Abstract
1. Whole-cell Ca2+ currents (ICa) from cultured rat melanotrophs were identified by their sensitivity to Ca2+ channel blockers, and their modulation by serotonin (5-HT) was studied. All cells displayed high voltage-activated (HVA; > -30 mV) Ca2+ currents. A low voltage-activated (LVA; > -60 mV) Ca2+ current was detected in 92% of the cells. 2. The whole-cell ICa was insensitive to omega-conotoxin GVIA (0.5-1 microM) indicating the absence of N-type Ca2+ channels. 3. At a holding potential (Vh) of -70 mV, the L-type channel blocker nifedipine reduced ICa in a dose-dependent manner with a half-maximal effective concentration (IC50) of 28 nM. The L-type current represented 39% of the total ICa. 4. omega-Agatoxin IVA (omega-Aga IVA) produced a biphasic dose-dependent inhibition of ICa, with IC50 values of 0.4 and 91 nM, indicating the presence of P-type and Q-type Ca2+ channels, which accounted respectively for 16 and 45% of the total ICa. The P-type current was also blocked by synthetic funnel-web spider toxin (sFTX 3.3; 1-10 microM) and was present only in a subpopulation (60-70%) of cells. 5. All cells possessed a Ca2+ current which was resistant to nifedipine (10 microM) and omega-Aga IVA (50 nM). This current was not affected by Ni2+ (40 microM) but was abolished by a low concentration of Cd2+ (10 microM) and by omega-conotoxin MVIIC (1 microM) indicating that it was a Q-type Ca2+ current. 