swelling and redness, reaching a significant level (P < 0.05) on day 3 (acute OA) and then exhibited a slight gradual decrease in the clinical score until day 14 (chronic OA) in MIA-administered rats. After the treatment of osteoarthritic rats with BMMSCs and/or HA, a significant decrease in arthritis score was observed in BMMSCs, HA, and BMMSCs + HA groups compared with osteoarthritic rats.
Effects of BMMSCs and HA on serum TNF-a, IL-17, and IL-4 levels in osteoarthritic rats
Data describing the effect of BMMSCs and/or HA on the levels of pro-inflammatory and anti-inflammatory cytokines in the serum of MIA-administered rats are presented in Table 3. The injection of MIA into rats induced a significant upregulation (P < 0.05) of serum TNF-α (39.9%) and IL-17 (26.5%) levels and a significant downregulation (P < 0.05) of serum IL-4 level (-56.91%) compared to the normal group. The treatment of osteoarthritic rats with BMMSCs and/or HA caused a significant improvement (P < 0.05) in serum TNF-α and IL-17 levels in relation to the osteoarthritic control. On the other hand, the serum IL-4 level was significantly enhanced due to BMMSC and/or HA treatments. Although the effects of BMMSCs, HA, and BMMSCs ± HA on serum IL-4 levels were similar, the effects of BMMSCs and HA on serum TNF-α and IL-17 levels were more potent than their combination.
Table 3.
Effect of bone marrow mesenchymal stem cell and/ or hyaluronic acid treatments on serum tumor necrosis factor alpha, interleukin-17 (pro-inflammatory cytokines) and interleukin-4 (anti-inflammatory cytokine) levels in osteoarthritic rat
|
Parameters groups
|
TNF-α (pg/mL)
|
% change
|
IL-17 (pg/mL)
|
% change
|
IL-4 (pg/mL)
|
% change
|
| Normal | 16.55 ± 0.95d | - | 35.47 ± 1.41e | - | 117.63 ± 1.06a | - |
| Osteoarthritic control | 82.65 ± 3.64a | 399 | 129.52 ± 1.45a | 265 | 48.77 ± 1.07c | -59 |
| Osteoarthritic + BMMSC | 30.38 ± 3.19c | -63 | 60.70 ± 2.05d | -53 | 96.40 ± 0.46b | 98 |
| Osteoarthritic + HA | 28.97 ± 1.52c | -65 | 70.43 ± 2.51c | -46 | 97.40 ± 1.57b | 100 |
| Osteoarthritic + BMMS + HA | 43.58 ± 4.04b | -47 | 76.04 ± 0.77b | -41 | 96.27 ± 4.01b | 97 |
P < 0.05, in the same column, values with b, c, d and e are significantly different;
P < 0.05, in the same column, values with a, c, d and e are significantly different;
P < 0.05, in the same column, values with a, b, d and e are significantly different;
P < 0.05, in the same column, values with a, b, c and e are significantly different;
P < 0.05, in the same column, values with a, b, c and d are significantly different.
Data are expressed as the mean ± standard error of the mean (SEM) of the mean (n = 6). Percentage changes were calculated by comparing osteoarthritic control with normal and osteoarthritic-treated groups with osteoarthritic control. BMMSCs: Bone marrow mesenchymal stem cells; HA: Hyaluronic acid; IL: Interleukin; TNF-α: Tumor necrosis factor alpha.
Effects of BMMSCs and HA on oxidative stress and antioxidant defense bioindicators in osteoarthritic rats
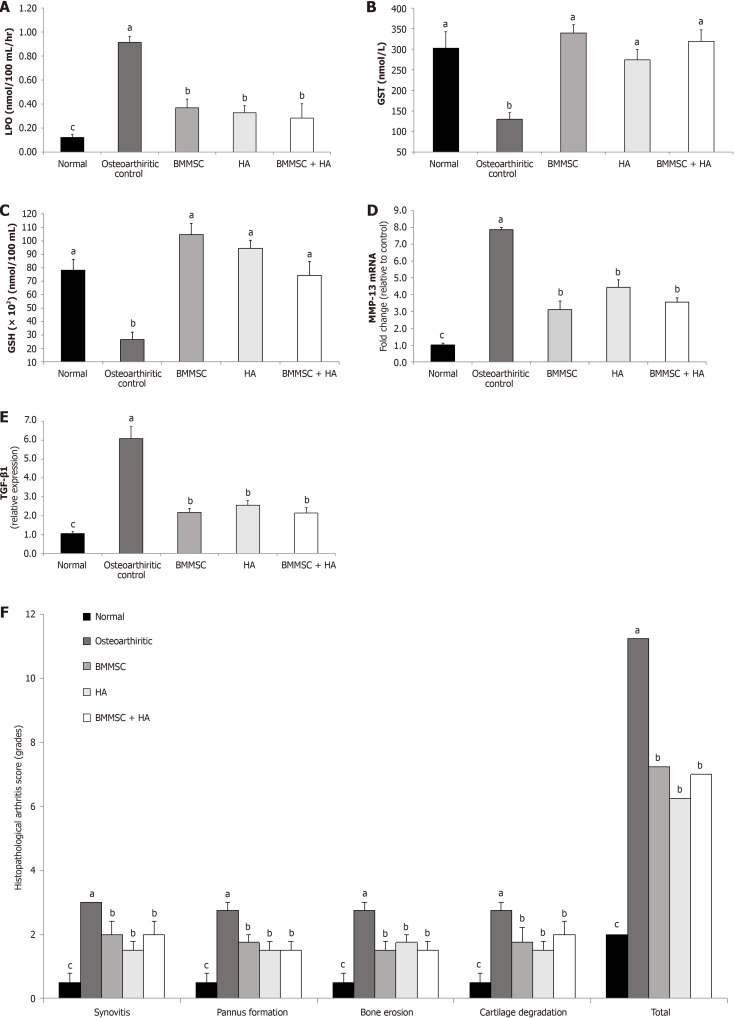
The effects of BMMSC and/or HA on serum oxidative stress and antioxidant defense biomarkers of MIA OA are presented in Figure 4A (LPO), Figure 4B (GST), and Figure 4C (GSH).
Figure 4.
