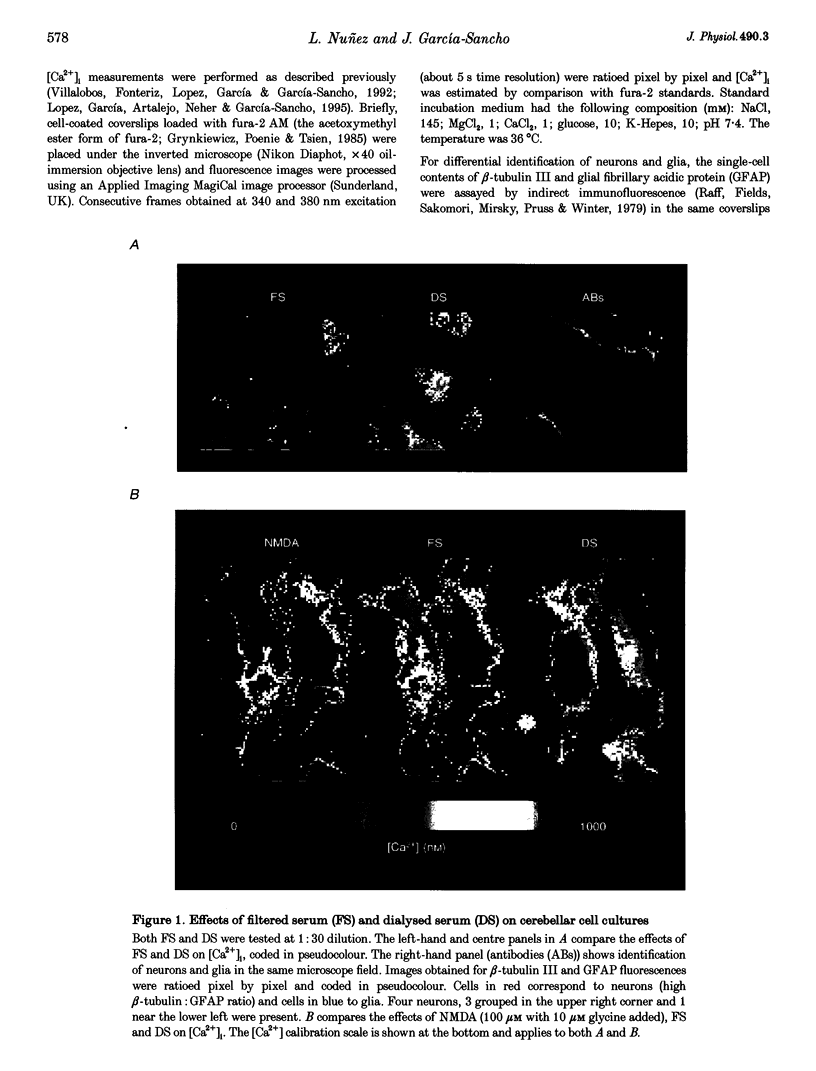
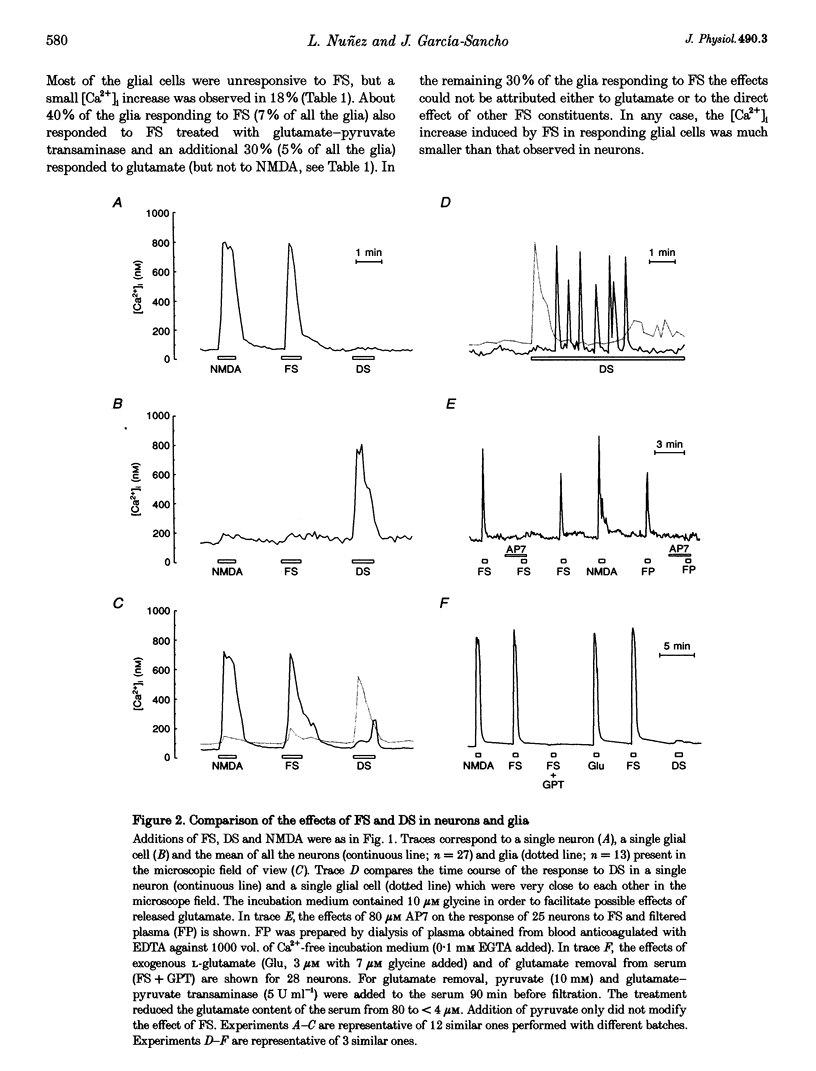
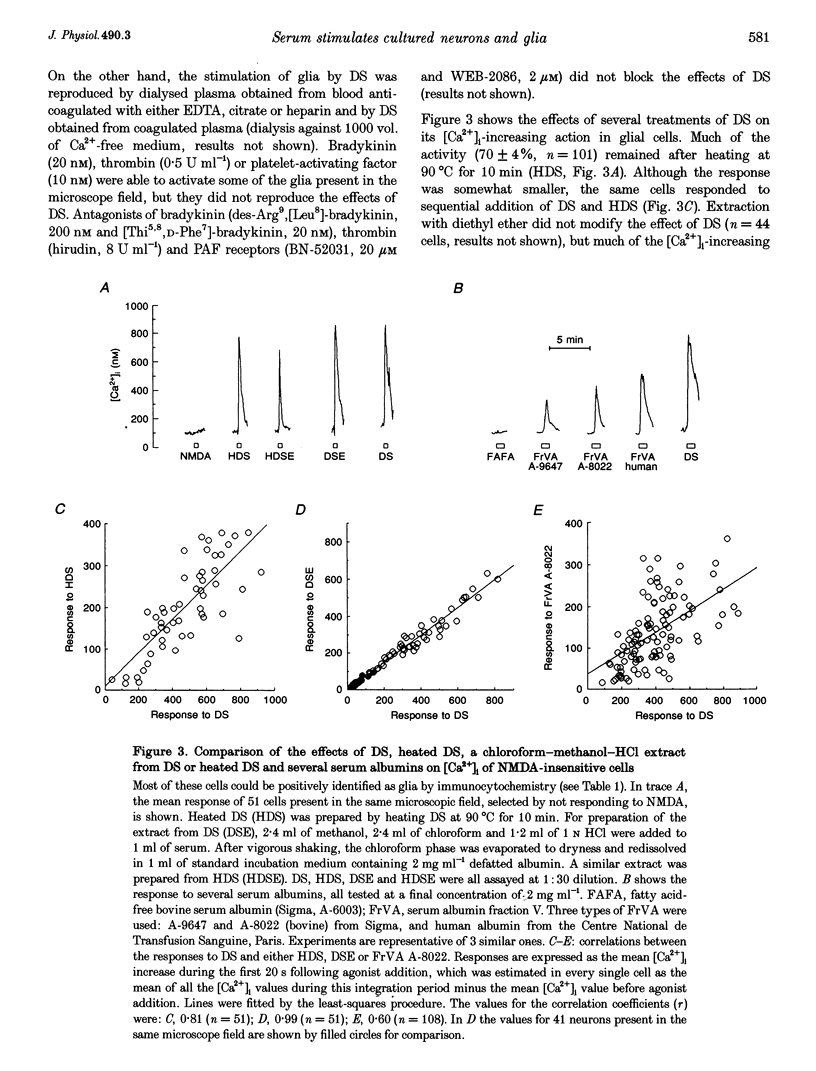
Abstract
1. The ability of several serum fractions to increase the cytosolic calcium concentration ([Ca2+]i) was tested in rat cerebellar cells maintained in primary culture. 2. Serum filtered through an ultrafiltration membrane with 3000 Da molecular mass cut-off (filtered serum, FS) selectively stimulated neurons whereas dialysed serum (DS) selectively stimulated glia. 3. The effects of FS were due to glutamate as they were reproduced by N-methyl-D-aspartate (NMDA), blocked by NMDA receptor antagonists and prevented by enzymatic removal of glutamate. 4. The effects of DS on glia were not reproduced by platelet-activating factor, thrombin or bradykinin. They were not lost on heating or extraction with diethyl ether. They were reproduced by a methanol-chloroform-HCl extract from DS and by several commercial fraction V plasma albumins. 5. These [Ca2+]i-increasing factors present in blood could contribute to brain damage during ischaemia if they reached the brain interstitium on disruption of the blood-brain barrier.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995 Feb;18(2):58–60. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- López M. G., Artalejo A. R., García A. G., Neher E., García-Sancho J. Veratridine-induced oscillations of cytosolic calcium and membrane potential in bovine chromaffin cells. J Physiol. 1995 Jan 1;482(Pt 1):15–27. doi: 10.1113/jphysiol.1995.sp020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. L., Lloyd H. G., Cowan A. I. