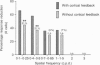Abstract
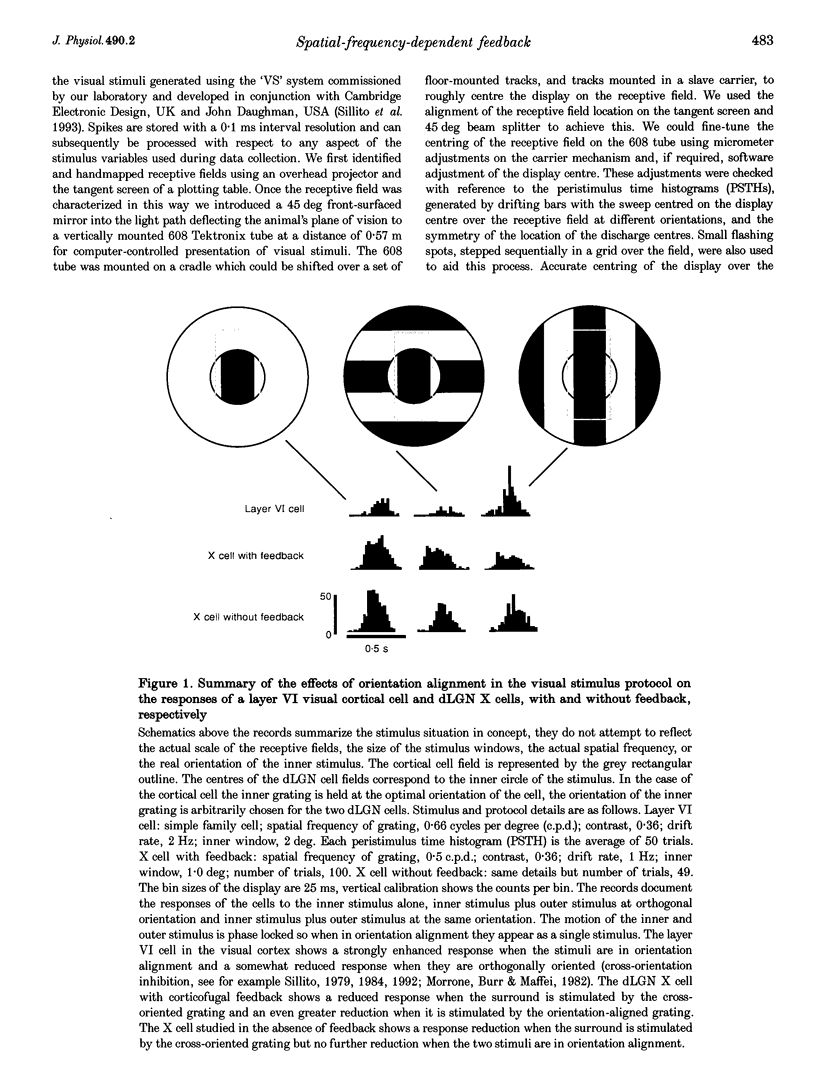
1. The influence of spatial frequency on the inhibitory component of the effects mediated by feedback from the visual cortex has been examined in X and Y cells in the A laminae of the feline dorsal lateral geniculate nucleus (dLGN). Experiments utilized a concentric, bipartite visual stimulus centered over the receptive fields of the cells studied. The responses of dLGN cells to selective stimulation of receptive field centre (with the inner window) were compared with those to stimulation of centre and surround mechanisms (both inner and outer window), with the stimuli either in or out of orientation alignment. 2. With these same stimuli, layer VI cells in the visual cortex showed a marked increase in response magnitude when the inner and outer components of the stimulus were in orientation alignment, and presented at the preferred orientation. In the case of dLGN X and Y cells we observed an enhancement of the surround antagonism of the centre response when the inner and outer sections of the stimulus were in orientation alignment. 3. The effects of varying spatial frequency on these responses were examined in dLGN cells in the presence of corticofugal feedback. With the stimulus sections in orientation alignment, surround stimulation produced a powerful and significant reduction in the response to stimulation of centre mechanism alone with the most marked effects for stimuli in the range 0.1-0.85 cycles per degree (c.p.d.). The reduction produced by surround stimulation in the range 0.1-0.5 c.p.d. was notably more potent in X cells than in Y cells. 