Effect of bone marrow mesenchymal stem cells and/ or hyaluronic acid treatments. A: Effect of bone marrow mesenchymal stem cells (BMMSCs) and/or hyaluronic acid (HA) treatments on serum lipid peroxidation (LPO) level of monosodium iodate-administered rats; B: Effect of BMMSC and/or HA treatments on serum glutathione S-transferase (GST) activity in monosodium iodate-administered rats; C: Effect of BMMSC and/or HA treatments on serum glutathione (GSH) content in monosodium iodate-administered rats; D: Effect of BMMSC and/or HA treatments on matrix metalloproteinase 13 (MMP-13) mRNA expression level in joint tissue of monosodium iodate-administered rat; E: Effect of BMMSCs and/or HA treatments on transforming growth factor-β (TGF-b) protein expression level in the joint tissue of monosodium iodate-administered rat; F: Effect of BMMSCs and/or HA treatments on the histopathological arthritis score monosodium iodate-administered rat. The histological lesion scores of synovitis (synovial inflammation), pannus formation, bone resorption, and cartilage destruction were demonstrated. aP < 0.05, compared values with b, c and d are significantly different; bP < 0.05, compared values with a, c and d are significantly different; cP < 0.05, compared values with a, b and d are significantly different; dP < 0.05, compared values with a, b, and c are significantly different.
LPO was increased in osteoarthritic control Wistar rats. The intra-articular injections of arthritic rats with BMMSCs, HA, and HA + BMMSCs produced a marked decrease in the elevated LPO. HA + BMMSCs were more potent in improving the LPO in osteoarthritic rats.
The intra-articular injection of MIA to Wistar rats significantly (P < 0.05) decreased the serum GSH content as well as GST activities as compared to normal animals. As a result of BMMSC treatment, all the deleterious effects of MIA on the antioxidants were significantly (P < 0.05) enhanced. In addition, HA significantly improved GSH content and GST activity. Therefore, BMMSC treatment seemed to be the most effective.
Effects of BMMSCs and HA on joint MMP-13 protein expression in osteoarthritic rats
Data describing the effect of BMMSCs, HA, and BMMSCs in combination with HA on joint tissue MMP-13 mRNA expression of MIA-administered Wistar rats are shown in Figure 4D. MMP-13 mRNA expression significantly increased in MIA-administered rats compared to the normal group, while in all treated groups, the level of MMP-13 was significantly decreased (P < 0.05).
Effects of BMMSCs and HA on joint TGF-β1 protein expression in osteoarthritic rats
The effect of BMMSC and/or HA treatments on joint TGF-β1of MIA-administered rats are presented in Figure 4E. MIA-induced osteoarthritic rats showed a significant (P < 0.05) increase in joint TGF-β1 protein expression in comparison with normal non-arthritic rats. Supplying the MIA-induced osteoarthritic rats with either BMMSCs and/or HA by intra-articular injection significantly (P < 0.05) resulted in TGF-β1 protein expression downregulation.
Effects of BMMSCs and HA on ankle histopathological score in osteoarthritic rats
Ankle histological lesions were scored on a 0-3 scale in H&E-stained sections by a blinded pathologist. The present data revealed a significant (P < 0.05) decrease in the total histopathological score and synovitis, pannus formation, bone erosion, and cartilage destruction of osteoarthritic rats treated with BMMSCs and/or HA compared to osteoarthritic control ones (Figure 4F).
Effects of BMMSCs and HA on ankle histopathological changes in osteoarthritic rats
H&E-stained section photomicrographs of ankle joint articular tissues of non-arthritic Wistar rats revealed the absence of inflammation and intact histological structures of the joints (bones, cartilages, and fibrous joint capsules) (Figure 5A and B). Photomicrographs of osteoarthritic control rats, however, showed striking histopathological changes, including synovial swelling, hyperplasia with infiltrations of numerous mononuclear leucocytic cells (lymphocytes, macrophages, and occasionally plasma cells), extensive pannus formation, and severe destruction (Figure 5C and D).
Figure 5.
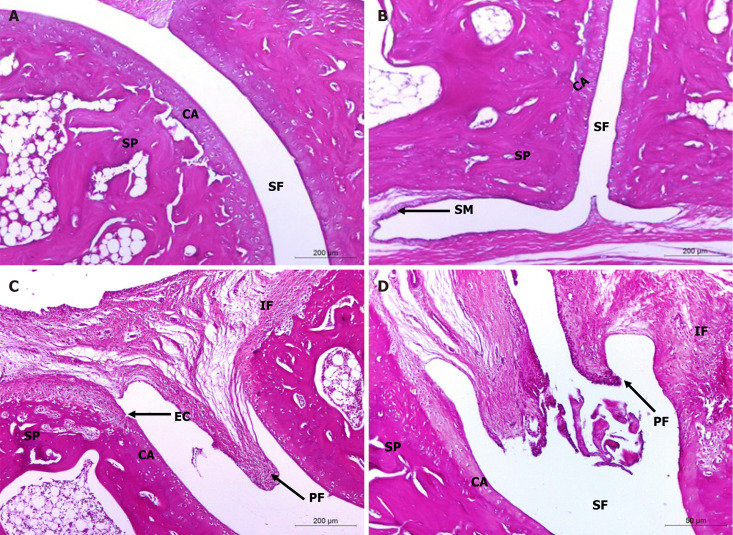
Histopathological evaluation of treatments on monosodium iodate-induced osteoarthritis in rat. A and B: Microphotographs showing the histological changes in hind ankle of normal control (photomicrographs); C and D: Osteoarthritic control rats (photomicrographs) in Hematoxylin and eosin (100 ×) stained sections. The integral histological architecture of hind ankle joints was found in normal control rats. Osteoarthritic control rats showed synovial hyperplasia, mononuclear inflammatory cell infiltration, marked pannus formation, cartilage destruction and bone erosion. CA: Articular cartilage; EC: Erosion of cartilage; IF: Inflammatory cells infiltration; PF: Pannus formation; SF: Synovial fluid; SM: Synovial membrane; SP: Spongy bone.