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994 Jun;17(6):251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Nadal A., Fuentes E., Pastor J., McNaughton P. A. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A., Reilly J. A., Lysko P. G., Henneberry R. C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988 Jun 7;451(1-2):205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Parpura V., Basarsky T. A., Liu F., Jeftinija K., Jeftinija S., Haydon P. G. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994 Jun 30;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Historical overview. Calcium, ischemia, and death of brain cells. Ann N Y Acad Sci. 1988;522:638–661. doi: 10.1111/j.1749-6632.1988.tb33410.x. [DOI] [PubMed] [Google Scholar]
- Szatkowski M., Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994 Sep;17(9):359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Villalobos C., Fonteriz R., López M. G., García A. G., García-Sancho J. Inhibition of voltage-gated Ca2+ entry into GH3 and chromaffin cells by imidazole antimycotics and other cytochrome P450 blockers. FASEB J. 1992 Jun;6(9):2742–2747. doi: 10.1096/fasebj.6.9.1319362. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Cull-Candy S. G. A comparison of non-NMDA receptor channels in type-2 astrocytes and granule cells from rat cerebellum. J Physiol. 1994 Feb 15;475(1):95–114. doi: 10.1113/jphysiol.1994.sp020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Finkbeiner S. M., Cornell-Bell A. H. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci. 1992 Jul;12(7):2648–2664. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]