Abstract
BACKGROUND
Primary squamous cell carcinoma (SCC) of the renal parenchyma is extremely rare, with only nine cases reported.
CASE SUMMARY
This study reports a 51-year-old man with primary SCC of the renal parenchyma. The patient was admitted with recurrent dull pain and discomfort in the right lumbar region, which had worsened over 2 weeks, accompanied by painful gross hematuria. SCC antigen (SCCA) levels were elevated, and imaging revealed a renal mass with associated calculi. The patient underwent laparoscopic unilateral nephrectomy and lymph node dissection. Postoperative pathology confirmed highly differentiated SCC with necrosis in the right renal parenchyma, with negative renal pelvis and ureter. The pathological stage was Pt3aN1M0. Four months after surgery, the tumor recurred with involvement of the liver, right psoas major muscle, and inferior vena cava. The patient refused chemotherapy and succumbed to the disease 6 months postoperatively due to disease progression.
CONCLUSION
We report a case of primary SCC of the renal parenchyma, a rare renal malignancy. The clinical symptoms, laboratory tests, and imaging findings are nonspecific, making accurate and timely diagnosis challenging. According to the literature, for patients with renal calculi accompanied by a renal mass, elevated serum SCCA levels, and magnetic resonance imaging showing cystic or cystic-solid masses within the kidney with pseudocapsules and heterogeneous mild enhancement, the possibility of this disease should be considered.
Keywords: Renal tumor, Renal parenchyma, Squamous cell carcinoma, Renal calculi, Computed tomography, Case report
Core Tip: Primary squamous cell carcinoma (SCC) of the renal parenchyma is extremely rare. This study reports a 51-year-old man with primary SCC of the renal parenchyma, accompanied by lymph node metastasis. A comprehensive exposition of its clinical trajectory and imaging manifestation is presented, aiming to enhance comprehension and management of this rare disease.
INTRODUCTION
Primary squamous cell carcinoma (SCC) of the renal parenchyma is rare, with only nine cases reported so far. Clinical symptoms, signs and auxiliary examinations lack specificity, and some patients are already at an advanced stage at the time of discovery, resulting in poor prognosis. Here, we report a case of primary SCC of the renal parenchyma. A review of recent related literature is provided as well.
CASE PRESENTATION
Chief complaints
Dull aching discomfort in the right lumbar region accompanied by painful gross hematuria for 2 weeks.
History of present illness
A 51-year-old male patient presented with recurrent dull aching discomfort in the right lumbar region for 2 weeks, accompanied by dysuria and gross hematuria, without frequency, urgency or fever.
History of past illness
The patient had a history of right renal calculi for > 20 years. He denied any history of radiation exposure or occupational chemical exposure. There was no history of fever, hypertension or diabetes.
Personal and family history
The patient denied any serious personal or family medical history.
Physical examination
Positive percussion pain in the right renal area was noted, with no other abnormalities found.
Laboratory examinations
Urinalysis showed: Red blood cell count: 62.80 cells/μL (normal range: 0-15 cells/μL); Blood tests revealed: White blood cell count: 10.44 × 109/L (normal range: 4 × 109-10 × 109/L); Neutrophil percentage: 93.0% (normal range: 50%-75%); Lymphocyte percentage: 5.6% (normal range: 20%-45%); and C-reactive protein 11.60 mg/L (normal range: 0-5 mg/L). Tumor markers α-fetoprotein (AFP), carbohydrate antigen (CA)-199, CA-125 and carcinoembryonic antigen (CEA) were within normal ranges, while SCC antigen (SCCA) was elevated at 3.7 nmol/L (normal range: < 1.65 ng/mL).