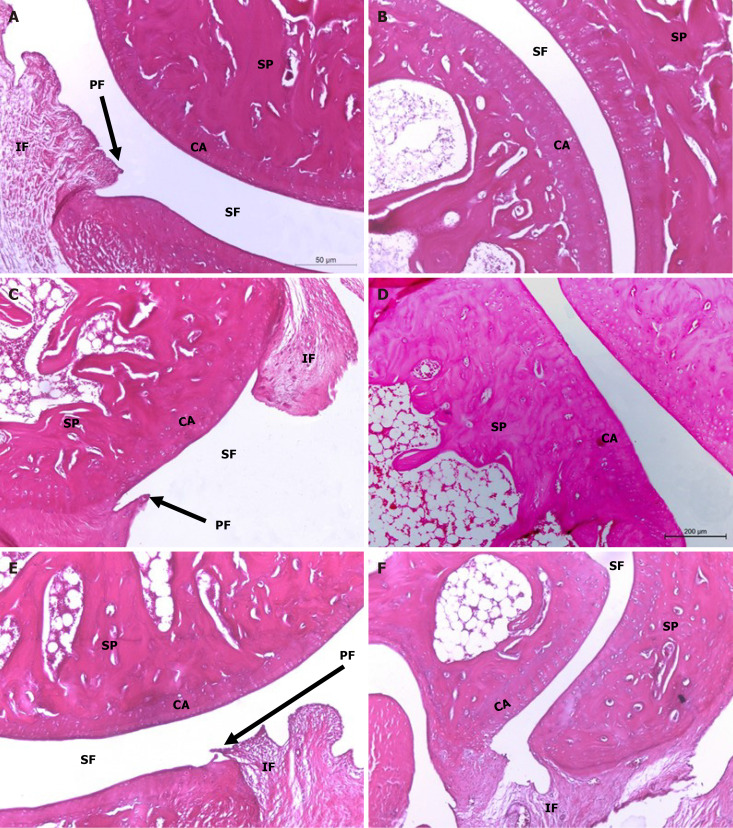
On the other hand, ankle joint photomicrographs of osteoarthritic rats supplemented with HA by intra-articular injection depicted mild signs of OA (Figure 6A and B) while those treated with BMMSCs (Figure 6C and D), as well as those concurrently treated with BMMSCs and HA (Figure 6E and F) exhibited less severe OA pathology with moderate degree of inflammation. Microscopically, osteoarthritic rats indicated inflammation of the synovial membrane (synovitis) characterized by multiplicities of synovial lining cells, in 2-3 layers as well as the proliferation of the underlying blood vessels, which was associated with peri-vascular edema and diffused cellular infiltrations consisting of mononuclear leucocytic cells. Many ankle specimens had lesions caused by inflammatory cells that spread to the surrounding muscles and connective tissue in the peri-articular region. There were modest lesions of proliferative fibroblast-like cells and synovial sloughing in several synovial membrane regions. At the edge of the articular cartilage and the level of the cartilage bone, pannus development in the form of a single or multiple proliferating granulation tissue containing hyperplastic synoviocytes and inflammatory cells was also seen. Some arthritis-affected rats' articular cartilages showed superficial fibrillation linked to cell deaths or proliferations, uneven articular surfaces, and in some cases, mid-zone articular cartilages. Additionally, osteoclast activity and fibroplasia were seen in conjunction with the destruction and degeneration of the articular bone. The HA-treated MIA-induced osteoarthritic Wistar rats exhibited milder forms of the previously known arthritic histological abnormalities. The histological lesions of arthritis in the osteoarthritic rats treated with BMMSCs and the group that received both BMMSCs and HA simultaneously exhibited mild to moderate severity.
Figure 6.
Histopathological evaluation of treatments on monosodium iodate-induced osteoarthritis in rat. Microphotographs showing the histological changes of hind ankle in hematoxylin and eosin (100 ×) stained sections. A and B: The histological picture of hind ankle joints of osteoarthritic rats treated with hyaluronic acid (HA); C and D: The histological picture of hind ankle joints of osteoarthritic rats treated with bone marrow mesenchymal stem cells (BMMSCs); E and F: Treated with BMMSCs + HA revealed improvements in the ankle joint articular tissue integrity and architecture with suppressed inflammation and reduced synovial membrane thickening. CA: Articular cartilage; EC: Erosion of cartilage; IF: Inflammatory cell infiltration; PF: Pannus formation; SF: Synovial fluid; SM: Synovial membrane; SP: Spongy bone.
Pb-stained ankle sections
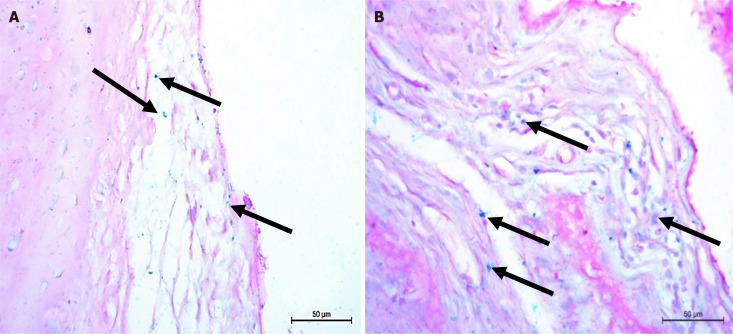
Rat ankle sections of osteoarthritic groups treated with BMMSCs showed multiple Pb positive (+ve) cells. Many stem cells were detected under the synovial membrane and within the joint capsule connective tissue and were stained blue with Pb reflecting that the cells contained iron (Figure 7).
Figure 7.
Osteoarthritic rats in bone marrow mesenchymal stem cells treated groups showing Prussian blue positive cells were distributed in the sub-synovial membrane and some positive cells between the connective tissue fibers and joint capsule (arrows) (Prussian blue, 400 ×).
DISCUSSION
Due to its short duration, simple measures, and similarities to human OA, MIA-induced OA is a popular experimental animal model for preclinical research, and has a high degree of validity for evaluating the anti-osteoarthritic properties of various agents[19]. On day 3, the mean clinical score for arthritis of the injected ankle was the greatest in the present study. Edema at the injection site decreased over time, and the lowest swelling was observed in the 6th week. These findings were consistent with those reported by Ma et al[20] in a previous investigation[20]. At the end of the experiment, the BMMSC-, HA-, and BMMSC + HA-treated rats were compared to the OA control.
The right hind leg paw circumference was used as a measure of paw edema and gross swelling rate. Typically, the anti-inflammatory effects of OA are assessed via changes in paw volume[21]. Compared to rats in the normal group, all osteoarthritic control and osteoarthritic-treated rats displayed considerable swelling of the injected ankle 3 days after MIA injection. Treatment with BMMSC, HA, or BMMSC + HA effectively decreased the enlarged right hind paw circumference in osteoarthritic rats. The reduction in circumference resulting from treatment with BMMSC and HA reflects a reduction in the swelling rate, which could be attributed to improved edema, attenuation of the inflammatory process, and suppression of synovial hyperplasia, as confirmed by the histopathological results of joints in this study and previous investigations[22,23].
