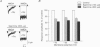Abstract
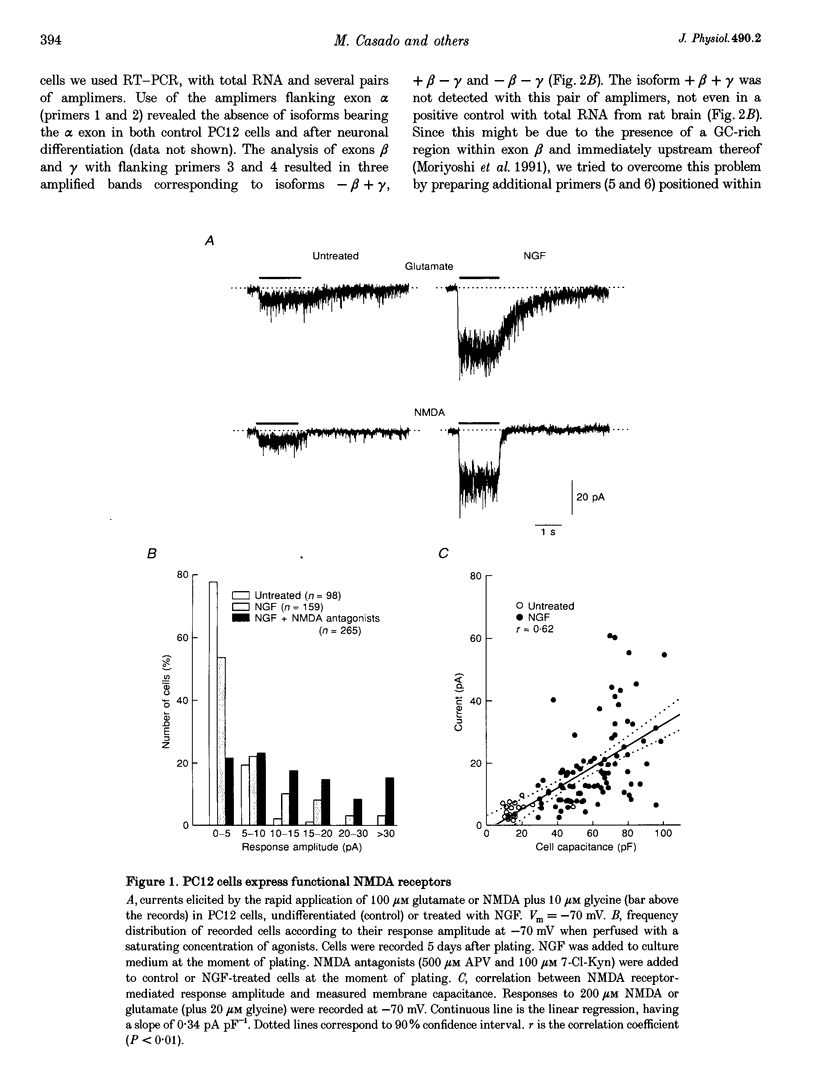
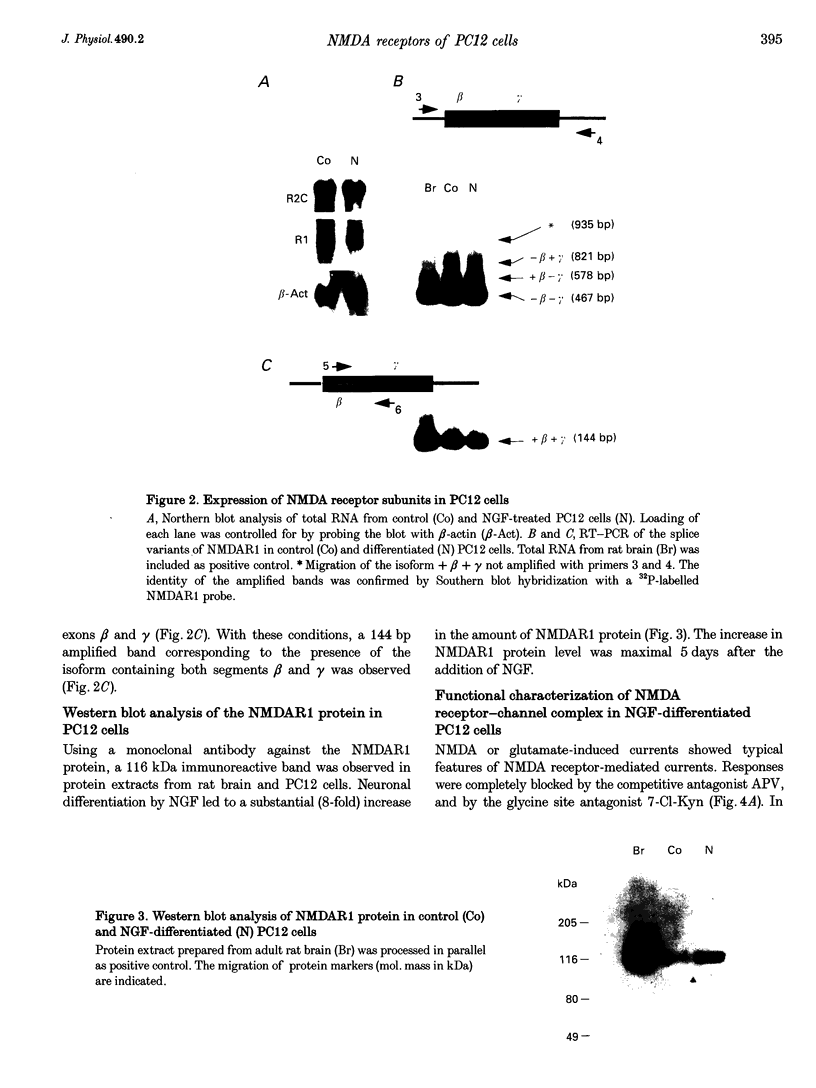
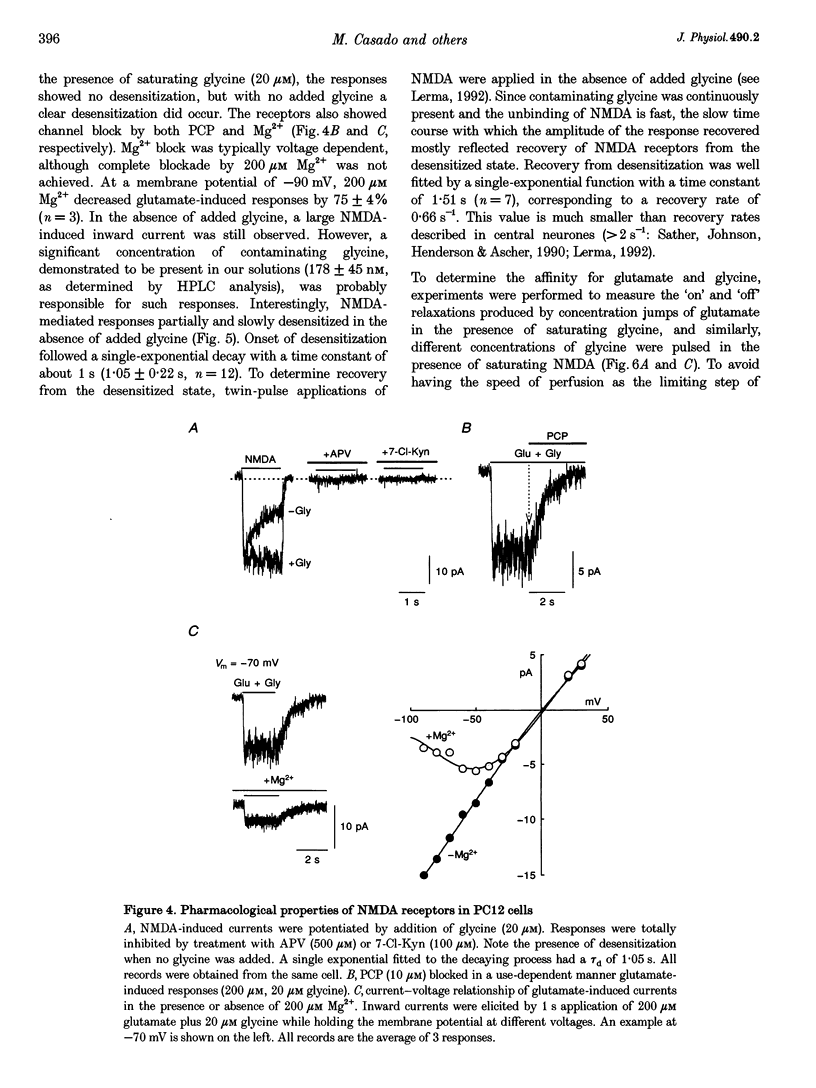
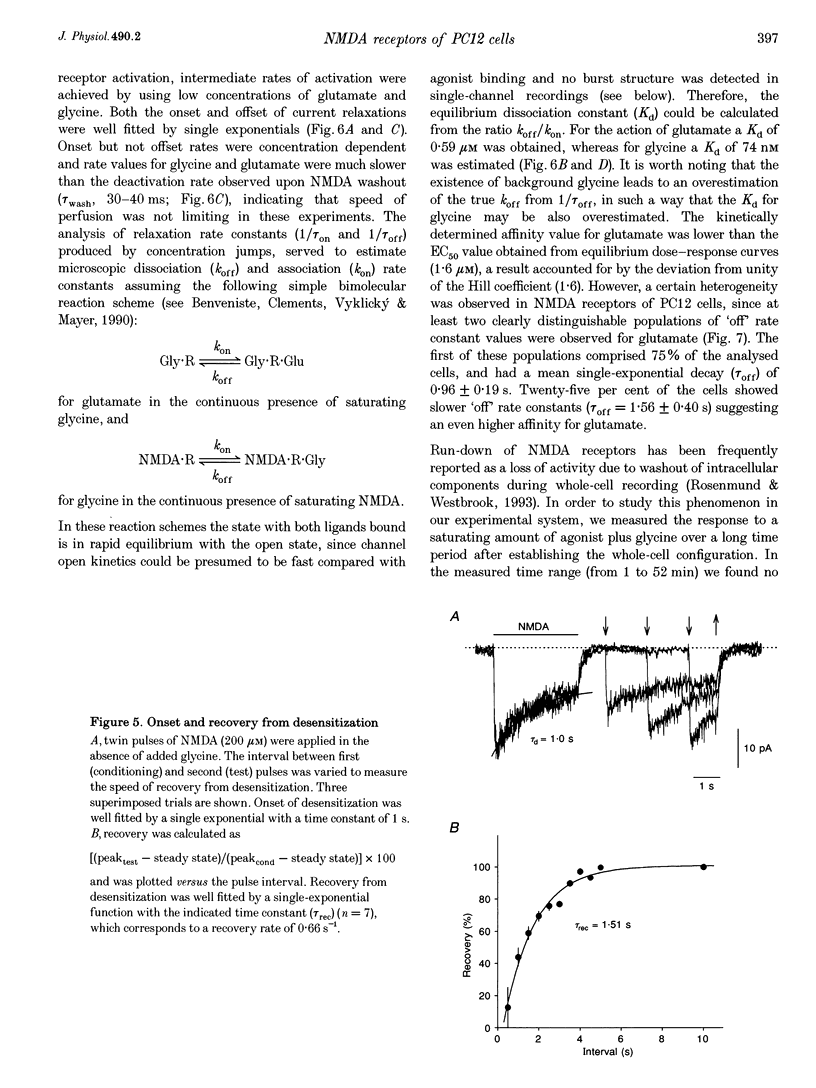
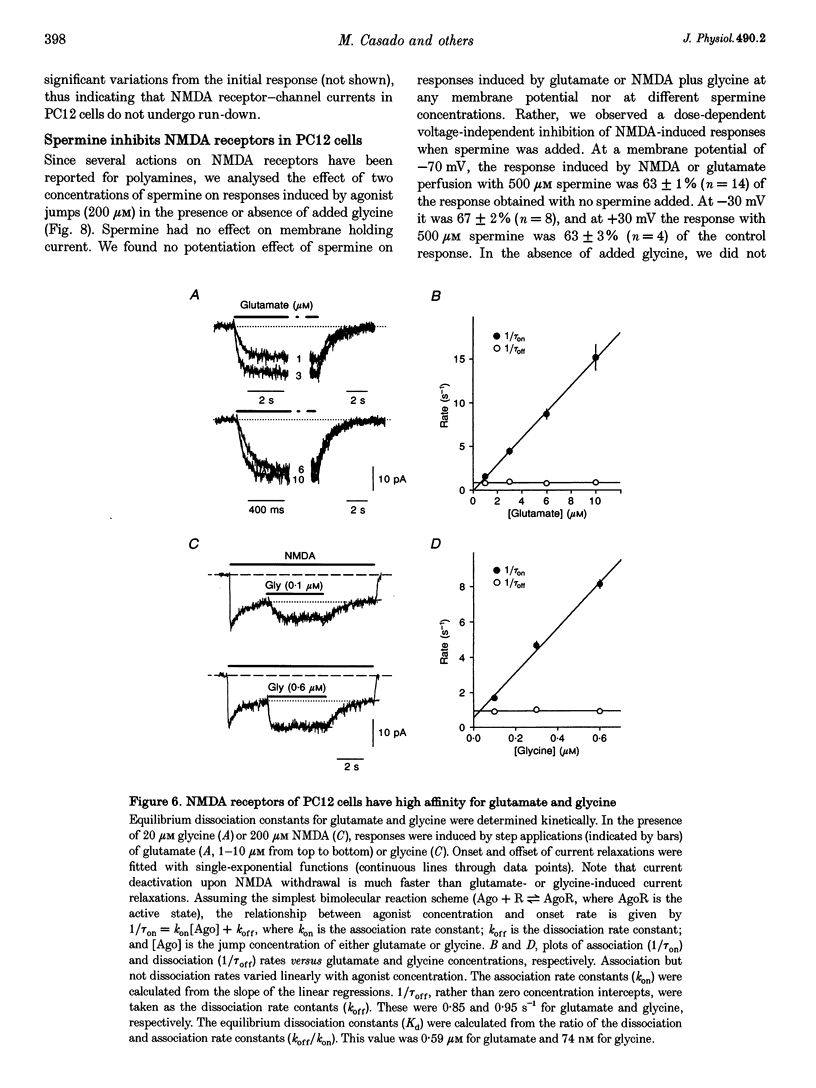
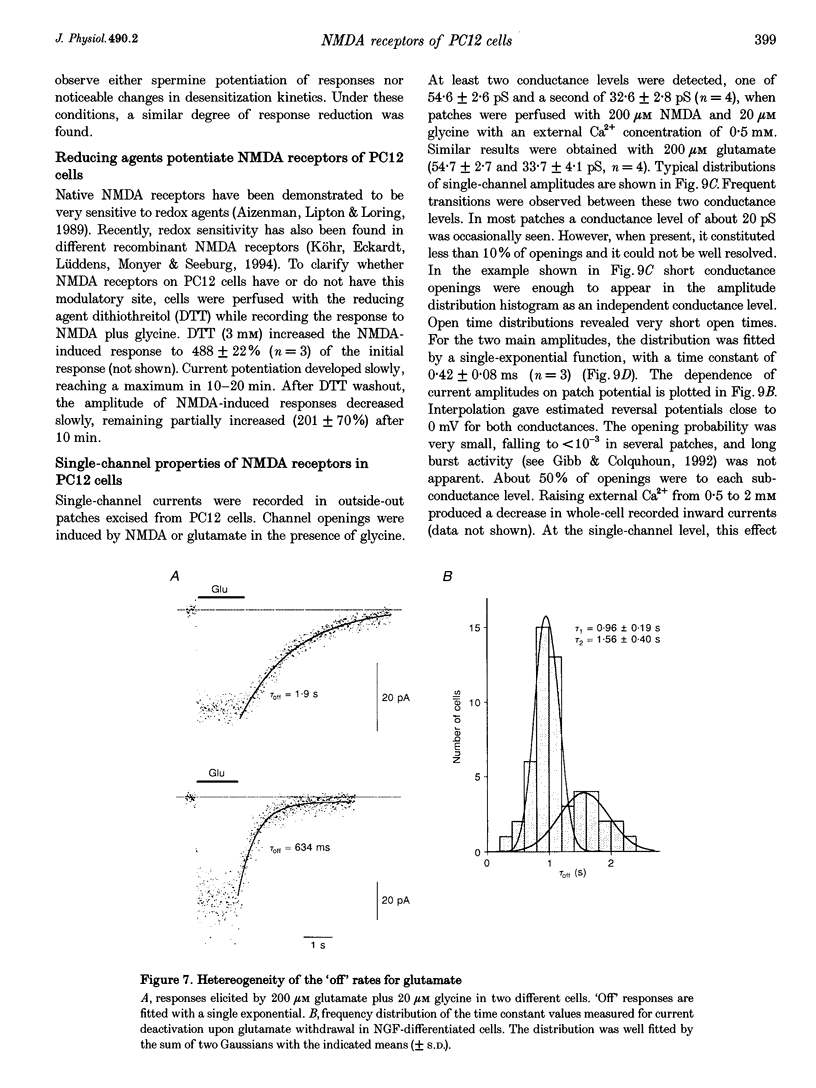
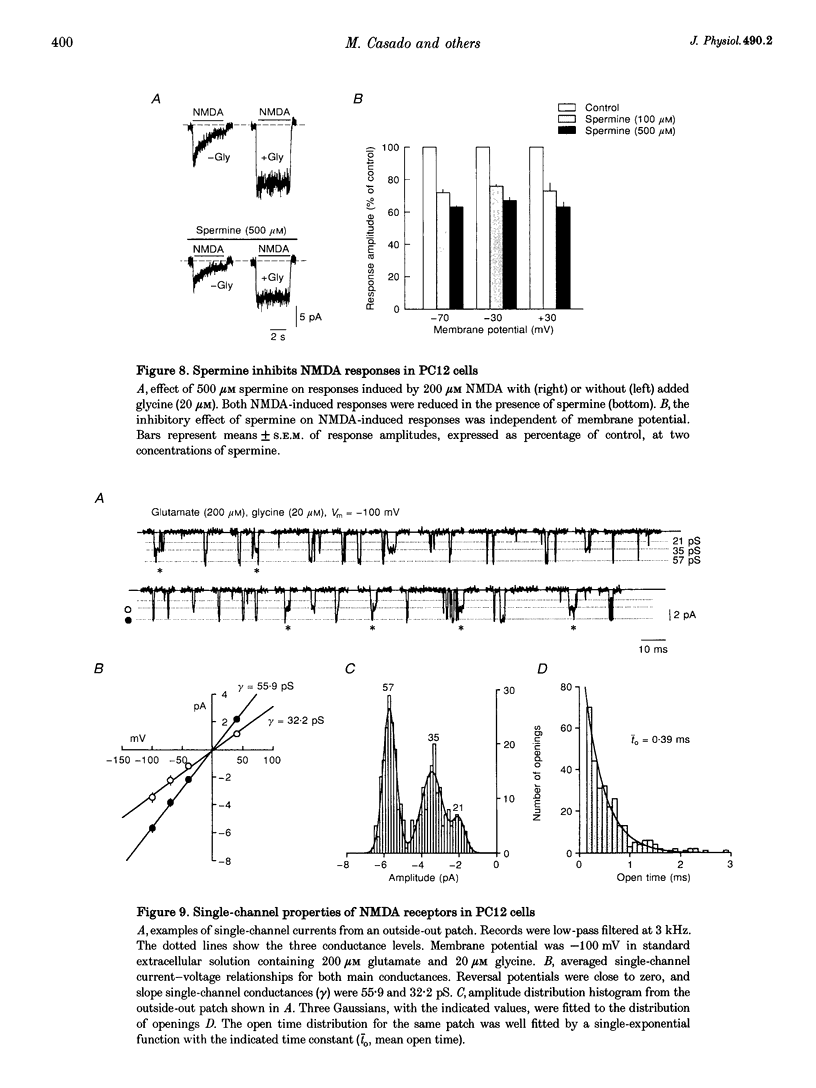
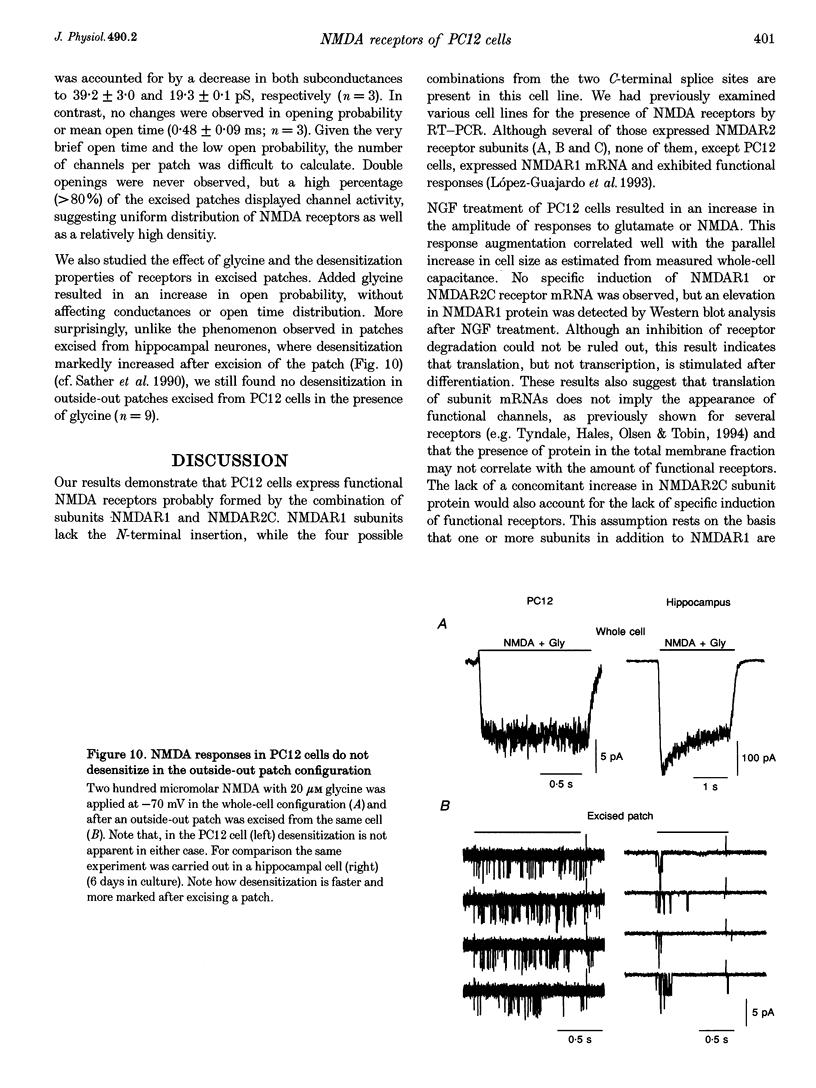