6. 5-HT (10 microM) inhibited the whole-cell ICa in 70% of the cells tested (n = 120) by activating 5-HT1A and 5-HT2C receptors. 5-HT produced either a kinetic slowing of the activation phase (37% of the cells) or a scaling down (14% of the cells) of ICa. In the majority of cells (49%) both types of inhibition were found to coexist. 7. The effects of 5-HT were voltage dependent, rendered irreversible when GTP-gamma-S (30 microM) was present in the pipette solution and abolished by pretreatment of the cells with pertussis toxin (PTX; 150 ng ml-1, 18 h). 8. Low concentrations of omega-Aga IVA (20 nM), which blocked mainly P-type channels, did not reduce the effect of 5-HT on ICa. The scaling down effect of 5-HT on ICa was eliminated in the presence of nifedipine (10 microM) and the kinetic slowing effect of 5-HT persisted after blockade of L- and P-type channels but was abolished by omega-conotoxin MVIIC (1 microM). 9. We conclude that rat melanotrophs possess functional L-, P- and Q-type Ca2+ channels and that 5-HT inhibits selectively L-type and Q-type Ca2+ currents with different modalities. These effects are voltage dependent and mediated by a PTX-sensitive G-protein.
Full text
PDF


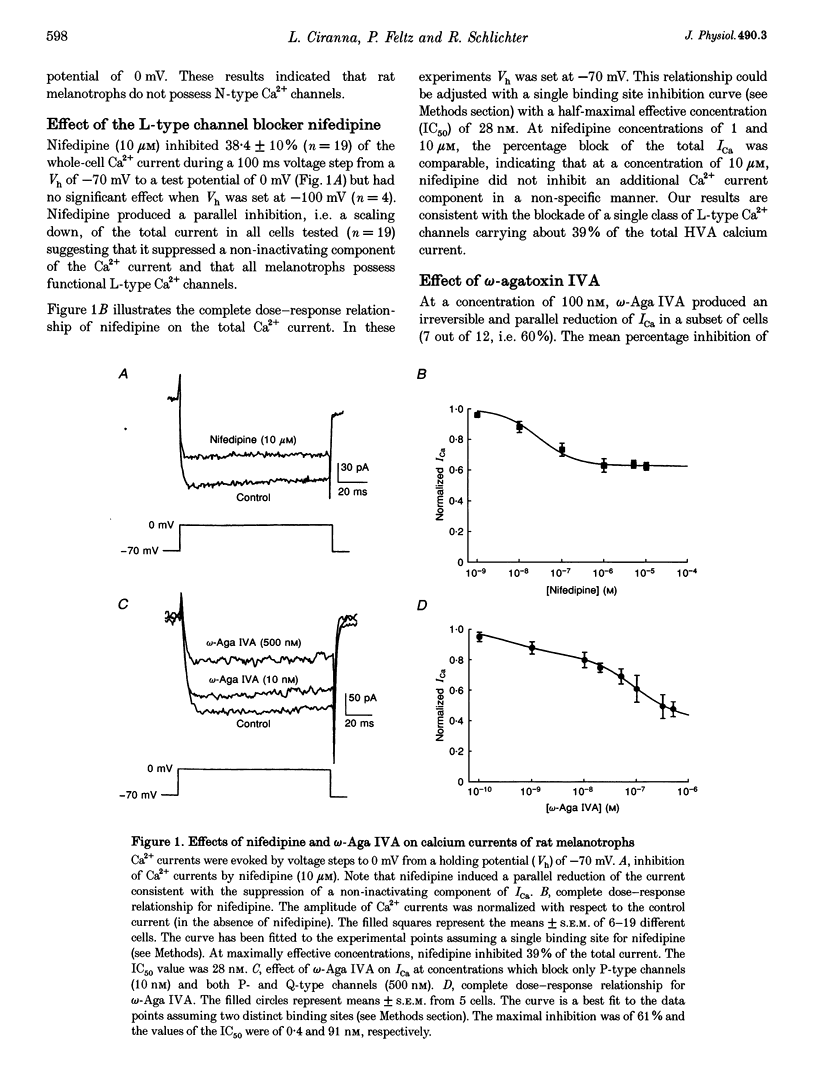





Selected References
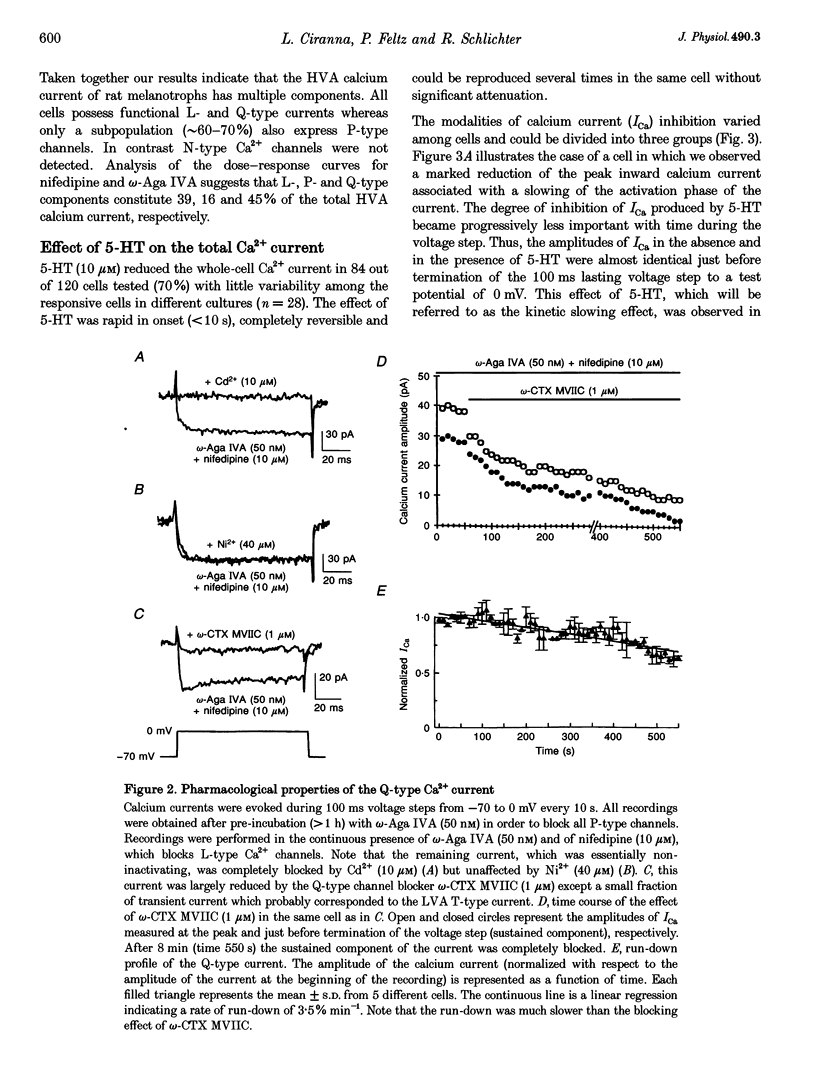
These references are in PubMed. This may not be the complete list of references from this article.
