Abstract
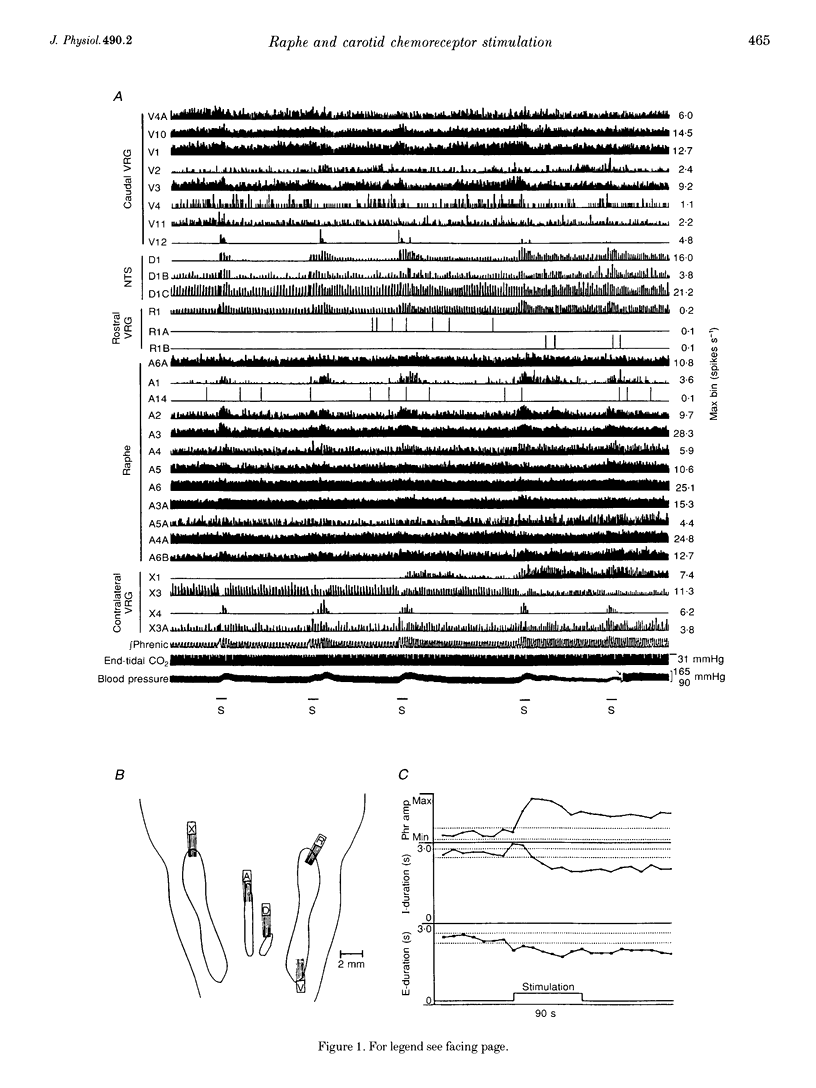
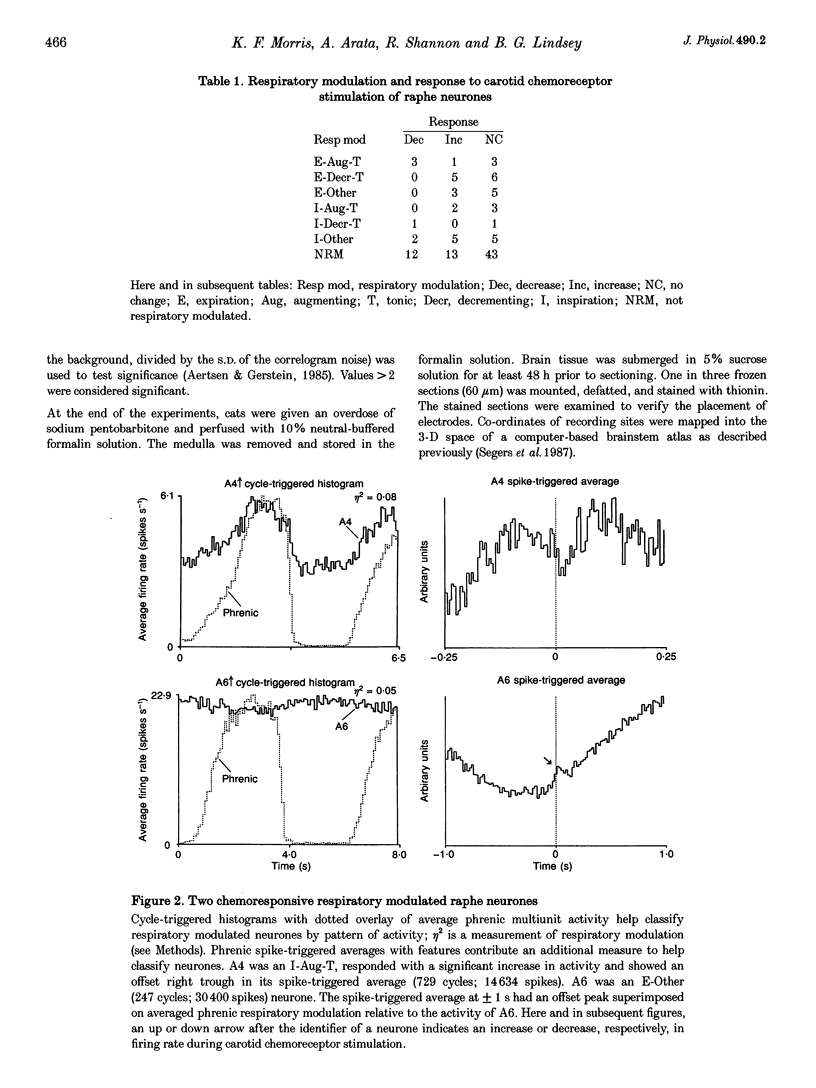
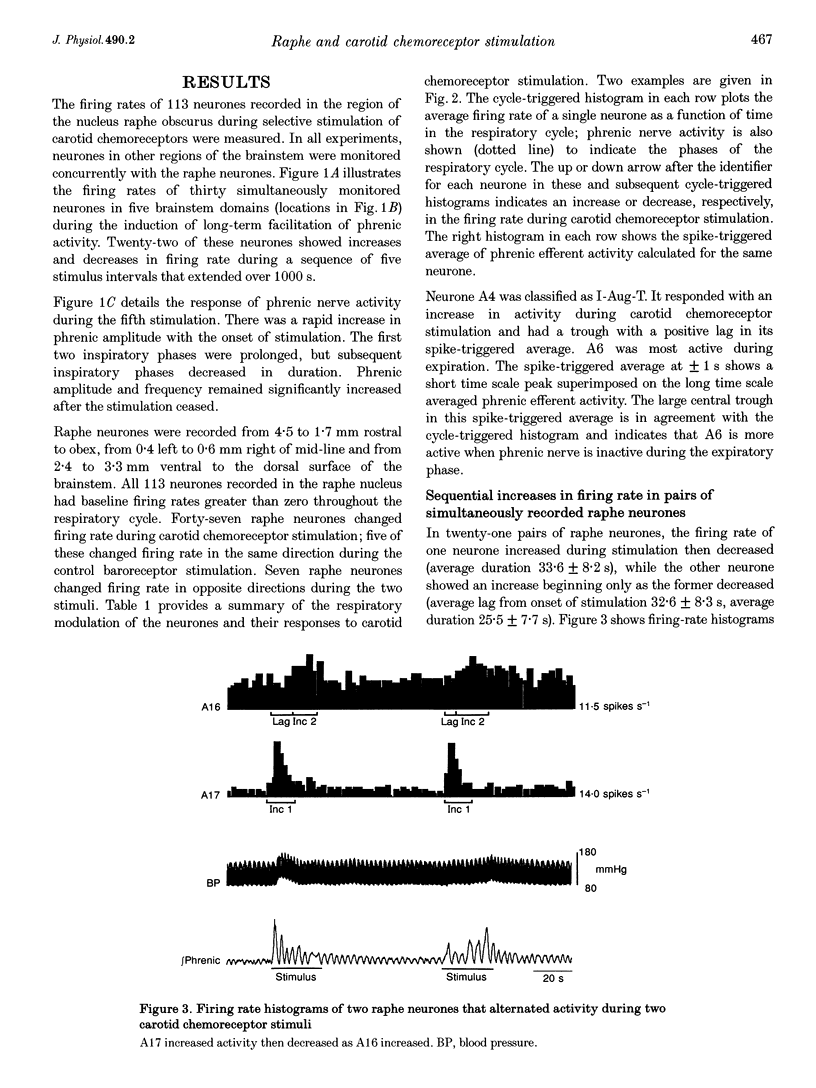
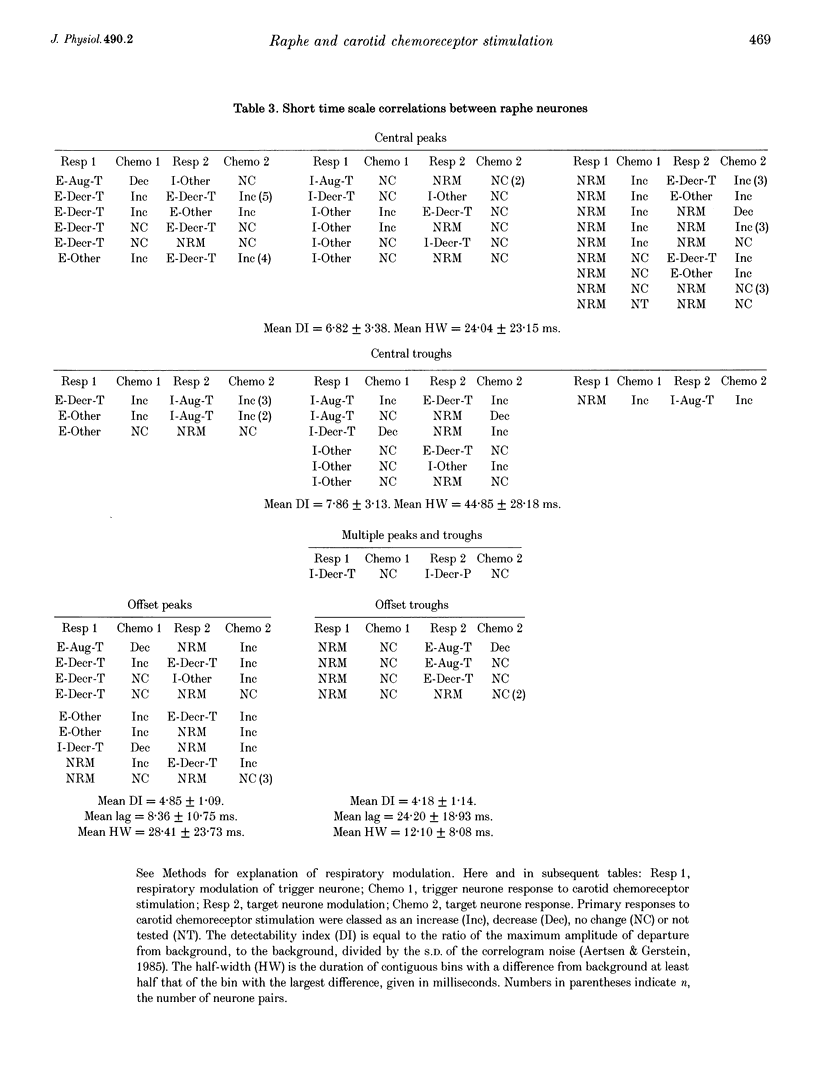
1. Stimulation of either peripheral chemoreceptors or nucleus raphe obscurus results in long-term facilitation of phrenic motoneurone activity. The first objective of this work was to measure the concurrent responses of neurones in the nucleus raphe obscurus, the nucleus tractus solitarii, and the regions of the retrofacial nucleus, nucleus ambiguus and nucleus retroambigualis during induction of long-term facilitation. A second goal was to assess functional relationships of the chemoresponsive raphe neurones with neurones in the other monitored locations and with phrenic motoneurones. 2. Up to thirty single medullary neurones and phrenic nerve efferent activity were recorded simultaneously in fifteen anaesthetized, paralysed, vagotomized, artificially ventilated adult cats. Carotid chemoreceptors were stimulated by close arterial injection of 200 microliters of CO2-saturated saline solution. Spike trains were analysed with cycle-triggered histograms and two statistical tests for respiratory modulation. Peristimulus-time histograms and cumulative sum histograms were used to assess responses to stimulation. Cross-correlation was used to test for non-random temporal relationships between spike trains. Spike-triggered average histograms provided evidence for functional associations with phrenic motoneurones. 3. One hundred and thirteen of 348 neurones were monitored in the nucleus raphe obscurus. The firing rates of twenty-nine raphe neurones increased during stimulation; eighteen decreased. In twenty-one pairs of concurrently monitored raphe neurones, the firing rate of one increased its activity during stimulation then decreased, while the other showed an increase that began as the rate of the former declined. Eighteen chemoresponsive raphe neurones had short time scale features in their phrenic spike-triggered averages. Short time scale features were found in cross-correlograms from 184 of 1407 neurone pairs. 