1. To characterize from a molecular and functional point of view the endogenous NMDA receptors expressed by phaeochromocytoma (PC12) cells, experiments involving polymerase chain reaction (PCR) amplification, Western blotting and patch-clamp analysis of undifferentiated and nerve growth factor (NGF)-differentiated PC12 cells were performed. 2. Analysis of PC12 mRNA demonstrated the presence of NMDAR1 and NMDAR2C transcripts. The NMDAR1 subunits lack the amino terminal insert of twenty-one amino acid residues, where as transcripts with and without deletions I and II at the 3' end of the coding region were detected. Thus, NMDA receptors of the PC12 cells might include NMDAR1A, NMDAR1E, NMDAR1C and NMDAR1D subunits. 3. Differentiation by NGF treatment of PC12 cells did not alter mRNA expression for NMDA receptor subunits significantly but induced an increase in both the NMDAR1 protein and the total amount of functional receptors that correlated well with a parallel increase in membrane area. 4. NMDA receptors in differentiated PC12 cells had a high affinity for both glutamate and glycine. These were estimated kinetically as 0.59 microM and 74 nM, respectively. Responses to glutamate or NMDA were non-desensitizing in the presence of saturating glycine, but slowly desensitized with low concentrations of glycine. Currents were completely blocked by D-aminophosphonovalerate (APV), 7-Cl-kynurenate and phencyclidine, and showed a voltage-dependent magnesium blockade. Spermine did not potentiate but inhibited NMDA receptor-mediated responses in a voltage-independent manner. 5. With 0.5 mM Ca2+, single-channel analysis revealed very brief openings (mean open time (t(o)) = 0.42 ms), with at least two conductive states, 55 and 33 pS, both having markedly low open probability. At 2 mM Ca2+, conductances were reduced to 39 and 19 pS, without an effect in open probability or mean open time. 6. The functional properties of NMDA receptors in PC12 cells were very similar to those described for NMDAR1A-NMDAR2C heteromers recombinantly expressed. The PC12 cell line provides a simple and reproducible system to analyse some specific NMDA receptor properties.