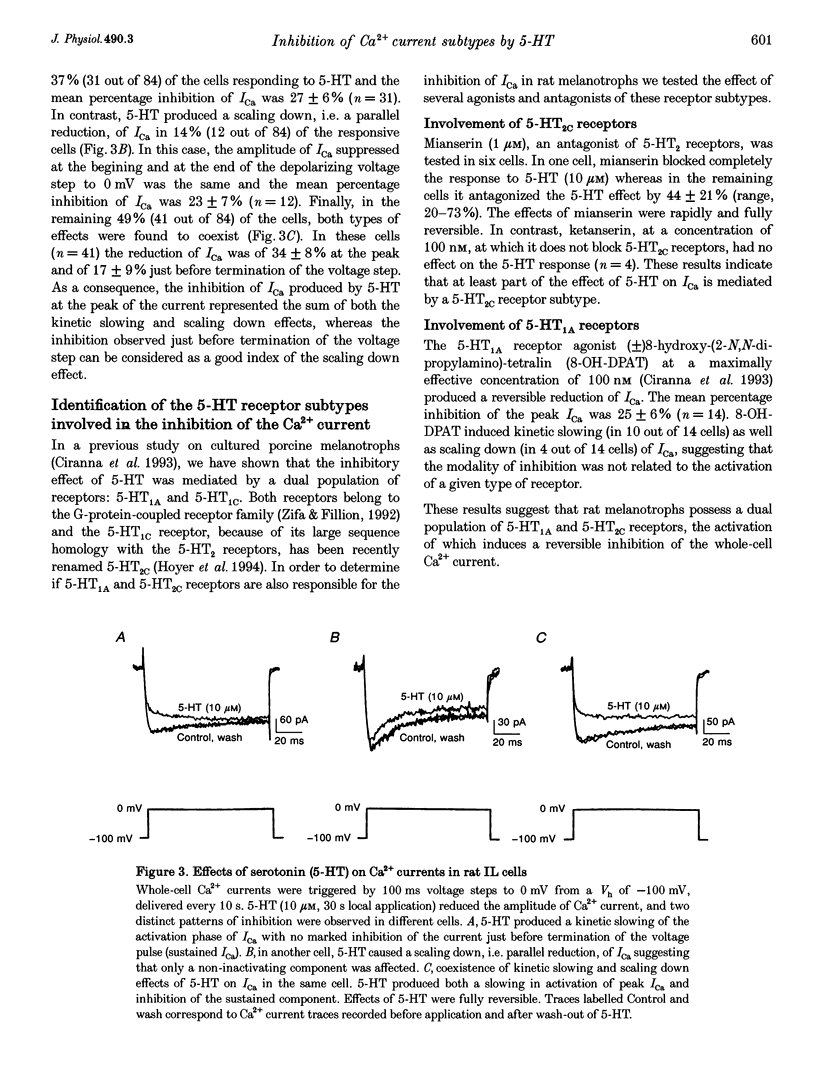
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
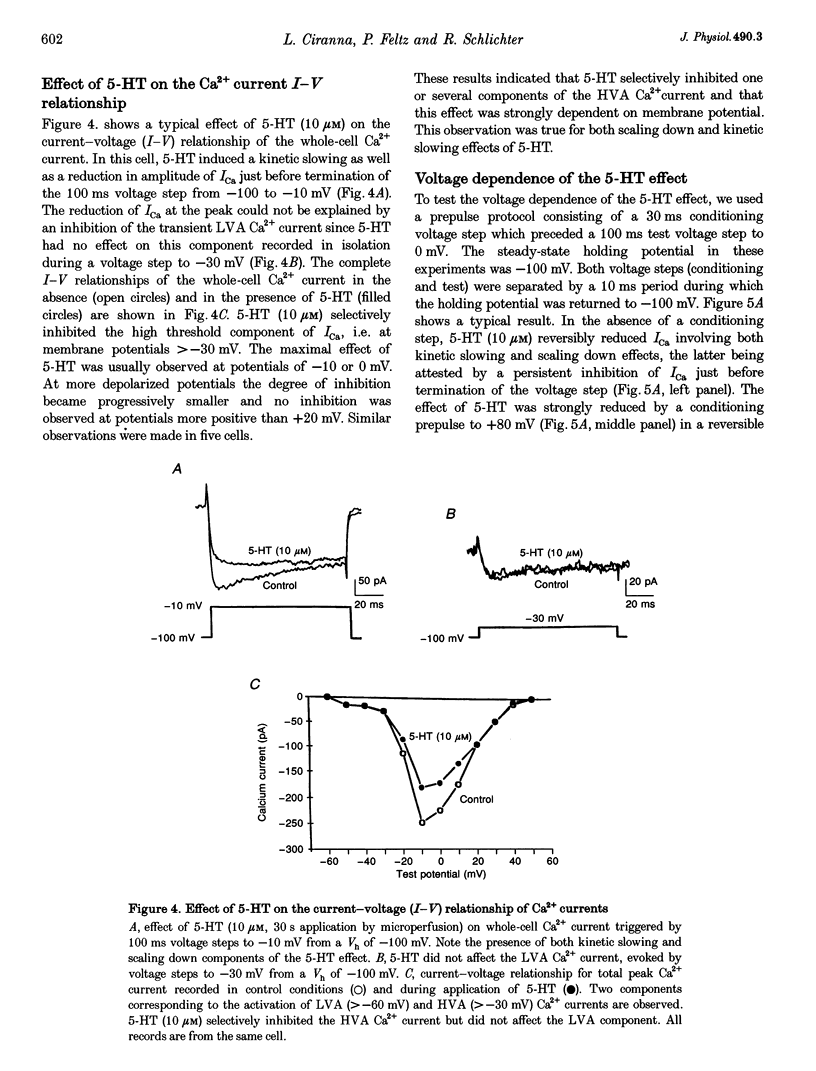
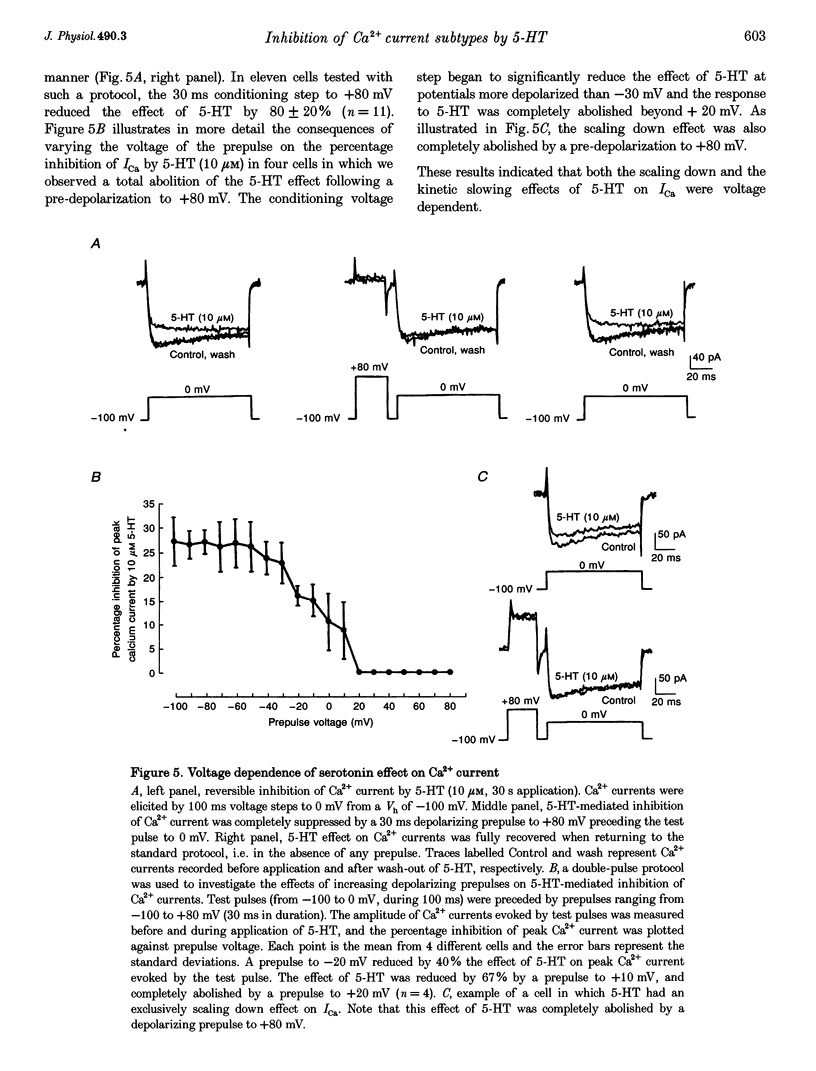
- Beech D. J., Bernheim L., Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992 Jan;8(1):97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
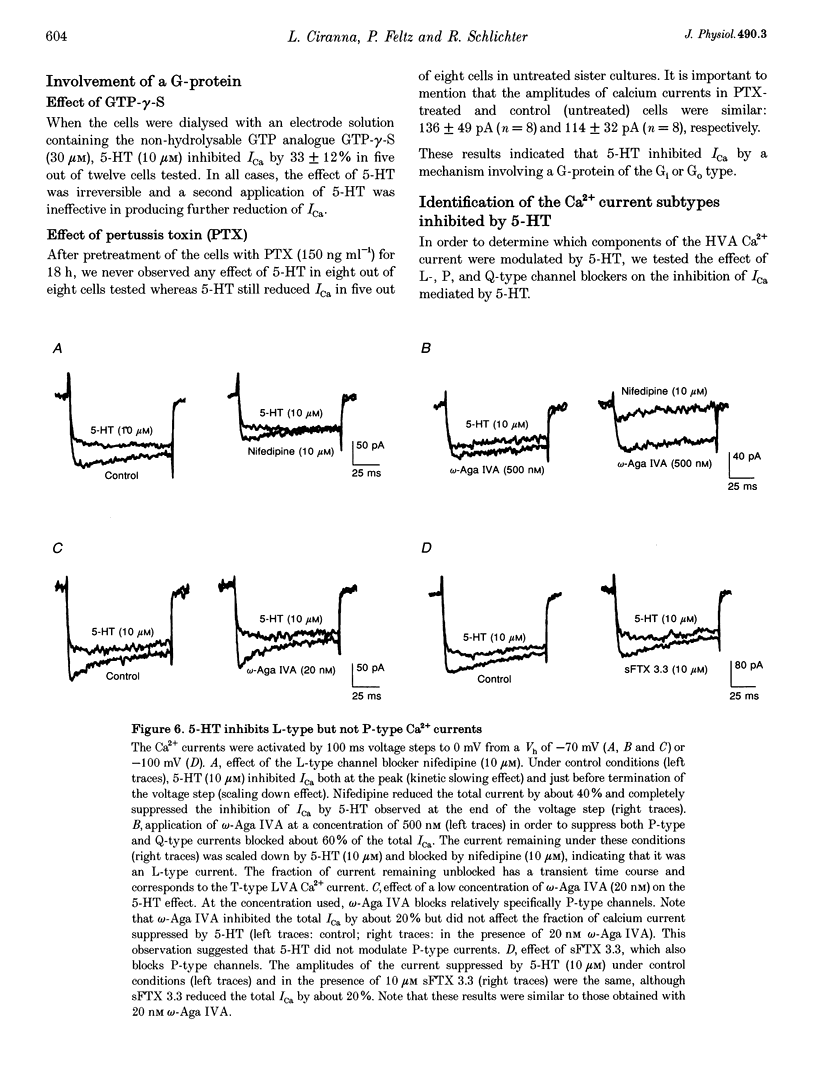
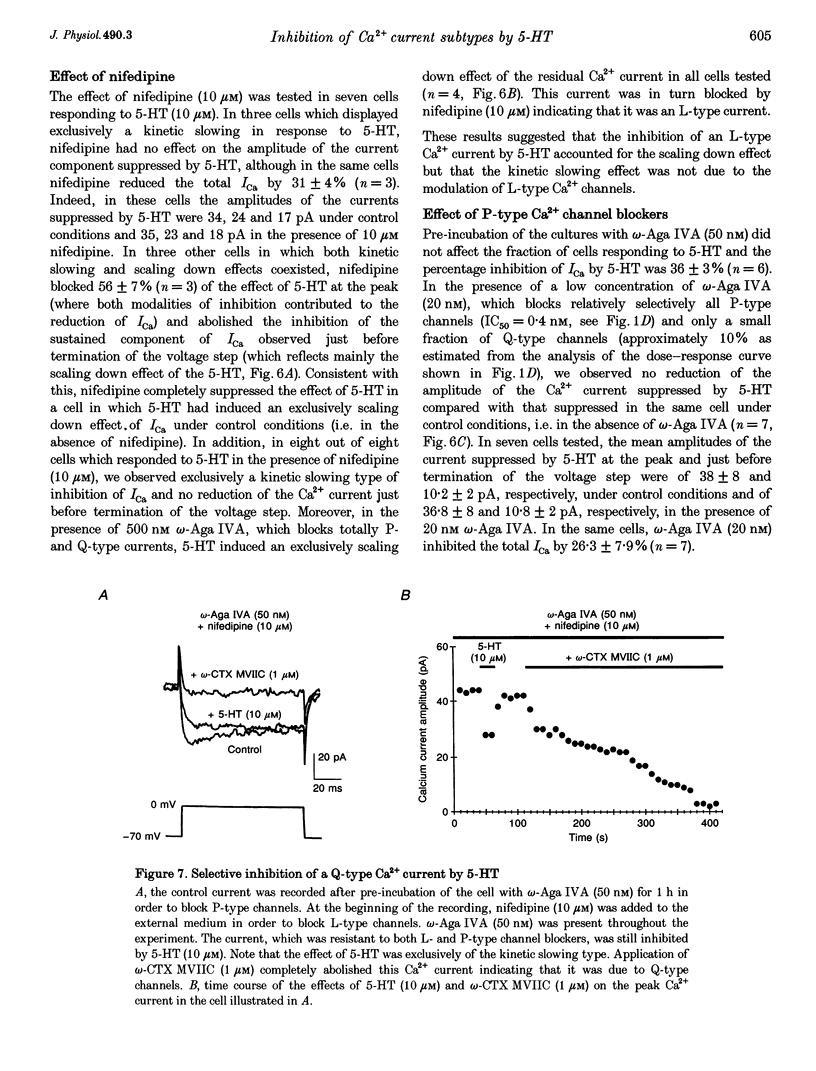
- Boland L. M., Bean B. P. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993 Feb;13(2):516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciranna L., Mouginot D., Feltz P., Schlichter R. Serotonin inhibits Ca2+ currents in porcine melanotrophs by activating 5-HT1C and 5-HT1A receptors. J Physiol. 1993 Apr;463:17–38. doi: 10.1113/jphysiol.1993.sp019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty R. J., McFadzean I. Noradrenaline-Induced Inhibition of Voltage-Sensitive Calcium Currents in NG108-15 Hybrid Cells. Eur J Neurosci. 1989 Mar;1(2):132–140. doi: 10.1111/j.1460-9568.1989.