Imaging examinations
Ultrasound: The right kidney mass measured approximately 5 cm × 5 cm, containing hyperechoic spots with posterior shadowing and a hypoechoic area within the mass. Color doppler flow imaging showed blood flow signals (Figure 1A). Computed tomography urography revealed a right kidney mass measuring approximately 6 cm × 4 cm; it contained septations and stones, with mild irregular enhancement of the posterior wall (Figure 1B and C). Enhanced magnetic resonance imaging (MRI) showed a cystic-solid mass in the right kidney, measuring about 6 cm × 4 cm; The tumor shows slightly low signal intensity on T1- weight ed imaging. (Figure 1D). the mass had a central stone signal with long T1 and short T2, approximately 17 mm in diameter, surrounded by fluid signal with long T1 and T2. The tumor had a clear pseudocapsule on the T2-weighted imaging (T2WI) sequence (Figure 1E). The cyst wall was thick and irregular, showing high signal intensity on diffusion-weighted imaging (DWI) (Figure 1F) and Enlarged lymph nodes were observed in the retroperitoneum (Figure 1F, arrow). low signal intensity on apparent diffusion coefficient (ADC) (Figure 1G), with mild irregular enhancement postcontrast (Figure 1H).
Figure 1.
Medical image. A: Ultrasound examination showing a mass in the right kidney with hypoechoic areas and echogenic stones within, accompanied by posterior acoustic shadowing. Color doppler flow imaging shows blood flow signals within the mass; B: Computed tomography urography views show the mass and internal stones Coronal; C: Sagittal; D: The tumor shows slightly low signal intensity on T1 weight ed imaging; E: Magnetic resonance T2-weighted imaging shows a clearly defined right renal tumor with a low signal pseudocapsule (arrow). The internal signal is heterogeneous, with nodular short T2 signal stones and long T2 cystic necrosis. The solid tumor tissue shows isointense T2 signal; F: Diffusion-weighted imaging shows high signal intensity in the solid tumor tissue and low signal intensity in the cystic necrotic areas. Enlarged lymph nodes are visible in the retroperitoneum (arrow); G: Apparent diffusion coefficient map shows low signal intensity in the solid tumor components; H: Contrast-enhanced scan shows heterogeneous mild enhancement of the solid tumor components.
FINAL DIAGNOSIS
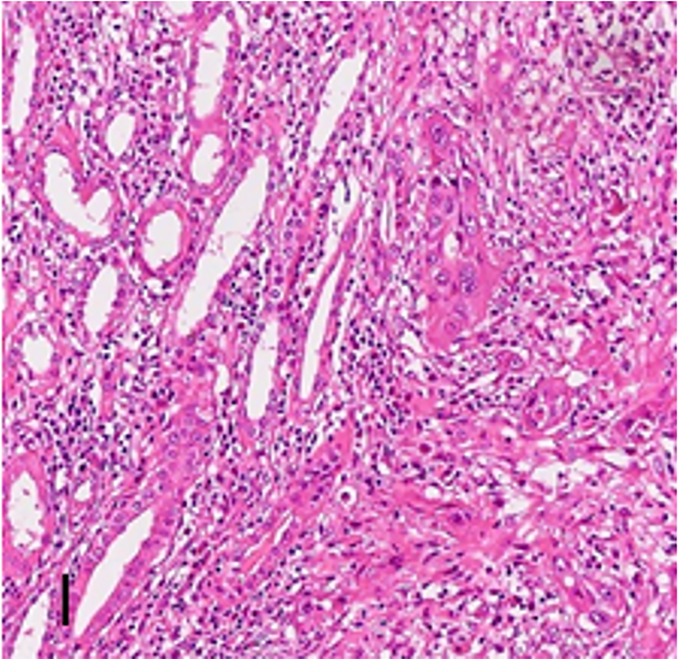
Right renal parenchyma: Well-differentiated SCC with extensive necrosis, invading blood vessels and nerves (Figure 2). The mass size was 6 cm × 2.5 cm × 3.0 cm. Renal pelvis and ureter were negative for malignancy. Associated findings included infectious interstitial nephritis and stones. Right renal hilum lymph nodes: One of two lymph nodes showed metastatic well-differentiated SCC, and the other lymph node showed reactive hyperplasia. Right ureter segment: Chronic ureteritis. Pathological stage: Pt3aN1M0.
Figure 2.
High-grade squamous cell carcinoma tissue within the renal parenchyma, with a significant inflammatory cell response in the stroma (Hematoxylin-eosin stains × 10).