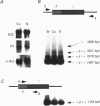
Full text
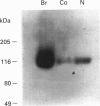
PDF





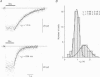
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993 May;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Mizuno N., Inagaki N., Kuromi H., Seino Y., Miyazaki J., Seino S. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994 Jun 24;269(25):16989–16992. [PubMed] [Google Scholar]
- Halegoua S., Armstrong R. C., Kremer N. E. Dissecting the mode of action of a neuronal growth factor. Curr Top Microbiol Immunol. 1991;165:119–170. doi: 10.1007/978-3-642-75747-1_7. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Cull-Candy S. G., Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991 Jan;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Jahr C. E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992 Jan 24;255(5043):470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Köhr G., Eckardt S., Lüddens H., Monyer H., Seeburg P. H. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994 May;12(5):1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Spray D. C., Bennett M. V., Zukin R. S. mRNA from NCB-20 cells encodes the N-methyl-D-aspartate/phencyclidine receptor: a Xenopus oocyte expression study. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1708–1711. doi: 10.1073/pnas.86.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Morales M., Ibarz J. M., Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci. 1994 Jul 1;6(7):1080–1088. doi: 10.1111/j.1460-9568.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Lerma J. Spermine regulates N-methyl-D-aspartate receptor desensitization. Neuron. 1992 Feb;8(2):343–352. doi: 10.1016/0896-6273(92)90300-3. [DOI] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Interaction of Mg2+ and phencyclidine in use-dependent block of NMDA channels. Neurosci Lett. 1991 Feb 25;123(2):187–191. doi: 10.1016/0304-3940(91)90927-l. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk J. F., Bennett M. V., Zukin R. S. Polyamines potentiate responses of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9971–9974. doi: 10.1073/pnas.87.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B., Naranjo J. R., Foulkes N. S., Lafarga M., Sassone-Corsi P. Transcriptional response to cAMP in brain: specific distribution and induction of CREM antagonists. Neuron. 1993 Apr;10(4):655–665. doi: 10.1016/0896-6273(93)90167-p. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Mewett K. N., Watkins J. C. Structure/activity relations of N-methyl-D-aspartate receptor ligands as studied by their inhibition of [3H]D-2-amino-5-phosphonopentanoic acid binding in rat brain membranes. Neuroscience. 1988 Jul;26(1):17–31. doi: 10.1016/0306-4522(88)90124-8. [DOI] [PubMed] [Google Scholar]
- Raditsch M., Ruppersberg J. P., Kuner T., Günther W., Schoepfer R., Seeburg P. H., Jahn W., Witzemann V. Subunit-specific block of cloned NMDA receptors by argiotoxin636. FEBS Lett. 1993 Jun 7;324(1):63–66. doi: 10.1016/0014-5793(93)81533-6. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Schubert D., Kimura H., Maher P. Growth factors and vitamin E modify neuronal glutamate toxicity. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8264–8267. doi: 10.1073/pnas.89.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P., Béhé P., Schoepfer R., Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992 Dec 22;250(1329):271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Sucher N. J., Brose N., Deitcher D. L., Awobuluyi M., Gasic G. P., Bading H., Cepko C. L., Greenberg M. E., Jahn R., Heinemann S. F. Expression of endogenous NMDAR1 transcripts without receptor protein suggests post-transcriptional control in PC12 cells. J Biol Chem. 1993 Oct 25;268(30):22299–22304. [PubMed] [Google Scholar]
- Turetsky D. M., Huettner J. E., Gottlieb D. I., Goldberg M. P., Choi D. W. Glutamate receptor-mediated currents and toxicity in embryonal carcinoma cells. J Neurobiol. 1993 Sep;24(9):1157–1169. doi: 10.1002/neu.480240904. [DOI] [PubMed] [Google Scholar]
- Tyndale R. F., Hales T. G., Olsen R. W., Tobin A. J. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994 Sep;14(9):5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Le Bourdelles B., Whiting P. J., Kemp J. A. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport. 1993 Sep 30;4(12):1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
- Younkin D. P., Tang C. M., Hardy M., Reddy U. R., Shi Q. Y., Pleasure S. J., Lee V. M., Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin R. S., Bennett M. V. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995 Jul;18(7):306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]