4. The responses to the same stimuli were examined in dLGN cells with the corticofugal feedback inactivated. Comparison of data from cells studied with and without feedback revealed a significant decrease in surround-mediated attenuation of the centre response in Y cells for spatial frequencies in the range 0.1-0.85 c.p.d. For X cells the decrease in strength of the surround antagonism was also clear and significant but only seen in the range 0.1-0.5 c.p.d. 5. The influence of the orientation alignment of inner and outer stimulus sections revealed a marked difference between cells studied with and without feedback. In the presence of feedback fully aligned stimuli enhanced surround antagonism of centre responses for spatial frequencies in the range 0.1-0.5 c.p.d., in X and Y cells. In the absence of corticofugal feedback this alignment effect was essentially eliminated. 6. These data show that surround antagonism of the centre response is influenced by orientation alignment of the stimulus sections at low spatial frequencies and in the presence of corticofugal feedback. They support a cortically driven enhancement of the inhibitory mechanisms reinforcing surround mechanisms in the dLGN. We propose that feedback enhances a low spatial frequency cut-off in the dLGN, that this effect is maximal for a continuous iso-orientated contour, but diminished whenever there is an orientation discontinuity. The hyperpolarizing influence underlying this effect may contribute to the recently described synchronizing influence of the direct corticofugal contacts onto relay cells. We suggest feedback of the cortical level of analysis refines the transfer of the visual input at geniculate level in a stimulus-context-dependent fashion.
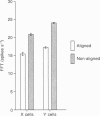
Full text
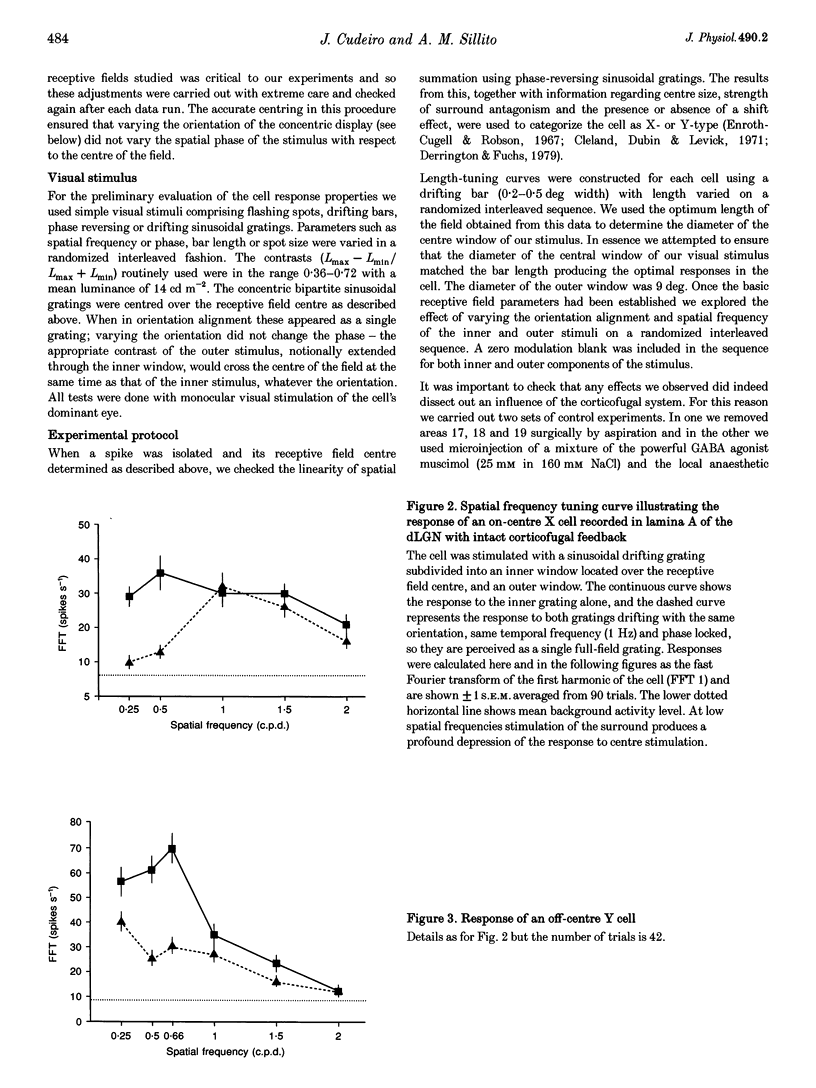
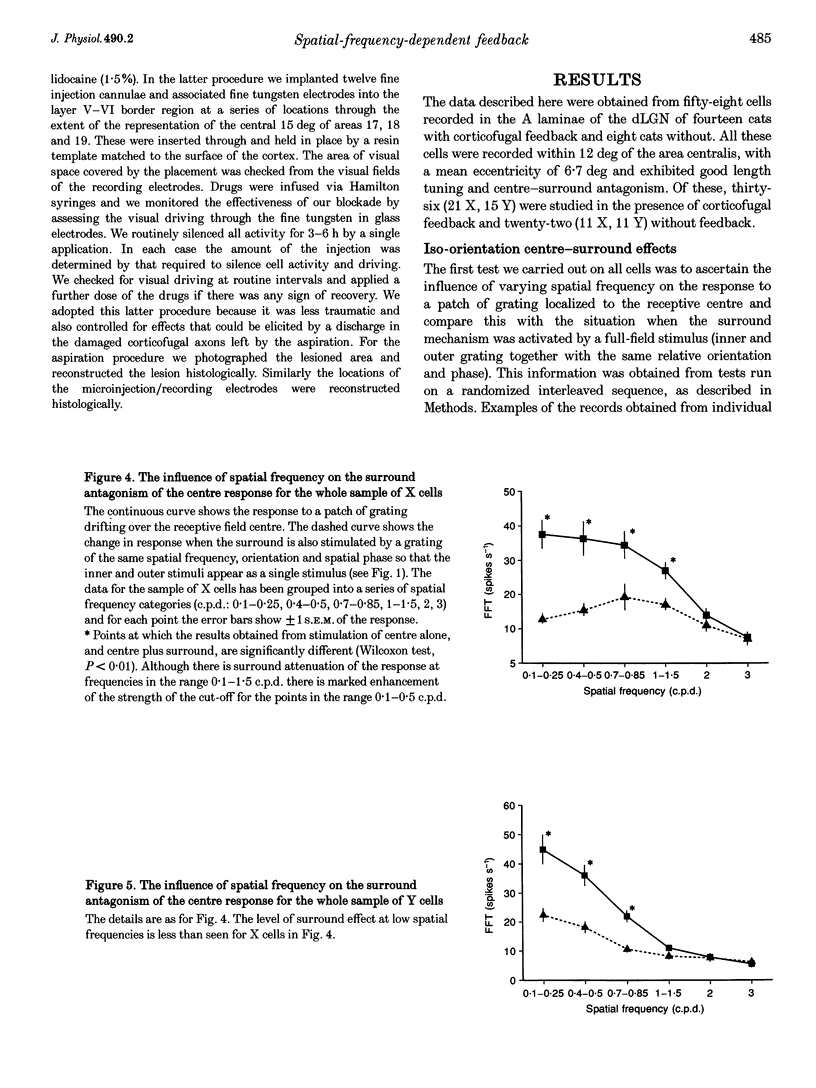
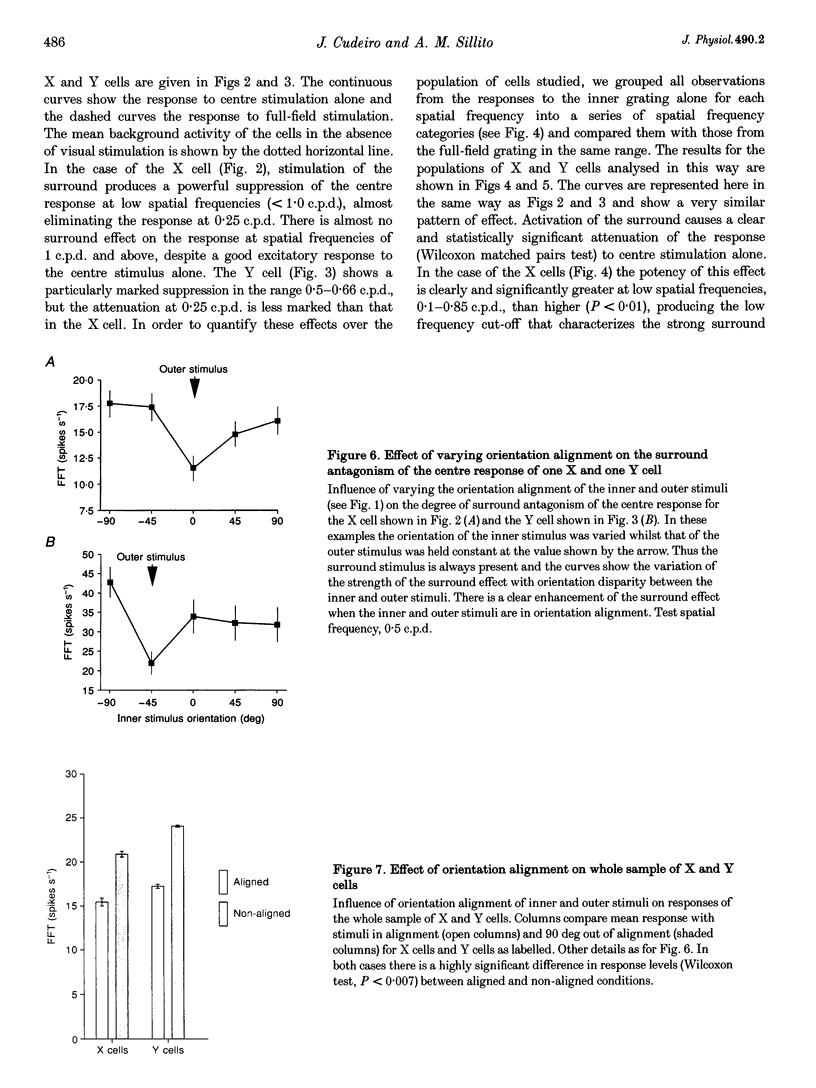
PDF

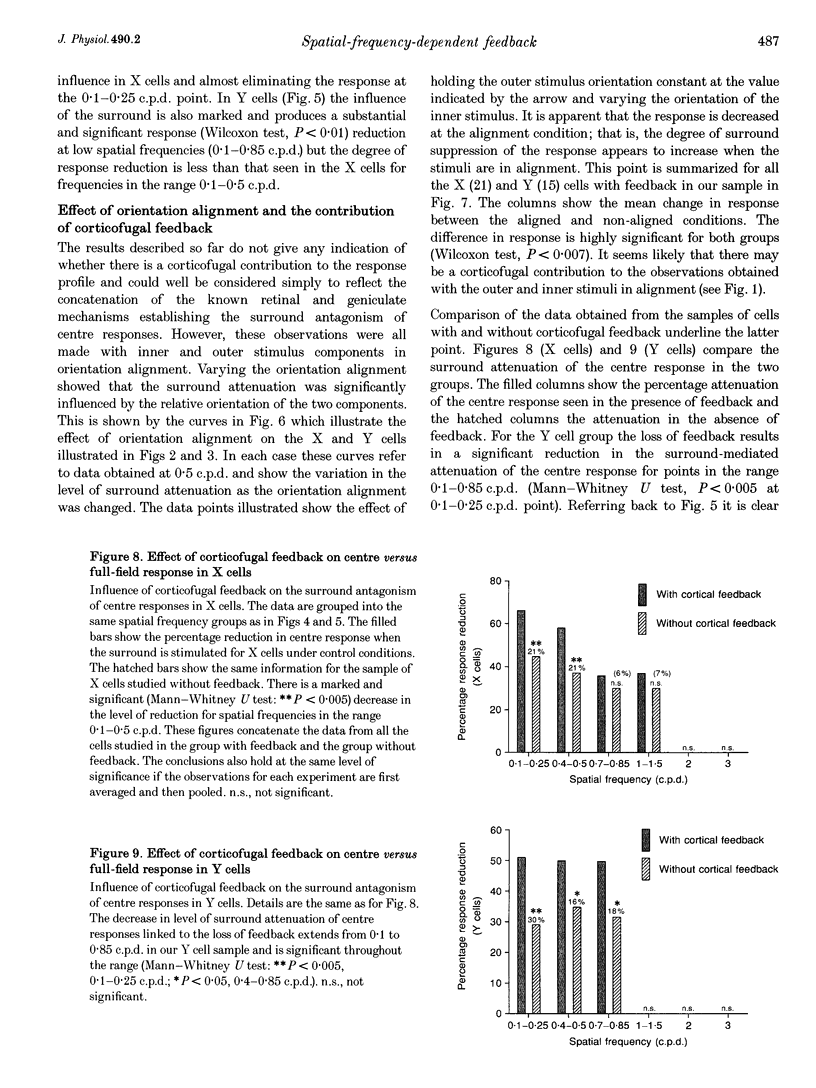
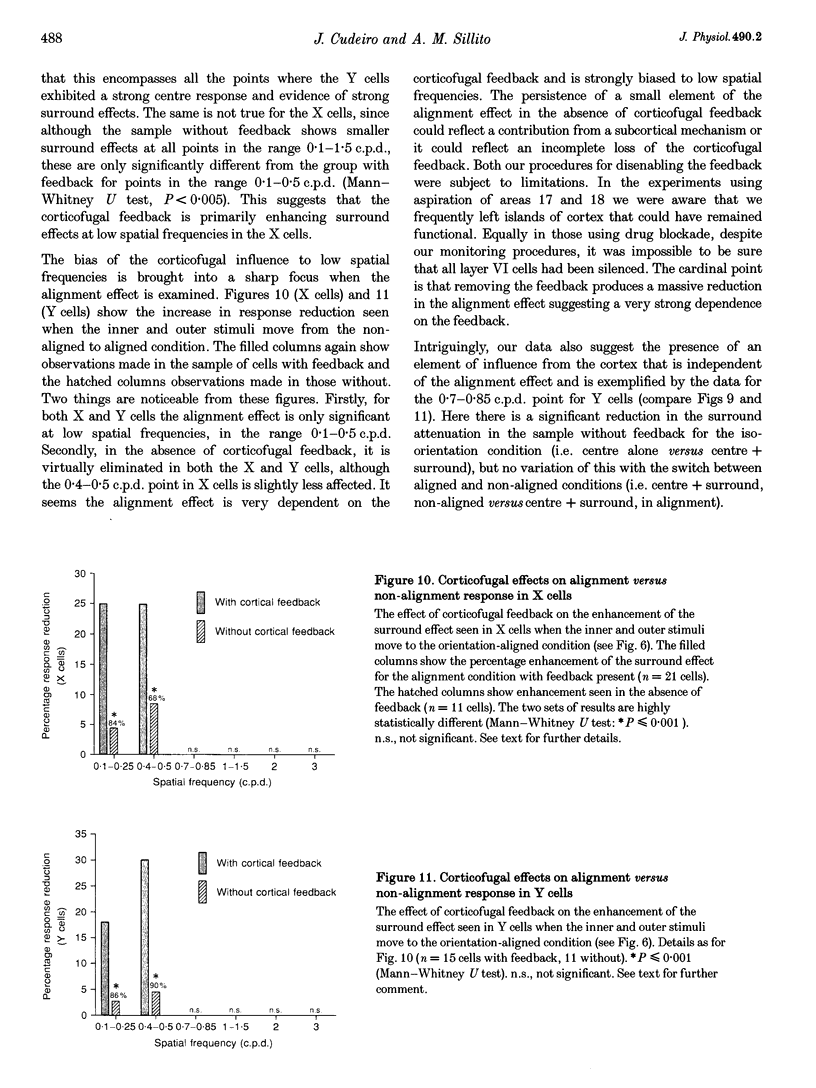




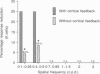
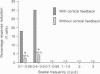
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardi N., Morrone M. C. The role of gamma-aminobutyric acid mediated inhibition in the response properties of cat lateral geniculate nucleus neurones. J Physiol. 1984 Dec;357:505–523. doi: 10.1113/jphysiol.1984.sp015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyapati J., Henry G. H. The duplex character of the corticofugal pathway from the striate cortex to the lateral geniculate complex of the cat. Vision Res. 1987;27(5):723–726. doi: 10.1016/0042-6989(87)90069-1. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Lee B. B., Vidyasagar T. R. Response of neurons in the cat's lateral geniculate nucleus to moving bars of different length. J Neurosci. 