Abstract
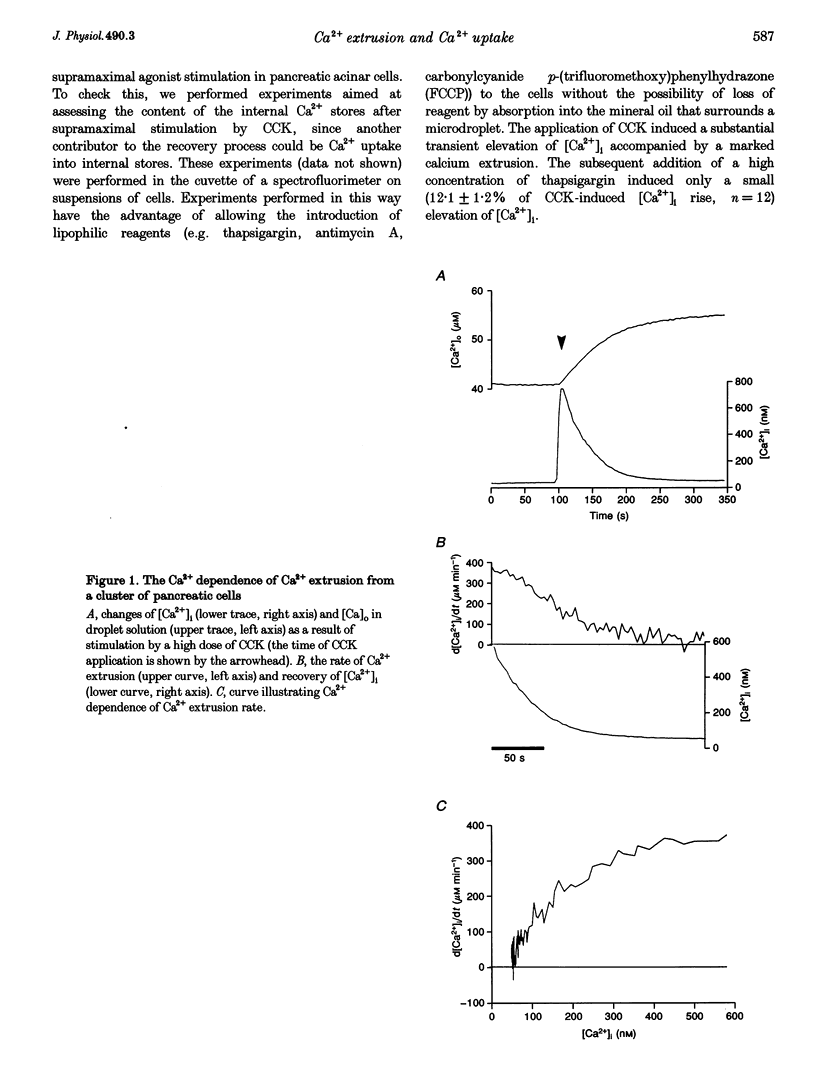
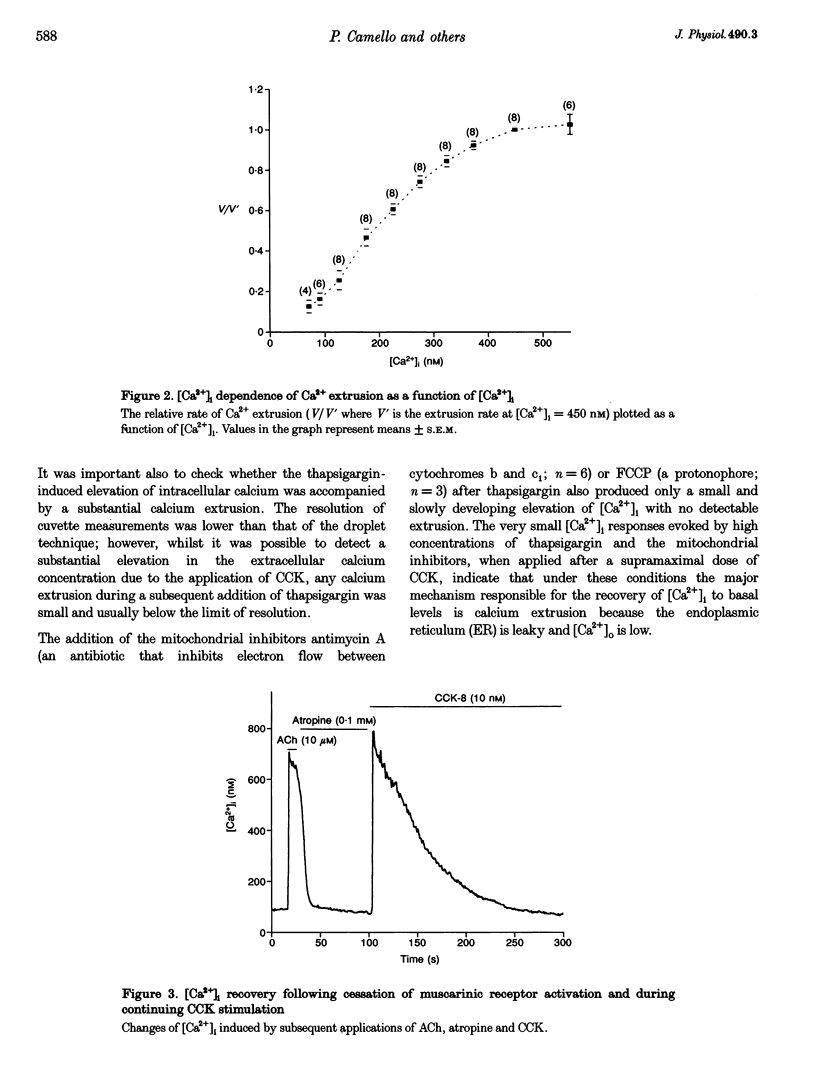
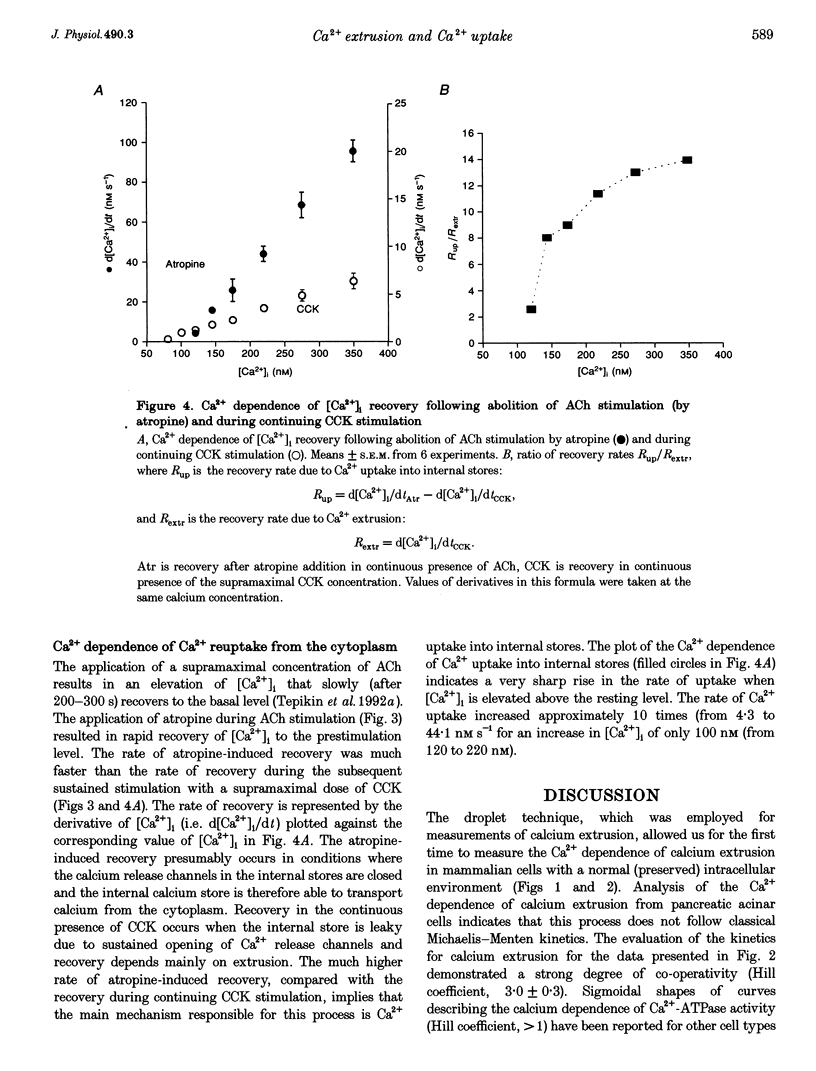
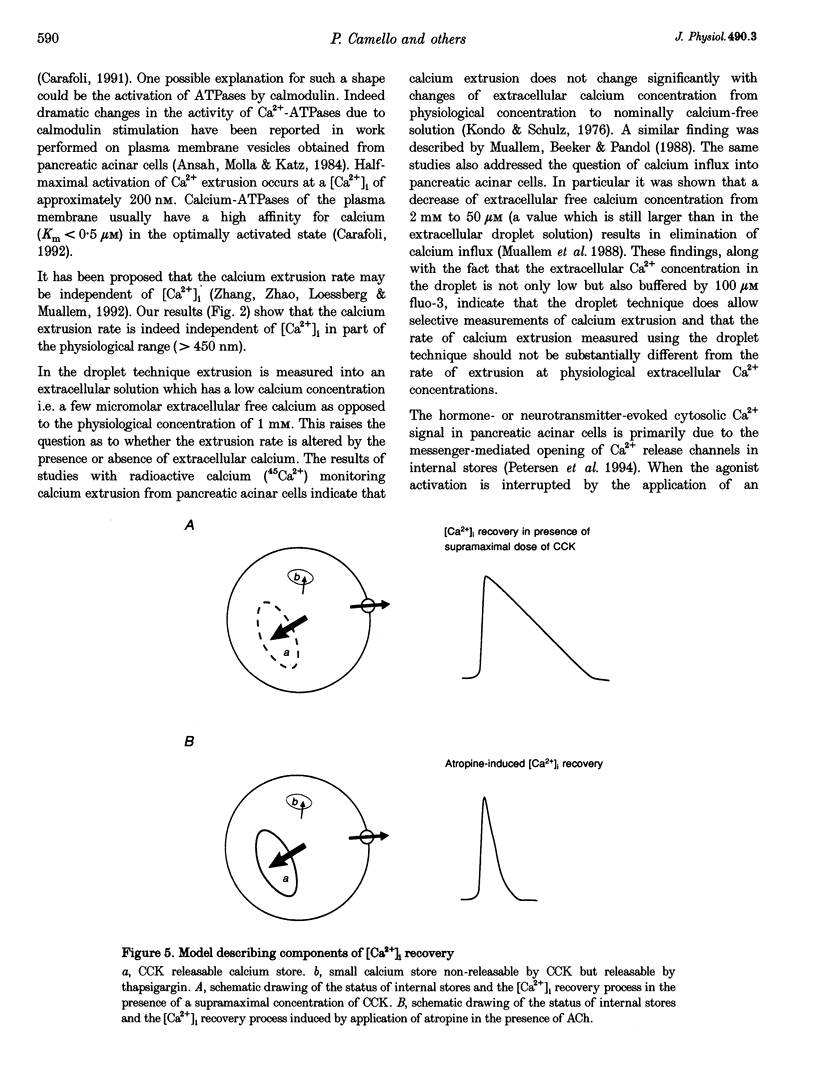
1. The droplet technique was used to investigate the calcium dependence of calcium extrusion from pancreatic acinar cells with preserved intracellular environments. The calcium dependence of calcium extrusion indicated a strong co-operativity (Hill coefficient, 3). The half-maximal rate of calcium extrusion occurred at an intracellular free calcium concentration ([Ca2+]i) of approximately 200 nM. At [Ca2+]i levels higher than 400 nM the calcium extrusion mechanism was almost completely saturated. 2. The rate of [Ca2+]i recovery was measured with the same cells under conditions where both calcium extrusion and calcium reuptake occurred simultaneously and under conditions when calcium reuptake was prevented and recovery depended entirely upon calcium extrusion. The rate of [Ca2+]i recovery due to calcium reuptake displayed a very sharp dependence on [Ca2+]i. The rate of [Ca2+]i recovery due to reuptake increased approximately 10 times (from 4.3 to 44.1 nM s-1) for an increase of [Ca2+]i of only 100 nM (from 120 to 220 nM). 