TREATMENT
The patient underwent laparoscopic unilateral nephrectomy with lymph node dissection. Intraoperatively, a right renal mass approximately 6 cm × 6 cm in size was observed, containing milky white fluid and a 2.5 cm × 2.0 cm stone. Postoperatively, the patient’s wound healed well.
OUTCOME AND FOLLOW-UP
Four months postsurgery, the patient presented with right abdominal discomfort. Follow-up serum SCCA was 4.2 nmol/L. Contrast-enhanced computed tomography (CT) revealed a mass at the original surgical site, invading liver segment VI, inferior vena cava, right abdominal wall, and psoas major muscle (Figure 3). A multidisciplinary discussion recommended genetic testing or chemotherapy with gemcitabine and carboplatin. However, the patient declined genetic testing due to cost and refused chemotherapy due to poor general condition, opting to discharge. Telephone follow-up 6 months postsurgery confirmed the patient’s death due to disease progression.
Figure 3.
Enhanced computed tomography scan four months post-surgery. A: The original surgical area shows a mass; B: The tumor invaded the psoas major muscle (arrow) and the right abdominal wall; C: The mass also invades the VI segment of the liver (asterisk) and the inferior vena cava (arrow).
DISCUSSION
Primary SCC of the kidney is clinically rare, accounting for 0.5%-0.8% of renal malignancies[1], with the majority being renal pelvic SCC. Experts generally believe that chronic persistent irritation from stones and inflammation can cause abnormal hyperplasia of the renal pelvis epithelium, leading to squamous metaplasia and carcinogenesis[2]. Primary SCC of the renal parenchyma is extremely rare and has only been reported in individual case reports. Its histological origin and mechanism remain unclear. To date, only nine cases of primary renal parenchyma SCC have been reported[2-10] (Table 1).
Table 1.
Literature report on primary parenchymal squamous cell carcinoma of the kidney
|
|
Ref.
|
Sex
|
Age (years)
|
Presentation
|
Location
|
Treatment
|
Tumor extent
|
Renal stone
|
Involvement of renal pelvis
|
Prognosis
|
| 1 | Terada[10] | M | 73 | Hematuria and lumbago | Multiple: Bladder, left ureter, and left kidney | Cystectomy and nephroureterectomy | Replacing entire kidney parenchyma | Absent | Absent | Alive and disease free at 3 months after surgery |
| 2 | Kulshreshtha et al[7] | F | 60 | Weight loss for 3 months | Mid and lower pole of the left kidney | Radical nephrectomy with lymph node dissection | 6.5 cm × 5.5 cm, with Gerota’s fascia invasion and para-aortic lymph node metastasis (pT4N1) | Absent | Absent | Alive and disease free at 13 months after surgery |
| 3 | Ghosh and Saha[2] | M | 51 | Dull and intermittent flank pain for 5 months | Lower pole of the right kidney | Radical nephrectomy | 5.8 cm × 5.5 cm (pT1bN0) | Absent | Absent | Alive and disease free at 12 months after surgery |
| 4 | Sahoo et al[8] | F | 50 | Right abdomen pain for 6 months | Upper pole of the right kidney | Radical nephrectomy | 8.0 cm × 6.0 cm (pT2aNx) | Absent | Absent | Alive and disease free at 6 months after surgery |
| 5 | Wang et al[3] | M | 61 | Hematuria and lumbago for 2 months | Right kidney | Radical nephrectomy | NA, with perirenal fat invasion (pT3aNx) | Absent | Absent | Alive and disease free at one month after surgery |
| 6 | Zhang et al[9] | F | 61 | Intermittent flank pain for 2 months | Lower pole of the right kidney | Radical nephrectomy | With perirenal fat invasion (pT3aNx) | Absent | Absent | Alive and disease free at 3 months after surgery |
| 7 | Fotovat et al[5] | F | 41 | Flank pain and dysuria for 3 months | Lower pole of the left kidney | Radical nephrectomy | With perirenal fat invasion and para-aortic lymph node metastasis (pT3aN1) | Present | Absent | Metastasis to ovary at 8 months after surgery |
| 8 | Present (2022) | M | 61 | Flank pain and weight loss for 2 months | Lower pole of the right kidney | Radical nephrectomy with right hemicolectomy | 9.0 cm × 8.0 cm, with ascending colon invasion (pT4N0) | Present | Absent | Alive and disease free at 5 months after surgery |
| 9 | Liang et al[4] | M | 52 | 1 week of renal cyst found in physical examination | Upper pole of the right kidney | Robot-assisted partial nephrectomy | 8.3 cm × 8.2 cm × 8.1 cm (pT2aNxM0) | Absent | Absent | Alive and disease free at 6 months after surgery |
