Abstract
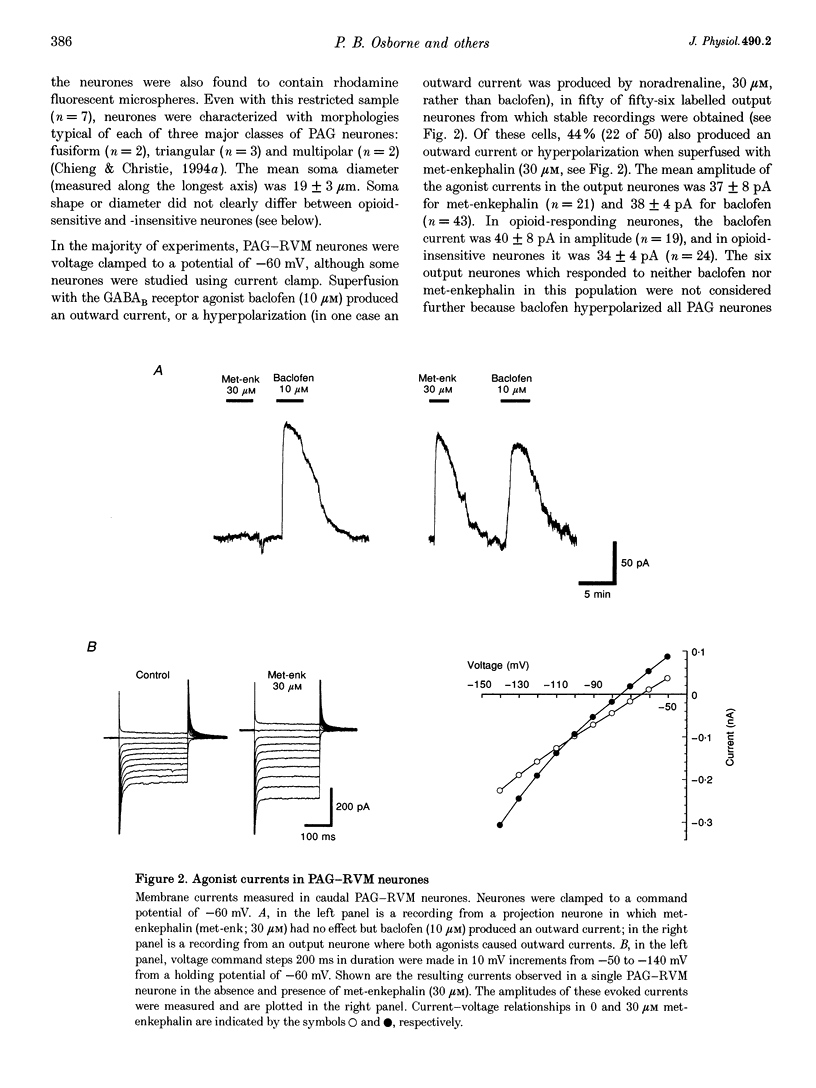
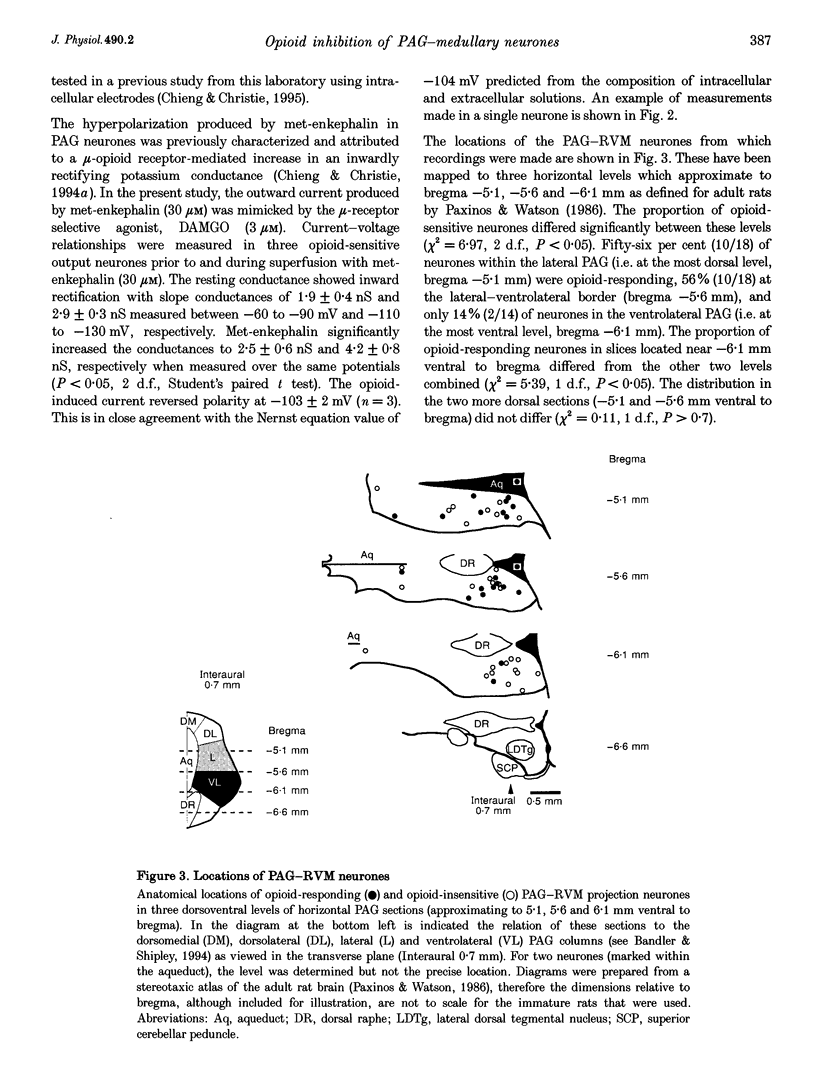
1. Rat caudal periaqueductal grey (PAG) output neurones containing rhodamine microspheres, retrogradely transported from an injection site in the rostral ventromedial medulla (RVM), were visualized in brain slices and recorded from using whole-cell patch clamp techniques. 2. The specific GABAB receptor agonist baclofen (10 microM) produced an outward current or hyperpolarization in fifty out of fifty-six caudal PAG output neurones. In 44% of these baclofen-sensitive neurones, the opioid agonist methionine enkephalin (30 microM) also produced an outward current or hyperpolarization. The opioid current reversed polarity at -104 mV and could also be produced by DAMGO, an agonist selective for the mu-subtype of opioid receptor. 3. Opioid-responding output neurones were not distributed uniformly in the caudal PAG. In horizontal slices containing lateral PAG, 56% of output neurones were inhibited by opioids, as compared with only 14% of the output neurones in slices containing ventrolateral PAG. 4. These observations are consistent with opioid disinhibition of ventrolateral PAG neurones projecting to the RVM as the predominant mechanism underlying opioid-induced analgesia in the PAG. The role of opioid receptors found on a major proportion of the output neurones in the lateral PAG remains to be established, but is assumed not be related to modulation of nociceptive function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandler R., Shipley M. T. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994 Sep;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Behbehani M. M., Jiang M., Chandler S. D. The effect of [Met]enkephalin on the periaqueductal gray neurons of the rat: an in vitro study. Neuroscience. 1990;38(2):373–380. doi: 10.1016/0306-4522(90)90035-3. [DOI] [PubMed] [Google Scholar]