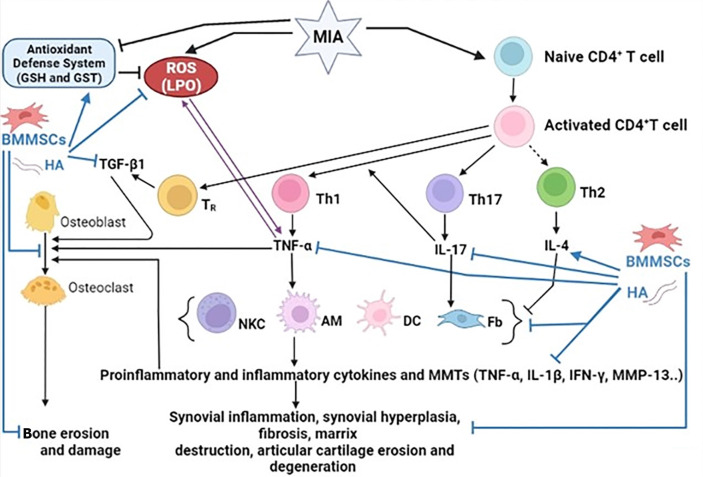
Inflammation and inflammatory responses are essential contributors to the development and progression of OA. Pro-inflammatory and inflammatory cytokines have long been recognized as important mediators in the development and progression of OA[23-26]. The dysregulation of these CD4 + T cell cytokines plays a crucial role in the pathogenesis of OA (Figure 8). T helper 1 (Th1) cells release pro-inflammatory cytokines, such as TNF-α, IL-1β, interferon gamma, IL-2, IL-12, IL-6, and IL-8, whereas Th2 cells release anti-inflammatory cytokines such as IL-4, IL-5, IL-10, and IL-13[27]. In addition, Th17 cells secrete the proinflammatory cytokine, IL-17. In the current study, the osteoarthritic rats exhibited a profound elevation in the serum concentrations of Th1 cytokine (TNF-α) and Th17 cytokine (IL-17) and a significant depletion in Th2 cytokine (IL-4) level after 6 weeks of MIA injection in comparison with those in the normal control. These findings confirmed the dominance of Th1 and Th17 on Th2 in MIA-induced OA (Figure 8) and are in similar to the result by Li et al[28], who revealed that after MIA treatment, serum TNF-α and IL-17 expression was considerably higher in rats with OA than in healthy rats. The increased amounts of pro-inflammatory cytokines may indicate their important impact on the pathophysiology of experimental arthritis and the immunomodulatory and regenerative roles of MSCs and their derived exosomes in rheumatoid arthritis (RA) pathology[24,25]. TNF-α is a key inflammatory mediator implicated in the induction of chronic inflammatory conditions[29] and has been reported to be linked to the progression of OA and RA[4,28,30]. IL-17 can cause matrix damage and inflammation in patients with OA[31]. The present results are also in accordance with those of Pal et al[30], Nazir et al[32], and Zhang et al[33], who reported a significant decrease in IL-4 expression in arthritic rats compared to that in normal controls.
Figure 8.
Schematic figure showing mechanisms of actions of bone marrow mesenchymal stem cells and hyaluronic acid in improving osteoarthritis in Wistar rats. BMMSCs: Bone marrow mesenchymal stem cells; GSH: Glutathione; GST: Glutathione S-transferase; HA: Hyaluronic acid; IL-17: Interleukin-17; LPO: Lipid peroxidation; MIA: Monosodium iodate; MMP-13: Matrix metalloproteinase 13; ROS: Reactive oxygen species; TGF-β1: Transforming growth factor beta 1; TNF-α: Tumor necrosis factor alpha.
In the present study, the administration of BMMSC, HA, and BMMSC + HA to osteoarthritic rats significantly downregulated the elevated levels of pro-inflammatory cytokines, TNF-α and IL-17, and upregulated the anti-inflammatory cytokines, including IL-4. These findings confirmed the dominance of Th2 over Th1 and Th17 cells in osteoarthritic rats treated with BMMSC, HA, or BMMSC + HA, which have anti-inflammatory effects (Figure 8). Despite the effects of BMMSCs, HA, and BMMSCs + HA on serum IL-4 levels being similar, the effects of BMMSCs and HA on serum TNF-α and IL-17 levels were more efficient than their combination. When Th2 cells are predominant in inflamed tissues, high amounts of IL-4 are produced to counteract the inflammatory effects by lowering the production and activity of pro-inflammatory cytokines. IL-4 is thought to have chondroprotective properties by reducing proteoglycan breakdown and MMP production in articular cartilage[34]. The results in the present study are consistent with Ahmed et al[4] who revealed a significant downregulation of ankle TNF-α expression and serum TNF-α level, and with Abd-Elhalem et al[35] and Endrinaldi et al[36], who reported a significant rise in IL-4 expression and blood levels in arthritic rats treated with MSCs. Furthermore, Mao et al[37] reported that MSC therapy successfully suppresses IL-17 expression in mice with collagen-induced arthritis, suggesting that MSCs may achieve their immunosuppressive activity by downregulating IL-17 expression. In concurrence with the presented study, Wang et al[38] also found that the treatment of osteoarthritic rats with HA and human amniotic MSCs separately or in combination effectively reduced TNF-α levels and increased IL-4 levels in plasma and synovial fluid in the knee of osteoarthritic rats after 28 days and 56 days after MIA injection. Overall, these findings show that the three therapies in the current study reduced MIA-induced inflammation during OA by downregulating pro-inflammatory mediators and increasing anti-inflammatory effects (Figure 8).
Oxidative stress appears to play a role in the pathogenesis of OA. Oxidative stress affects both cells and the extracellular matrix. In the presence of antioxidant defense system attenuation, the elevated levels of reactive oxygen species (ROS) signal disease development[39]. Several studies have found that ROS not only can oxidize and thereby cause damage to many of the components of a joint but also play a role in regulating different inflammatory processes that contribute to the disease's etiology (Figure 8)[40].
The level of LPO, a marker of a degenerative process in which oxidative stress destroys cell membranes and other lipid-containing structures, increased considerably in the serum of osteoarthritic control rats compared to the normal group in the current study. Many studies have demonstrated that the amount of LPO is elevated in MIA-induced OA, which is consistent with our findings[40].
GSH and GST, are endogenous antioxidant systems that protect cells from free radicals and avoid oxidative damage by neutralizing ROS before other molecules may become targets[41]. In the current study, a significant reduction in the levels of these antioxidants in osteoarthritic rats provided substantial evidence for the role of oxidative damage in MIA-induced OA. GSH is an important antioxidant since it has been proven to have a vital function in cellular resistance to oxidative stress. It is endogenously produced in the liver and serves as the initial line of defense against peroxidation. Consequently, in chronic arthritis, GSH appears to be a reflux mechanism that protects against extracellular free radicals. The osteoarthritic group showed a substantial drop in serum GSH levels in the current investigation. This is consistent with the results of previous studies[42]. GST is one of the most important elements in the cellular defense against oxidative stress[42]. In the current study, we discovered that GST activity was significantly reduced in MIA-induced osteoarthritic rats compared to that in normal rats. Similar effects on GST and other antioxidant enzyme levels were noted by Hamdalla et al[42] in the serum of rats with OA, which was associated with an increase in MIA levels as a marker of LPO. Due to enhanced turnover to detoxify extra lipoperoxidation products, GST activity was lower in MIA-induced OA.