3. With a decrease of [Ca2+]i the ratio of rate of calcium extrusion to rate of calcium uptake into internal stores increased, indicating that extrusion plays a more important role at low [Ca2+]i levels. Data for [Ca2+]i recovery rates due to extrusion and due to reuptake allowed us to evaluate the absolute rate of calcium translocation into the internal stores during the recovery process. When [Ca2+]i = 350 nM the total (i.e. bound and free) calcium concentration in the cytosol decreased by approximately 100 microM s-1 due to calcium uptake into internal stores. The rate of uptake was approximately 20 times slower when [Ca2+]i = 120 nM.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Ansah T. A., Molla A., Katz S. Ca2+-ATPase activity in pancreatic acinar plasma membranes. Regulation by calmodulin and acidic phospholipids. J Biol Chem. 1984 Nov 10;259(21):13442–13450. [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O., Moody M. M., Depaoli A. M., Sharp A. H., Ross C. A., Swift H., Bell G. I. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992 Feb 5;267(4):2115–2118. [PubMed] [Google Scholar]
- Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995 Feb 10;80(3):439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kondo S., Schulz I. Ca++ fluxes in isolated cells of rat pancreas. effect of secretagogues and different Ca++ concentrations. J Membr Biol. 1976 Oct 20;29(1-2):185–203. doi: 10.1007/BF01868959. [DOI] [PubMed] [Google Scholar]