- Cannon J. T., Prieto G. J., Lee A., Liebeskind J. C. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982 Jul 15;243(2):315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Hyperpolarization by GABAB receptor agonists in mid-brain periaqueductal gray neurones in vitro. Br J Pharmacol. 1995 Sep;116(1):1583–1588. doi: 10.1111/j.1476-5381.1995.tb16376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994 Sep;113(1):121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaulis A., Keay K. A., Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90(2):307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Depaulis A., Keay K. A., Bandler R. Quiescence and hyporeactivity evoked by activation of cell bodies in the ventrolateral midbrain periaqueductal gray of the rat. Exp Brain Res. 1994;99(1):75–83. doi: 10.1007/BF00241413. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Barbaro N. M., Heinricher M. M. Brain stem neuronal circuitry underlying the antinociceptive action of opiates. Prog Brain Res. 1988;77:245–257. doi: 10.1016/s0079-6123(08)62792-2. [DOI] [PubMed] [Google Scholar]
- Kangrga I. M., Loewy A. D. Whole-cell patch-clamp recordings from visualized bulbospinal neurons in the brainstem slices. Brain Res. 1994 Apr 4;641(2):181–190. doi: 10.1016/0006-8993(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993 May;40(5):631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Morgan M. M., Heinricher M. M., Fields H. L. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience. 1992;47(4):863–871. doi: 10.1016/0306-4522(92)90036-2. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., Basbaum A. I. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: I. GABA-immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J Comp Neurol. 1990 Dec 8;302(2):370–377. doi: 10.1002/cne.903020213. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Yeung J. C., Rudy T. A. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976 Sep 10;114(1):83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]