Treatment with BMMSC, HA, and BMMSC plus HA resulted in profound downregulation of LPO levels in the current investigation; the combined effects of BMMSC plus HA seemed to be the most potent. Compared to the osteoarthritic control group, GSH content, and GST activity were dramatically improved as a result of treatment with BMMSC, HA, and BMMSC + HA, and increased to near-normal levels. These findings are in agreement with those of previous studies showing that MSCs improved the oxidative stress environment by lowering LPO and increasing GSH levels[35,43]. According to Aniss et al[22], HA treatment decreases oxidative stress and boosts the antioxidant defense system, which is consistent with the findings of the present study. The TNF-α-stimulated gene/proteins generated by MSCs, which reduce ROS production and, as a result, reduce chronic inflammation, are thought to play a role in the antioxidant function of MSCs. Other studies showed that HA decreased the harmful effects of ROS and reactive nitrogen species on mitochondrial DNA integrity and repair, ATP generation, and cell survival[44].
Numerous variables contributing to the pathophysiology of OA have been identified. Degradation of the extracellular matrix, which is a direct result of the activity of matrix-degrading enzymes, is a characteristic of OA. By reducing the extracellular matrix, MMPs, such as MMP13, are important enzymes that target cartilage for breakdown[45]. This MMP is produced in response to inflammatory mediators found in tissues and OA joint fluid, such as IL-1 and TNF-α (Figure 8). MMP13 mRNA expression was considerably higher in the joints of MIA-OA control rats than that in our study compared to normal rats. This is consistent with the findings of[46], who reported that high MMP-13 levels in MIA-induced OA imply ongoing cartilage disintegration. The enhanced inflammatory cytokines, such as TNF-α, which were demonstrated in the current study, may have stimulatory effects that led to the rise in MMP-13 expression. BMMSC, HA, and BMMSC + HA substantially decreased the elevated MMP-13 mRNA synthesis in the joints of osteoarthritic rats. These findings are consistent with those of several previous studies. MMP-13 mRNA expression was dramatically reduced in rats with OA following MSC therapy, according to Li et al[47]. By limiting the expression of enzymes that break down the extracellular matrix, other researchers found that administering HA to OA-affected rats led to a substantial decrease in the mRNA expression levels of MMP-3, MMP-9, and MMP-13[48].
TGF-β is a cytokine that affects both healthy and OA-affected joints. However, the function of this cytokine in normal healthy joints differs significantly from that in OA-affected joints[49]. TGF-1β signaling plays a crucial role in the growth, remodeling, and maintenance of bone and cartilage in a healthy joint[49]. However, persistent and high levels of active TGF-β1 were noticed in OA-affected joints. Chondrocyte apoptosis and cartilage degradation in OA were reported to be caused by mechanical stress-induced upregulation of TGF-β1 (Figure 8). Multiple intra-articular injections of TGF-1β, although it appears to enhance chondrogenesis, may also worsen OA[50]. When compared to control rats in the current investigation, MIA-induced osteoarthritic rats had a significantly higher production of the TGF-β1 protein in the joints. This effect was similar to the one observed by Waly et al[51], who reported that serum TGF-β1 levels were significantly elevated in the OA group after MIA administration. When compared to osteoarthritic control rats, the administration of BMMSC and/or HA to MIA-induced rats dramatically reduced the production of the TGF-β1 protein. This is consistent with the findings of a prior study by Abdelmawgoud and Saleh[43], who found that injecting MSCs into arthritic rats led to a rapid reversal of tissue inflammation and a decreased tissue level of TNF-α and TGF-β1. This drastic effect was accompanied by a reduced tissue level of TNF-α and TGF-β1. The current findings are in line with those of the study by Kanazawa et al[52], which showed that TGF-β1 was much lower in the group of arthritis sufferers treated with HA than in the group of arthritis sufferers in the control condition. Treatment with BMMSC plus HA resulted in a decrease in the elevated TGF-β1 protein expression, but it is still significantly higher than normal. It can be suggested that the decrease in TGF-β1 protein expression following treatment of osteoarthritic rats with BMMSCs and HA could have an important impact on the prevention of chondrocyte apoptosis and cartilage damage (Figure 8).
In the present study, rats with MIA-induced OA exhibited detrimental histological alterations in their ankle joints, including synovial hyperplasia, degraded articulating cartilage, and pannus development. These histological alterations may be brought on by an increase in oxidative stress, a weakened antioxidant defense system, an increase in pro-inflammatory and inflammatory cytokines (TNF-α and IL-17), and a reduction in anti-inflammatory cytokine levels (IL-4). Histopathological analysis of rats administered MIA revealed periarticular inflammation, severe synovitis, bone resorption, and cartilage degradation. These findings are consistent with prior research that found degenerative articular cartilage alterations in the MIA group (e.g., synovial thickening, cartilage loss, and osteophyte development)[53]. These results are consistent with those of Jimbo et al[54] and Chiang et al[3], who identified the potential anti-osteoarthritic effects of BMMSCs. Treatment with BMMSC and/or HA was associated with a significant reduction in joint cartilage and synovial membrane inflammation, as well as a significant restoration of histopathological alterations compared to the osteoarthritic control group. Lower levels of pro-inflammatory cytokines (TNF-α and IL-17), MMP-13, and greater levels of anti-inflammatory cytokines were associated with these advantages (IL-4). According to our findings, all treatments significantly reduced paw edema, arthritic scores, and system and ankle measurements. The enhancement of the antioxidant defense system and repression of oxidative stress and inflammation may be responsible for these advantages (Figure 8).
Compared to osteoarthritic rats, treatment with HA decreased the severity of MIA-induced OA by inhibiting inflammation, pannus development, cartilage degradation, and bone resorption. This result was similar to that reported by Jimbo et al[54], who discovered that HA has chondroprotective properties. Histological analysis showed that cartilage degradation was considerably decreased in MIA-induced osteoarthritic rats treated with HA compared to that in MIA-induced osteoarthritic control rats. Intra-articular HA injections are also thought to cause synovial cells to secrete endogenous HA, thereby increasing the joint fluid viscosity. Another proposed mechanism involves the chondroprotective effects of HA and aggrecan when used together. This results in the formation of strongly negatively charged aggregates that absorb water and improve cartilage durability. The biochemical actions of HA prevent the inflammatory process from building up to the point at which it destroys the joints[54]. In contrast to our findings, Chiang et al[3] found that HA alone had no meaningful therapeutic advantage in slowing the progression of OA. This ineffectiveness may be attributed to the use of a suboptimal HA dosage for cartilage regeneration, single-dose injections, or a long interval between OA induction and HA injection.