| 10 | This study | M | 51 | Hematuria and lumbago for 2 weeks | Right kidney | Radical nephrectomy with lymph node dissection | 6.0 cm × 6.0 cm, with perirenal fat invasion and para-aortic lymph node metastasis (pT3aN1) | Present | Absent | Metastasis to Liver and inferior vena cava metastases at 4 months after surgery |
A review of the cases revealed that all nine presented with flank pain and urinary difficulties, with no specific clinical symptoms. Three cases were associated with stones, and preoperative diagnosis could not exclude xanthogranulomatous pyelonephritis (XGP). Two cases underwent MRI, which revealed tumors with pseudocapsules. All patients underwent nephrectomy, with normal renal pelvic histology. Three cases had lymph node metastasis and poor prognosis.
Radiological examination is an essential tool for evaluating renal tumors. Ultrasound can detect lesions but has limitations in determining the nature of the lesions. CT scans can clearly show the location of the lesion and the presence of urinary obstruction, as well as the number and size of stones. All cases in the literature underwent CT, but the imaging of SCC lacks specificity, and patients with concomitant stones are often misdiagnosed with XGP. MRI can provide more diagnostic information; SCC tends to necrotize, often presenting as a cystic-solid mass. Eight previous cases and our case underwent MRI, revealing a clear pseudocapsule on the T2WI sequence, mild irregular crab-like enhancement on contrast-enhanced scans, high signal intensity on the DWI sequence, and low intensity on the ADC. Flu-deoxy glucose-positron emission tomography/CT is valuable for diagnosing primary renal carcinoma but cannot distinguish between tumors and inflammation[3].
In laboratory tests, urinalysis may show elevated red blood cell counts, but this is nonspecific. Tumor markers CA-199, AFP, CEA and CA-125 are not elevated. In this case, preoperative and recurrent serum SCCA levels were elevated, suggesting their significance for diagnosing renal parenchymal SCC[11]. Serum SCCA is a tumor-associated antigen extracted from cervical SCC, and elevated serum SCCA can also be seen in patients with uremia, azotemia, diabetic nephropathy and nephrotic syndrome. Excluding these diseases, elevated serum SCCA has more diagnostic value[12].
When the tumor is accompanied by stones, it needs to be differentiated from focal XGP[3]. The presence of adipose tissue in the lesion is a specific sign of XGP, typically presenting as the bear paw sign. Focal XGP involving the renal parenchyma does not present with a pseudocapsule, and enhanced scans show increased inflammatory vessels around the lesion with significant marginal enhancement, aiding in differentiation[13]. The most common primary tumor of the renal parenchyma is clear cell carcinoma, which often undergoes necrotic cystic changes. Enhanced scans show significant enhancement during the cortical phase and significant reduction during the medullary and excretory phases[13]. In contrast, SCC presents as a cystic or cystic-solid mass with mild continuous enhancement, showing distinct enhancement patterns.
There are no standard guidelines for the treatment of primary renal parenchymal SCC. Currently, surgery remains the primary treatment method. For patients with gene mutations, targeted drug therapy can also be used, but its efficacy still lacks clinical follow-up validation.
CONCLUSION
Primary renal parenchymal SCC is a rare malignancy that lacks specific symptoms, signs and auxiliary examinations. Some cases are discovered late and already have metastases, leading to short-term postoperative recurrence. Therefore, early diagnosis and treatment of this disease are particularly important. For patients with renal stones who present with gross hematuria and elevated serum SCCA, and imaging shows a cystic-solid mass with a pseudocapsule on T2WI sequences and mild, irregular enhancement of the solid component, primary renal parenchymal SCC should be considered.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this case report.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Zhai QL S-Editor: Fan M L-Editor: A P-Editor: Yu HG
Contributor Information
Zhi-Hui Zheng, Department of Ultrasound, The Second People’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China.