- Konishi M., Baylor S. M. Myoplasmic calcium transients monitored with purpurate indicator dyes injected into intact frog skeletal muscle fibers. J Gen Physiol. 1991 Feb;97(2):245–270. doi: 10.1085/jgp.97.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Beeker T., Pandol S. J. Role of Na+/Ca2+ exchange and the plasma membrane Ca2+ pump in hormone-mediated Ca2+ efflux from pancreatic acini. J Membr Biol. 1988 May;102(2):153–162. doi: 10.1007/BF01870453. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M. S., Fimmel C. J., Pandol S. J. Agonist-sensitive calcium pool in the pancreatic acinar cell. I. Permeability properties. Am J Physiol. 1988 Aug;255(2 Pt 1):G221–G228. doi: 10.1152/ajpgi.1988.255.2.G221. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Petersen C. C., Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Tepikin A. V., Llopis J., Snitsarev V. A., Gallacher D. V., Petersen O. H. The droplet technique: measurement of calcium extrusion from single isolated mammalian cells. Pflugers Arch. 1994 Oct;428(5-6):664–670. doi: 10.1007/BF00374591. [DOI] [PubMed] [Google Scholar]
- Tepikin A. V., Voronina S. G., Gallacher D. V., Petersen O. H. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J Biol Chem. 1992 Feb 25;267(6):3569–3572. [PubMed] [Google Scholar]
- Tepikin A. V., Voronina S. G., Gallacher D. V., Petersen O. H. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J Biol Chem. 1992 Jul 15;267(20):14073–14076. [PubMed] [Google Scholar]
- Thorn P., Gerasimenko O., Petersen O. H. Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J. 1994 May 1;13(9):2038–2043. doi: 10.1002/j.1460-2075.1994.tb06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E. C., Lawrie A. M., Petersen O. H., Gallacher D. V. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J. 1992 Apr;11(4):1623–1629. doi: 10.1002/j.1460-2075.1992.tb05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule D. I., Lawrie A. M., Gallacher D. V. Acetylcholine and cholecystokinin induce different patterns of oscillating calcium signals in pancreatic acinar cells. Cell Calcium. 1991 Feb-Mar;12(2-3):145–151. doi: 10.1016/0143-4160(91)90016-8. [DOI] [PubMed] [Google Scholar]
- Zhang B. X., Zhao H., Loessberg P., Muallem S. Activation of the plasma membrane Ca2+ pump during agonist stimulation of pancreatic acini. J Biol Chem. 1992 Aug 5;267(22):15419–15425. [PubMed] [Google Scholar]