In addition, when MIA-induced osteoarthritic rats were treated with a combination of HA and BMMSC for 6 weeks following MIA induction, OA development was significantly reduced compared to osteoarthritic rats. These findings suggest that intra-articular injections of BMMSCs paired with HA may effectively slow the development of OA and promote cartilage repair. These findings corroborate those of Chiang et al[3], who found that combining HA with MSCs effectively suppressed OA-related histological alterations in rabbits. According to Wang et al[38], HA synergistically enhances the effects of MSCs on cartilage healing. This restorative process is thought to occur because the addition of HA to MSCs boosts their overall activity, including chondrogenic differentiation, proliferation, colonization, and regenerative modulation. Astachov et al[55] found that hyaluronan had complex effect on MSC during intra-articular injections of HA-MSCs. While, Choong et al[56] found that HA has no additional effects on MSC during intra-articular injections. The local tissue environment is responsible for the transition of MSCs into functional lineage-committed cells, such as chondrocytes. This may explain why BMMSC did not deliver the predicted improved regeneration potential in the HA-BMMSC combination. The combination of the two had no extra impact on the ability of BMMSCs to heal injured cartilage, as indicated by the fact that the histopathological scores in the HA-BMMSC group were not statistically different from those independently treated with HA and BMMSCs in the current investigation.
CONCLUSION
The current findings demonstrated that BMMSCs and HA have substantial anti-inflammatory, antioxidant, and anti-arthritic effects in Wistar rats with MIA-induced OA. When BMMSC and HA were administered together, there were no further preventative or therapeutic effects of either BMMSC or HA against MIA-induced OA in Wistar rats, suggesting that the use of either HA or BMMSCs alone successfully lowered OA development. The anti-osteoarthritic actions of BMMSC and/or HA may be mediated by their ability to regulate inflammation and oxidative stress as well as diminish MMP-13 production. However, further research is required to determine the efficacy and safety of BMMSCs and HA in the treatment of OA in both animals and humans.
ACKNOWLEDGEMENTS
The authors are thankful to Professor Laila Rashed, Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt for performing Western blot analysis and Professor Dina Sabry, Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt for performing qRT-PCR analysis. The author appreciates the help of the Department of Chemistry and Basic and Applied Scientific Research Center (BASRC), at Imam Abdul Rahman Bin Faisal University.
Footnotes
Institutional animal care and use committee statement: All animal approaches and the experiment are in harmony with the standard guidelines of the Institutional Animal Care and Use Committee, Beni-Suef University, Beni-Suef, Egypt, No. BSU/020-107.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: Saudi Arabia
Peer-review report’s classification
Scientific Quality: Grade B, Grade D
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Che JM; Zhang C S-Editor: Li L L-Editor: Filipodia P-Editor: Zhao YQ
Contributor Information
Usama Ismaeil Hagag, Department of Surgery, Beni-Suef University, Beni Suef 62111, Egypt.
Fatma Mohamed Halfaya, Department of Surgery, Anesthesiology and Radiology, Beni-Suef University, Beni Suef 62111, Egypt.
Hessah Mohammed Al-Muzafar, Department of Chemistry, College of Science, Basic and Applied Scientific Research Center, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia.
Suhailah Saud Al-Jameel, Department of Chemistry, College of Science, Basic and Applied Scientific Research Center, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia.
Kamal Adel Amin, Department of Chemistry, Biochemistry, College of Science, Basic and Applied Scientific Research Center (BASRC), Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia. kaothman@iau.edu.sa.
Wael Abou El-Kheir, Department of Immunology, Military Medical Academy, Cairo 11511, Al Qāhirah, Egypt.
Emad A Mahdi, Department of Pathology, Beni-Suef University, Beni Suef 62111, Egypt.
Gamal Abdel-Nasser Ragab Hassan, Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Beni-Suef University, Beni Suef 62521, Egypt.
Osama Mohamed Ahmed, Physiology Division, Department of Zoology, Faculty of Science, Beni-Suef University, Beni-Suef 62521, Egypt.
Data sharing statement
No additional data are available.
References
- 1.Fan MP, Si M, Li BJ, Hu GH, Hou Y, Yang W, Liu L, Tang B, Nie L. Cell therapy of a knee osteoarthritis rat model using precartilaginous stem cells. Eur Rev Med Pharmacol Sci. 2018;22:2119–2125. doi: 10.26355/eurrev_201804_14745. [DOI] [PubMed] [Google Scholar]
- 2.Kimmerling KA, Gomoll AH, Farr J, Mowry KC. Amniotic Suspension Allograft Modulates Inflammation in a Rat Pain Model of Osteoarthritis. J Orthop Res. 2020;38:1141–1149. doi: 10.1002/jor.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. Allogeneic Mesenchymal Stem Cells in Combination with Hyaluronic Acid for the Treatment of Osteoarthritis in Rabbits. PLoS One. 2016;11:e0149835. doi: 10.1371/journal.pone.0149835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed EA, Ahmed OM, Fahim HI, Ali TM, Elesawy BH, Ashour MB. Potency of Bone Marrow-Derived Mesenchymal Stem Cells and Indomethacin in Complete Freund's Adjuvant-Induced Arthritic Rats: Roles of TNF-α, IL-10, iNOS, MMP-9, and TGF-β1. Stem Cells Int. 2021;2021:6665601. doi: 10.1155/2021/6665601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragab GH, Halfaya FM, Ahmed OM, Abou El-Kheir W, Mahdi EA, Ali TM, Almehmadi MM, Hagag U. Platelet-Rich Plasma Ameliorates Monosodium Iodoacetate-Induced Ankle Osteoarthritis in the Rat Model via Suppression of Inflammation and Oxidative Stress. Evid Based Complement Alternat Med. 2021;2021:6692432. doi: 10.1155/2021/6692432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. 2021;13:448–461. [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang XN, Zhu SY, He HC, Yu X, Xu Y, He CQ. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022;13:14. doi: 10.1186/s13287-021-02689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuenschwander HM, Moreira JJ, Vendruscolo CP, Fülber J, Seidel SRT, Michelacci YM, Baccarin RYA. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J Vet Sci. 2019;20:e67. doi: 10.4142/jvs.2019.20.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, Haugen IK, Herrero-Beaumont G, Jonsson H, Kjeken I, Maheu E, Ramonda R, Ritt MJ, Smeets W, Smolen JS, Stamm TA, Szekanecz Z, Wittoek R, Carmona L. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16–24. doi: 10.1136/annrheumdis-2018-213826. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Jiang H, Yang H. In vitro culture of bone marrow mesenchymal stem cells in rats and differentiation into retinal neural-like cells. J Huazhong Univ Sci Technolog Med Sci. 2007;27:598–600. doi: 10.1007/s11596-007-0531-1. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed OM, Hassan MA, Saleh AS. Combinatory effect of hesperetin and mesenchymal stem cells on the deteriorated lipid profile, heart and kidney functions and antioxidant activity in STZ-induced diabetic rats. Biocell. 2020;44:27–29. [Google Scholar]
