Abstract
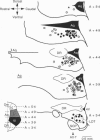
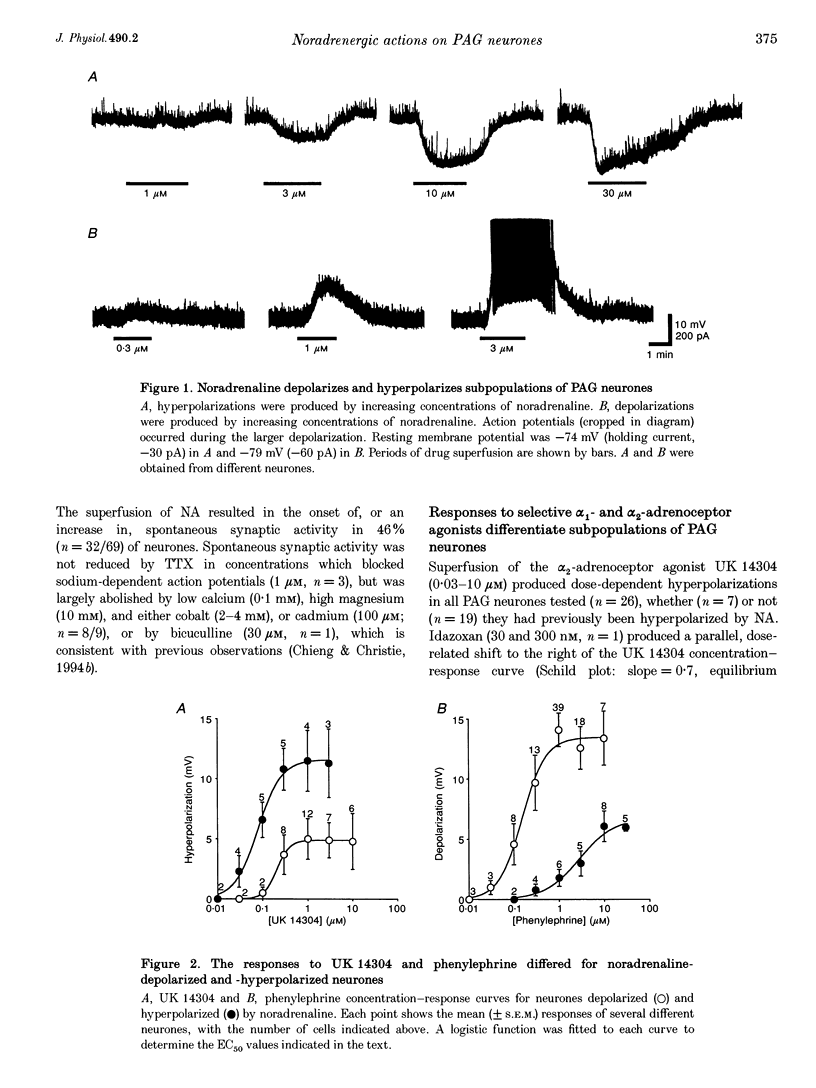
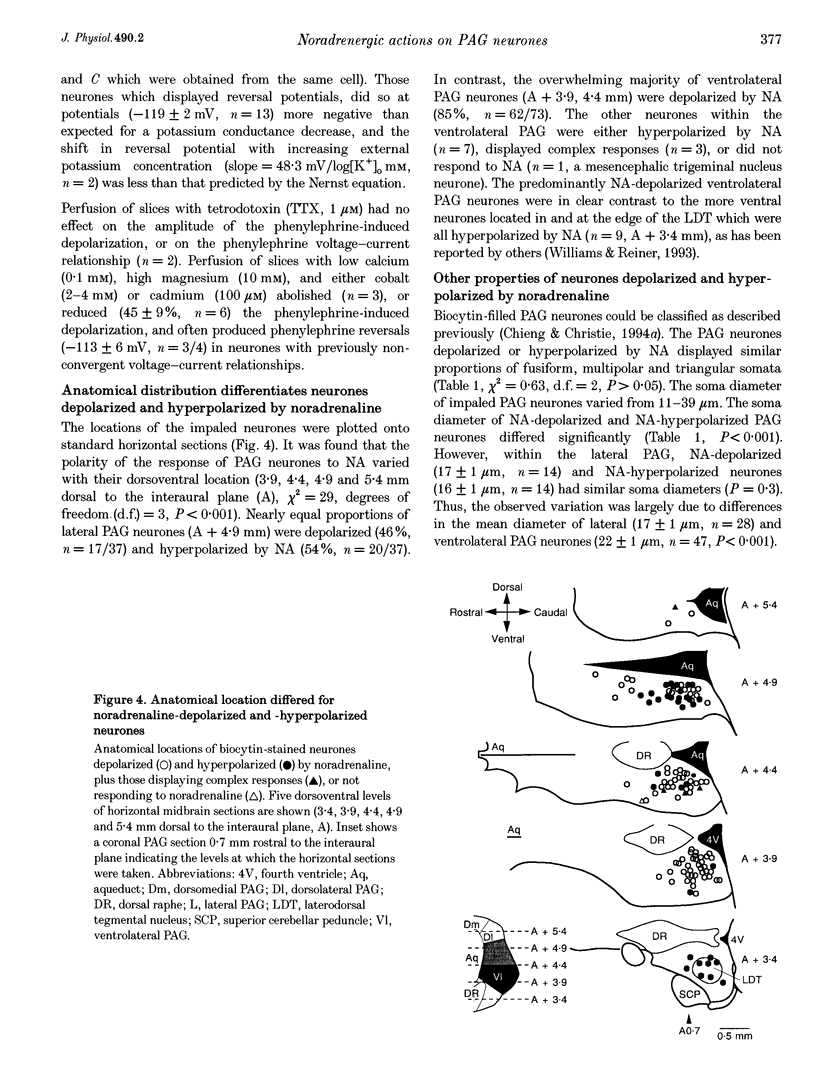
1. The action of noradrenaline on the membrane properties of rat periaqueductal grey (PAG) neurones was examined using intracellular recordings in brain slices maintained in vitro. Morphological properties and the anatomical location of neurones were characterized by use of intracellular staining within biocytin. 2. Noradrenaline (0.3-100 microM) depolarized 66% (81/123) and hyperpolarized 30% (37/123) of neurones. The alpha 1- and alpha 2-adrenoceptor agonists phenylephrine and UK 14304 produced depolarizations and hyperpolarizations in all PAG neurones tested, respectively. Neurones depolarized by noradrenaline were more responsive to phenylephrine, whereas neurones hyperpolarized by noradrenaline were more responsive to UK 14304. 3. The UK 14304-induced hyperpolarizations reversed polarity at -108 +/- 2 mV (n = 11). The reversal potential increased when the extracellular potassium concentration was raised (slope = 57.8 mV/log[K+]o mM) in a manner similar to that predicted for potassium conductance. 4. The phenylephrine-induced depolarizations did not reverse polarity at negative potentials (n = 25), or did so at potentials (-119 +/- 2 mV, n = 13) more negative than the UK 14304-induced hyperpolarizations. Superfusion with low calcium (0.1 mM), high magnesium (10 mM) and either cobalt (2-4 mM), or cadmium (100 microM) usually reduced the response to phenylephrine and produced reversals near that predicted for potassium conductance. 5. The majority of the ventrolateral PAG neurones were depolarized by noradrenaline (85%, 62/73). In contrast, almost equal proportions of the lateral PAG neurones were hyperpolarized (54%, 20/37) and depolarized (46%, n = 17/37) by noradrenaline. PAG neurones depolarized or hyperpolarized by noradrenaline could not be differentiated on morphological grounds. 6. These results suggest that the net effect of noradrenaline on lateral and ventrolateral PAG neurones is to bias activity in favour of a ventrolateral PAG-mediated response pattern, which includes quiescence, hyporeactivity, hypotension and bradycardia.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasu T., Gallagher J. P., Nakamura T., Shinnick-Gallagher P., Yoshimura M. Noradrenaline hyperpolarization and depolarization in cat vesical parasympathetic neurones. J Physiol. 1985 Apr;361:165–184. doi: 10.1113/jphysiol.1985.sp015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R., Carrive P., Zhang S. P. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- Bandler R., Shipley M. T. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994 Sep;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P., Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985 Feb;84(2):561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyajian C. L., Loughlin S. E., Leslie F. M. Anatomical evidence for alpha-2 adrenoceptor heterogeneity: differential autoradiographic distributions of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987 Jun;241(3):1079–1091. [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994 Sep;113(1):121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994 Sep;113(1):303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaulis A., Keay K. A., Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90(2):307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Depaulis A., Keay K. A., Bandler R. Quiescence and hyporeactivity evoked by activation of cell bodies in the ventrolateral midbrain periaqueductal gray of the rat. Exp Brain Res. 1994;99(1):75–83. doi: 10.1007/BF00241413. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Minami T., Nabekura J., Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987 Dec;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. K., D'Angelo D. D., Zeng D. W., Lynch K. R. Pharmacological characterization of rat alpha 2-adrenergic receptors. Mol Pharmacol. 1991 Sep;40(3):407–412. [PubMed] [Google Scholar]
- Herbert H., Saper C. B. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992 Jan 1;315(1):34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- Jiang M., Chandler S. D., Ennis M., Shipley M. T., Behbehani M. M. Actions of epinephrine on neurons in the rat midbrain periaqueductal gray maintained in vitro. Brain Res Bull. 1992 Dec;29(6):871–877. doi: 10.1016/0361-9230(92)90158-t. [DOI] [PubMed] [Google Scholar]
- Jones L. S., Gauger L. L., Davis J. N. Anatomy of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985 Jan 8;231(2):190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Keay K. A., Clement C. I., Owler B., Depaulis A., Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994 Aug;61(4):727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Kwiat G. C., Basbaum A. I. Organization of tyrosine hydroxylase- and serotonin-immunoreactive brainstem neurons with axon collaterals to the periaqueductal gray and the spinal cord in the rat. Brain Res. 1990 Sep 24;528(1):83–94. doi: 10.1016/0006-8993(90)90198-k. [DOI] [PubMed] [Google Scholar]
- Larkman P. M., Kelly J. S. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol. 1992 Oct;456:473–490. doi: 10.1113/jphysiol.1992.sp019347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Dupouy B., Vincent J. D. Excitatory effect of noradrenaline on pacemaker cells in spinal cord primary cultures. Neuroscience. 1988 Feb;24(2):647–658. doi: 10.1016/0306-4522(88)90358-2. [DOI] [PubMed] [Google Scholar]