1983 Jan;3(1):108–116. doi: 10.1523/JNEUROSCI.03-01-00108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979 Aug;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Andreas K., König Peter, Gray Charles M., Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Inter-Columnar Interaction as Determined by Cross-Correlation Analysis. Eur J Neurosci. 1990;2(7):588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve K. L., Sillito A. M. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J Neurosci. 1995 Jul;15(7 Pt 1):4868–4874. doi: 10.1523/JNEUROSCI.15-07-04868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve K. L., Sillito A. M. The length summation properties of layer VI cells in the visual cortex and hypercomplex cell end zone inhibition. Exp Brain Res. 1991;84(2):319–325. doi: 10.1007/BF00231452. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Sillito A. M. The length-response properties of cells in the feline dorsal lateral geniculate nucleus. J Physiol. 1991 Dec;444:329–348. doi: 10.1113/jphysiol.1991.sp018881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Purpura K., Shapley R. M. Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. J Physiol. 1987 Oct;391:267–288. doi: 10.1113/jphysiol.1987.sp016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Sprague J. M., Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1974 Dec 1;158(3):339–362. doi: 10.1002/cne.901580308. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Spatial frequency rows in the straite visual cortex. Vision Res. 1977 Feb;17(2):257–264. doi: 10.1016/0042-6989(77)90089-x. [DOI] [PubMed] [Google Scholar]