4. The data suggest parallel routes by which carotid chemoreceptors influence medullary raphe neurones and support the hypotheses that mid-line respiratory-related neuronal assemblies transform information from those receptors and regulate the gain of respiratory motor output.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aertsen A. M., Gerstein G. L. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 1985 Aug 12;340(2):341–354. doi: 10.1016/0006-8993(85)90931-x. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Bayliss D. A., Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992 Aug 31;143(1-2):164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Brodin E., Linderoth B., Goiny M., Yamamoto Y., Gazelius B., Millhorn D. E., Hökfelt T., Ungerstedt U. In vivo release of serotonin in cat dorsal vagal complex and cervical ventral horn induced by electrical stimulation of the medullary raphe nuclei. Brain Res. 1990 Dec 10;535(2):227–236. doi: 10.1016/0006-8993(90)91605-g. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Piercey M. F., Gootman P. M., Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res. 1974 Dec 6;81(2):319–324. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Ellenberger H. H., Feldman J. L. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989 Oct 23;105(1-2):34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H., Stein R. B. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986 Aug;17(2-3):153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Edwards M. W., Jr Medullary relay neurons in the carotid-body chemoreceptor pathway of cats. Respir Physiol. 1975 Jun;24(1):69–79. doi: 10.1016/0034-5687(75)90122-x. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Felder R. B., Jordan D., Spyer K. M. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol. 1984 Feb;347:397–409. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. C., Katz D. M. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992 Feb 14;572(1-2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Grélot L., Barillot J. C., Bianchi A. L. Central distributions of the efferent and afferent components of the pharyngeal branches of the vagus and glossopharyngeal nerves: an HRP study in the cat. Exp Brain Res. 1989;78(2):327–335. doi: 10.1007/BF00228904. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986 Apr;6(4):1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Marion L. J., Speck D. F. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37(2):541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. Structure and function of the brain serotonin system. Physiol Rev. 1992 Jan;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Nisimaru N., Sears T. A. Monosynaptic excitation of bulbospinal respiratory neurones by chemoreceptor afferents in the carotid sinus nerve [proceedings]. J Physiol. 1979 Aug;293:35P–36P. [PubMed] [Google Scholar]
- Kubin L., Tojima H., Davies R. O., Pack A. I. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992 May 25;139(2):243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lalley P. M. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986 Nov;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A. D., Feldman J. L. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993 Feb;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol. 1992 Apr;67(4):890–904. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R., Gerstein G. L. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol. 1992 Apr;67(4):923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Segers L. S., Morris K. F., Hernandez Y. M., Saporta S., Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J Neurophysiol. 1994 Oct;72(4):1830–1851. doi: 10.1152/jn.1994.72.4.1830. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980 Dec;42(3):171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol. 1986 Dec;381:169–179. doi: 10.1113/jphysiol.1986.sp016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. S., Sloan H. E., Jiang C., Miletic V., Hayashi F., Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neurosci Lett. 1992 Jul 6;141(1):75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Miura M., Reis D. J. Termination and secondary projections of carotid sinus nerve in the cat brain stem. Am J Physiol. 1969 Jul;217(1):142–153. doi: 10.1152/ajplegacy.1969.217.1.142. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hennequin S., Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990 Mar 26;111(1-2):127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Orem J., Dick T. Consistency and signal strength of respiratory neuronal activity. J Neurophysiol. 1983 Nov;50(5):1098–1107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Brownstein M., Saavedra J. M. Serotonin content of the brain stem nuclei in the rat. Brain Res. 1974 Nov 15;80(2):237–249. doi: 10.1016/0006-8993(74)90688-x. [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., de Castro D., Llewellyn-Smith I., Lipski J., Voss M. D. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990 Apr;10(4):1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers L. S., Shannon R., Saporta S., Lindsey B. G. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol. 1987 Apr;57(4):1078–1100. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Morrison D. E., Ellenberger H. H., Otto M. R., Feldman J. L. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989 Mar 1;281(1):69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- White S. R., Fung S. J. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989 Nov 20;502(2):205–213. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neuroscience. 1993 Aug;55(3):849–867. doi: 10.1016/0306-4522(93)90446-m. [DOI] [PubMed] [Google Scholar]
- Zhan W. Z., Ellenberger H. H., Feldman J. L. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31(1):105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]