- 12.Aboul-Fotouh GI, Zickri MB, Metwally HG, Ibrahim IR, Kamar SS, Sakr W. Therapeutic Effect of Adipose Derived Stem Cells versus Atorvastatin on Amiodarone Induced Lung Injury in Male Rat. Int J Stem Cells. 2015;8:170–180. doi: 10.15283/ijsc.2015.8.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-amir Ahmad Ghanayem Y, Ahmad Ashour F, Ahmad Rashed L, Abul Hasan Zoair M, Mohammad Abd El-Hai O. The Role of Bone Marrow Derived Mesenchymal Stem Cells in Attenuation of Renal Failure in Adult Male Albino Rats. Al-Azhar Med J. 2016;45:135–148. [Google Scholar]
- 14.Suranji Wijekoon HM, Kim S, Bwalya EC, Fang J, Aoshima K, Hosoya K, Okumura M. Anti-arthritic effect of pentosan polysulfate in rats with collagen-induced arthritis. Res Vet Sci. 2019;122:179–185. doi: 10.1016/j.rvsc.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Yilgor A, Demir C. Determination of oxidative stress level and some antioxidant activities in refractory epilepsy patients. Sci Rep. 2024;14:6688. doi: 10.1038/s41598-024-57224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yassin NYS, AbouZid SF, El-Kalaawy AM, Ali TM, Almehmadi MM, Ahmed OM. Silybum marianum total extract, silymarin and silibinin abate hepatocarcinogenesis and hepatocellular carcinoma growth via modulation of the HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways. Biomed Pharmacother. 2022;145:112409. doi: 10.1016/j.biopha.2021.112409. [DOI] [PubMed] [Google Scholar]
- 17.Allam G, Mahdi EA, Alzahrani AM, Abuelsaad AS. Ellagic acid alleviates adjuvant induced arthritis by modulation of pro- and anti-inflammatory cytokines. Cent Eur J Immunol. 2016;41:339–349. doi: 10.5114/ceji.2016.65132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lours C, Cottin L, Wiber M, Andrieu V, Baccini V, Baseggio L, Brouzes C, Chatelain B, Daliphard S, Fenneteau O, Geneviève F, Girard S, Leymarie V, Maloum K, Rieu JB, Sebahoun G, Sudaka I, Troussard X, Wagner-Ballon O, Wuilleme S, Bardet V, Lesesve JF. Perls' Stain Guidelines from the French-Speaking Cellular Hematology Group (GFHC) Diagnostics (Basel) 2022;12:1698. doi: 10.3390/diagnostics12071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.South S, Crabtree K, Vijayagopal P, Averitt D, Juma S. Dose Dependent Effects of Whole Blueberry on Cartilage Health and Pain in a Monosodium Iodoacetate (MIA) Induced Rat Model of Osteoarthritis. Cur Dev Nutr. 2020;4:nzaa045_110. [Google Scholar]
- 20.Ma Y, Guo H, Bai F, Zhang M, Yang L, Deng J, Xiong L. A rat model of knee osteoarthritis suitable for electroacupuncture study. Exp Anim. 2018;67:271–280. doi: 10.1538/expanim.17-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gui B, Zhang J, Wang S, Rong G. Anti-Osteoarthritic and Anti-Inflammatory Activities of Diazine: In Vitro and In Vivo Studies. Med Sci Monit. 2018;24:76–83. doi: 10.12659/MSM.905661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aniss NN, Zaazaa AM, Saleh MRA. Anti-arthritic Effects of Platelets Rich Plasma and Hyaluronic Acid on Adjuvant-induced Arthritis in Rats. Int J Pharmacol. 2019;16:33–46. [Google Scholar]
- 23.Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, Rod E, Čukelj F, Vrdoljak T, Vidović D, Starešinić M, Sabalić S, Dobričić B, Petrović T, Antičević D, Borić I, Košir R, Zmrzljak UP, Primorac D. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22179208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jrad AIS, Trad M, Bzeih W, El Hasbani G, Uthman I. Role of pro-inflammatory interleukins in osteoarthritis: a narrative review. Connect Tissue Res. 2023;64:238–247. doi: 10.1080/03008207.2022.2157270. [DOI] [PubMed] [Google Scholar]
- 25.Sardana Y, Bhatti GK, Singh C, Sharma PK, Reddy PH, Bhatti JS. Progression of pre-rheumatoid arthritis to clinical disease of joints: Potential role of mesenchymal stem cells. Life Sci. 2023;321:121641. doi: 10.1016/j.lfs.2023.121641. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Feng X, Zhang H, Wei Y, Yang Y, Tian Y, Bai L. Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect Tissue Res. 2021;62:508–518. doi: 10.1080/03008207.2020.1780218. [DOI] [PubMed] [Google Scholar]
- 27.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4⁺T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Xie S, Qi Y, Li H, Zhang R, Lian Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp Ther Med. 2018;16:4737–4744. doi: 10.3892/etm.2018.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han L, Song JH, Yoon JH, Park YG, Lee SW, Choi YJ, Nam SW, Lee JY, Park WS. TNF-α and TNF-β Polymorphisms are Associated with Susceptibility to Osteoarthritis in a Korean Population. Korean J Pathol. 2012;46:30–37. doi: 10.4132/KoreanJPathol.2012.46.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal RR, Rajpal V, Singh N, Singh S, Mishra N, Singh P, Maurya P, Alka, Saraf SA. Downregulation of pro-inflammatory markers IL-6 and TNF-α in rheumatoid arthritis using nano-lipidic carriers of a quinone-based phenolic: an in vitro and in vivo study. Drug Deliv Transl Res. 2023;13:627–641. doi: 10.1007/s13346-022-01221-7. [DOI] [PubMed] [Google Scholar]