- Martin K. A. The Wellcome Prize lecture. From single cells to simple circuits in the cerebral cortex. Q J Exp Physiol. 1988 Sep;73(5):637–702. doi: 10.1113/expphysiol.1988.sp003190. [DOI] [PubMed] [Google Scholar]
- Montero V. M. A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Exp Brain Res. 1991;86(2):257–270. doi: 10.1007/BF00228950. [DOI] [PubMed] [Google Scholar]
- Morrone M. C., Burr D. C., Maffei L. Functional implications of cross-orientation inhibition of cortical visual cells. I. Neurophysiological evidence. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):335–354. doi: 10.1098/rspb.1982.0078. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987 Oct 22;329(6141):727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Cudeiro J., Murphy P. C. Orientation sensitive elements in the corticofugal influence on centre-surround interactions in the dorsal lateral geniculate nucleus. Exp Brain Res. 1993;93(1):6–16. doi: 10.1007/BF00227775. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. GABA mediated inhibitory processes in the function of the geniculo-striate system. Prog Brain Res. 1992;90:349–384. doi: 10.1016/s0079-6123(08)63622-5. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Jones H. E., Gerstein G. L., West D. C. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994 Jun 9;369(6480):479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J., Thompson I. D. On the variety of spatial frequency selectivities shown by neurons in area 17 of the cat. Proc R Soc Lond B Biol Sci. 1981 Oct 14;213(1191):183–199. doi: 10.1098/rspb.1981.0061. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T., Creutzfeldt O. D., Legéndy C. R. Functional organization of the corticofugal system from visual cortex to lateral geniculate nucleus in the cat (with an appendix on geniculo-cortical mono-synaptic connections). Exp Brain Res. 1978 Jul 14;32(3):345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. The patterns of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1975 Oct 15;163(4):377–395. doi: 10.1002/cne.901630402. [DOI] [PubMed] [Google Scholar]
- Vidnyánszky Z., Hámori J. Quantitative electron microscopic analysis of synaptic input from cortical areas 17 and 18 to the dorsal lateral geniculate nucleus in cats. J Comp Neurol. 1994 Nov 8;349(2):259–268. doi: 10.1002/cne.903490208. [DOI] [PubMed] [Google Scholar]
- Weber A. J., Kalil R. E., Behan M. Synaptic connections between corticogeniculate axons and interneurons in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1989 Nov 1;289(1):156–164. doi: 10.1002/cne.902890113. [DOI] [PubMed] [Google Scholar]
- Weber A. J., Kalil R. E. Development of corticogeniculate synapses in the cat. J Comp Neurol. 1987 Oct 8;264(2):171–192. doi: 10.1002/cne.902640204. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Friedlander M. J., Sherman S. M. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]