Bo Shao, Department of Radiology, Shulan (Quzhou) Hospital, Quzhou 324000, Zhejiang Province, China.
Chao-Min Xu, Department of Ultrasound, The Second People’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China.
Ke Wang, Department of Radiology, The Second People’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China.
Jia-Zhu Wen, Department of Radiology, The Second People’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China.
Li-Kang Luo, Department of Pathology, The Second people’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China.
Jia-Cheng Guan, Department of Radiology, The Second People’s Hospital of Quzhou, Quzhou 324000, Zhejiang Province, China. gjcqzey@126.com.
References
- 1.Li MK, Cheung WL. Squamous cell carcinoma of the renal pelvis. J Urol. 1987;138:269–271. doi: 10.1016/s0022-5347(17)43116-8. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh P, Saha K. Primary intraparenchymal squamous cell carcinoma of the kidney: a rare and unique entity. Case Rep Pathol. 2014;2014:256813. doi: 10.1155/2014/256813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Yan B, Wei YB, Hu NA, Shen Q, Li D, Yang JR, Yang X. Primary kidney parenchyma squamous cell carcinoma mimicking xanthogranulomatous pyelonephritis: A case report. Oncol Lett. 2016;11:2179–2181. doi: 10.3892/ol.2016.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang K, Yuan Y, Lv B, Ke Z. Primary squamous cell carcinoma of renal parenchyma: A case report and literature review. Front Oncol. 2023;13:1037156. doi: 10.3389/fonc.2023.1037156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fotovat A, Gheitasvand M, Amini E, Ayati M, Nowroozi MR, Sharifi L. Primary squamous cell carcinoma of renal parenchyma: Case report and review of literature. Urol Case Rep. 2021;37:101627. doi: 10.1016/j.eucr.2021.101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh CK, Kim JY. Primary squamous cell carcinoma of the kidney parenchyma with ascending colon invasion: A case report and literature review. Int J Surg Case Rep. 2022;91:106762. doi: 10.1016/j.ijscr.2022.106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulshreshtha P, Kannan N, Bhardwaj R, Batra S. Primary squamous cell carcinoma of the renal parenchyma. Indian J Pathol Microbiol. 2012;55:370–371. doi: 10.4103/0377-4929.101747. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo TK, Das SK, Mishra C, Dhal I, Nayak R, Ali I, Panda D, Majumdar SK, Parida DK. Squamous cell carcinoma of kidney and its prognosis: a case report and review of the literature. Case Rep Urol. 2015;2015:469327. doi: 10.1155/2015/469327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang Y, Ge C, Zhang J, Liang P. Squamous cell carcinoma of the renal parenchyma presenting as hydronephrosis: a case report and review of the recent literature. BMC Urol. 2020;20:107. doi: 10.1186/s12894-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T. Synchronous squamous cell carcinoma of the kidney, squamous cell carcinoma of the ureter, and sarcomatoid carcinoma of the urinary bladder: a case report. Pathol Res Pract. 2010;206:379–383. doi: 10.1016/j.prp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Qi C, He S, Cai L, Zhang L, Ding H, Chen Y. A study on the clinical value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography combined with serum squamous cell carcinoma antigen in diagnosing recurrence/metastases in patients with early metaphase cervical cancer. Oncol Lett. 2021;22:746. doi: 10.3892/ol.2021.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Wang J, Zhang L. Serum SCCA levels in patients suffering cancers or other diseases. Prog Mol Biol Transl Sci. 2019;162:165–175. doi: 10.1016/bs.pmbts.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Ma W, Xu X, Guan Z, Pang P. A convention-radiomics CT nomogram for differentiating fat-poor angiomyolipoma from clear cell renal cell carcinoma. Sci Rep. 2021;11:4644. doi: 10.1038/s41598-021-84244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]