- 31.Xiao J, Zhang P, Cai FL, Luo CG, Pu T, Pan XL, Tian M. IL-17 in osteoarthritis: A narrative review. Open Life Sci. 2023;18:20220747. doi: 10.1515/biol-2022-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazir S, Ahmad I, Mobashar A, Sharif A, Shabbir A, Chaudhary WA. Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete Freund's adjuvant-induced arthritic rats. Front Pharmacol. 2023;14:1346054. doi: 10.3389/fphar.2023.1346054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Yang Y, Li X, Zhang H, Gang Y, Bai L. Alterations of autophagy in knee cartilage by treatment with treadmill exercise in a rat osteoarthritis model. Int J Mol Med. 2019;43:336–344. doi: 10.3892/ijmm.2018.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Meegeren ME, Roosendaal G, Jansen NW, Wenting MJ, van Wesel AC, van Roon JA, Lafeber FP. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage. 2012;20:764–772. doi: 10.1016/j.joca.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Abd-Elhalem SS, Haggag NZ, El-Shinnawy NA. Bone marrow mesenchymal stem cells suppress IL-9 in adjuvant-induced arthritis. Autoimmunity. 2018;51:25–34. doi: 10.1080/08916934.2018.1428956. [DOI] [PubMed] [Google Scholar]
- 36.Endrinaldi E, Darwin E, Zubir N, Revilla G. The Effect of Mesenchymal Stem Cell Wharton's Jelly on Matrix Metalloproteinase-1 and Interleukin-4 Levels in Osteoarthritis Rat Model. Open Access Maced J Med Sci. 2019;7:529–535. doi: 10.3889/oamjms.2019.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao F, Xu WR, Qian H, Zhu W, Yan YM, Shao QX, Xu HX. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219–225. doi: 10.1007/s00011-009-0090-y. [DOI] [PubMed] [Google Scholar]
- 38.Wang AT, Zhang QF, Wang NX, Yu CY, Liu RM, Luo Y, Zhao YJ, Xiao JH. Cocktail of Hyaluronic Acid and Human Amniotic Mesenchymal Cells Effectively Repairs Cartilage Injuries in Sodium Iodoacetate-Induced Osteoarthritis Rats. Front Bioeng Biotechnol. 2020;8:87. doi: 10.3389/fbioe.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada EF, Salgueiro AF, Goulart ADS, Mendes VP, Anjos BL, Folmer V, da Silva MD. Evaluation of monosodium iodoacetate dosage to induce knee osteoarthritis: Relation with oxidative stress and pain. Int J Rheum Dis. 2019;22:399–410. doi: 10.1111/1756-185X.13450. [DOI] [PubMed] [Google Scholar]
- 40.Zahan OM, Serban O, Gherman C, Fodor D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Rep. 2020;93:12–22. doi: 10.15386/mpr-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Gao J, Lu L, Bold T, Li X, Wang S, Chang Z, Chen J, Kong X, Zheng Y, Zhang M, Tang J. Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles. NanoImpact. 2021;23:100338. doi: 10.1016/j.impact.2021.100338. [DOI] [PubMed] [Google Scholar]
- 42.Hamdalla HM, Ahmed RR, Galaly SR, Ahmed OM, Naguib IA, Alghamdi BS, Abdul-Hamid M. Assessment of the Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells against a Monoiodoacetate-Induced Osteoarthritis Model in Wistar Rats. Stem Cells Int. 2022;2022:1900403. doi: 10.1155/2022/1900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdelmawgoud H, Saleh A. Anti-inflammatory and antioxidant effects of mesenchymal and hematopoietic stem cells in a rheumatoid arthritis rat model. Adv Clin Exp Med. 2018;27:873–880. doi: 10.17219/acem/73720. [DOI] [PubMed] [Google Scholar]
- 44.He Z, Hua J, Qian D, Gong J, Lin S, Xu C, Wei G, Meng H, Yang T, Zhou B, Song Z. Intravenous hMSCs Ameliorate Acute Pancreatitis in Mice via Secretion of Tumor Necrosis Factor-α Stimulated Gene/Protein 6. Sci Rep. 2016;6:38438. doi: 10.1038/srep38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Santis M, Di Matteo B, Chisari E, Cincinelli G, Angele P, Lattermann C, Filardo G, Vitale ND, Selmi C, Kon E. The Role of Wnt Pathway in the Pathogenesis of OA and Its Potential Therapeutic Implications in the Field of Regenerative Medicine. Biomed Res Int. 2018;2018:7402947. doi: 10.1155/2018/7402947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi DJ, Choi SI, Choi BR, Lee YS, Lee DY, Kim GS. Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats. BMC Complement Altern Med. 2019;19:325. doi: 10.1186/s12906-019-2746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Zhu X, Shao Q, Xu F, Sun G. Allogeneic adipose-derived stem cell transplantation on knee osteoarthritis rats and its effect on MMP-13 and DDR2. Exp Ther Med. 2019;18:99–104. doi: 10.3892/etm.2019.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SE, Lee JY, Shim KS, Lee S, Min K, Bae JH, Kim HJ, Park K, Song HR. Attenuation of inflammation and cartilage degradation by sulfasalazine-containing hyaluronic acid on osteoarthritis rat model. Int J Biol Macromol. 2018;114:341–348. doi: 10.1016/j.ijbiomac.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 49.Fang J, Xu L, Li Y, Zhao Z. Roles of TGF-beta 1 signaling in the development of osteoarthritis. Histol Histopathol. 2016;31:1161–1167. doi: 10.14670/HH-11-779. [DOI] [PubMed] [Google Scholar]
- 50.Seidel SRT, Vendruscolo CP, Moreira JJ, Fülber J, Ottaiano TF, Oliva MLV, Michelacci YM, Baccarin RYA. Does Double Centrifugation Lead to Premature Platelet Aggregation and Decreased TGF-β1 Concentrations in Equine Platelet-Rich Plasma? Vet Sci. 2019;6:68. doi: 10.3390/vetsci6030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waly NE, Refaiy A, Aborehab NM. IL-10 and TGF-β: Roles in chondroprotective effects of Glucosamine in experimental Osteoarthritis? Pathophysiology. 2017;24:45–49. doi: 10.1016/j.pathophys.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Kanazawa K, Hagiwara Y, Tsuchiya M, Yabe Y, Sonofuchi K, Koide M, Sekiguchi T, Itaya N, Ando A, Saijo Y, Itoi E. Preventing effects of joint contracture by high molecular weight hyaluronan injections in a rat immobilized knee model. Int J Clin Exp Pathol. 2015;8:3426–3440. [PMC free article] [PubMed] [Google Scholar]
- 53.Ängeby Möller K, Klein S, Seeliger F, Finn A, Stenfors C, Svensson CI. Monosodium iodoacetate-induced monoarthritis develops differently in knee versus ankle joint in rats. Neurobiol Pain. 2019;6:100036. doi: 10.1016/j.ynpai.2019.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimbo S, Terashima Y, Teramoto A, Takebayashi T, Ogon I, Watanabe K, Sato T, Ichise N, Tohse N, Yamashita T. Antinociceptive effects of hyaluronic acid on monoiodoacetate-induced ankle osteoarthritis in rats. J Pain Res. 2019;12:191–200. doi: 10.2147/JPR.S186413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Astachov L, Vago R, Aviv M, Nevo Z. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Biosci (Landmark Ed) 2011;16:261–276. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 56.Choong PF, Jee CSY, Leong CF, Cheong SK. Effect of hyaluronan on mesenchymal stem cells. Med J Malaysia. 2010;65 Suppl B:80–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.