- Lomasney J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Molecular biology of alpha-adrenergic receptors: implications for receptor classification and for structure-function relationships. Biochim Biophys Acta. 1991 Oct 26;1095(2):127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993 May;40(5):631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol. 1986 Mar;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- McCune S. K., Voigt M. M., Hill J. M. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience. 1993 Nov;57(1):143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- Nicholas A. P., Pieribone V., Hökfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993 Feb 22;328(4):575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- North R. A., Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984 Apr;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. Z., Grudt T. J., Williams J. T. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol. 1994 Aug 1;478(Pt 3):437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone V. A., Nicholas A. P., Dagerlind A., Hökfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994 Jul;14(7):4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. R., McCarley R. W., Greene R. W. The mechanism of noradrenergic alpha 1 excitatory modulation of pontine reticular formation neurons. J Neurosci. 1994 Nov;14(11 Pt 1):6481–6487. doi: 10.1523/JNEUROSCI.14-11-06481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnerstall J. R., Kopajtic T. A., Kuhar M. J. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res. 1984 Mar;319(1):69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Grant S. J., Burkhart B. A., Christoph G. R. In vitro electrophysiology of neurons in the lateral dorsal tegmental nucleus. Brain Res Bull. 1989 Mar;22(3):557–560. doi: 10.1016/0361-9230(89)90111-1. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Reiner P. B. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J Neurosci. 1993 Sep;13(9):3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., Henderson G., North R. A. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Marshall K. C. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J Neurosci. 1987 Nov;7(11):3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Polosa C., Nishi S. Slow IPSP and the noradrenaline-induced inhibition of the cat sympathetic preganglionic neuron in vitro. Brain Res. 1987 Sep 1;419(1-2):383–386. doi: 10.1016/0006-8993(87)90613-5. [DOI] [PubMed] [Google Scholar]