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Kammermeier P. J., Jones S. W. Calcium current modulation in frog sympathetic neurones: L-current is relatively insensitive to neurotransmitters. J Physiol. 1992 Oct;456:107–123. doi: 10.1113/jphysiol.1992.sp019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golard A., Siegelbaum S. A. Kinetic basis for the voltage-dependent inhibition of N-type calcium current by somatostatin and norepinephrine in chick sympathetic neurons. J Neurosci. 1993 Sep;13(9):3884–3894. doi: 10.1523/JNEUROSCI.13-09-03884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Ikeda S. R. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992 Dec;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keja J. A., Kits K. S. Single-channel properties of high- and low-voltage-activated calcium channels in rat pituitary melanotropic cells. J Neurophysiol. 1994 Mar;71(3):840–855. doi: 10.1152/jn.1994.71.3.840. [DOI] [PubMed] [Google Scholar]
- Keja J. A., Kits K. S. Voltage dependence of G-protein-mediated inhibition of high-voltage-activated calcium channels in rat pituitary melanotropes. Neuroscience. 1994 Sep;62(1):281–289. doi: 10.1016/0306-4522(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Kaczmarek L. K., Levitan E. S. Neuropeptide inhibition of voltage-gated calcium channels mediated by mobilization of intracellular calcium. Neuron. 1991 Apr;6(4):557–563. doi: 10.1016/0896-6273(91)90058-8. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Lopez H. S., Brown A. M. Correlation between G protein activation and reblocking kinetics of Ca2+ channel currents in rat sensory neurons. Neuron. 1991 Dec;7(6):1061–1068. doi: 10.1016/0896-6273(91)90350-9. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E., Léránth C., Brownstein M. J., Friedman E., Krieger D. T., Palkovits M. On the origin of the serotonergic input to the intermediate lobe of the rat pituitary. Brain Res. 1984 Mar 5;294(2):231–237. doi: 10.1016/0006-8993(84)91034-5. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Taraskevich P. S., Douglas W. W. Cytosolic Ca2+ in melanotrophs: pharmacological insights into regulatory influences of electrical activity and ion channels. Endocrinology. 1990 Feb;126(2):754–758. doi: 10.1210/endo-126-2-754. [DOI] [PubMed] [Google Scholar]
- Penington N. J., Kelly J. S., Fox A. P. A study of the mechanism of Ca2+ current inhibition produced by serotonin in rat dorsal raphe neurons. J Neurosci. 1991 Nov;11(11):3594–3609. doi: 10.1523/JNEUROSCI.11-11-03594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara Y., Westbrook G. L. Modulation of calcium currents by a metabotropic glutamate receptor involves fast and slow kinetic components in cultured hippocampal neurons. J Neurosci. 1993 Jul;13(7):3041–3050. doi: 10.1523/JNEUROSCI.13-07-03041.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Schwindt P. C., Crill W. E. Metabotropic glutamate receptor-mediated suppression of L-type calcium current in acutely isolated neocortical neurons. J Neurophysiol. 1992 Sep;68(3):833–842. doi: 10.1152/jn.1992.68.3.833. [DOI] [PubMed] [Google Scholar]
- Schultz G., Rosenthal W., Hescheler J., Trautwein W. Role of G proteins in calcium channel modulation. Annu Rev Physiol. 1990;52:275–292. doi: 10.1146/annurev.ph.52.030190.001423. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Pearson H. A., Dolphin A. C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36(6):485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sweeney M. I., Kobrinsky E. M., Pearson H. A., Timms G. H., Pullar I. A., Wedley S., Dolphin A. C. Actions of arginine polyamine on voltage and ligand-activated whole cell currents recorded from cultured neurones. Br J Pharmacol. 1992 May;106(1):199–207. doi: 10.1111/j.1476-5381.1992.tb14315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J., Surprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. J Physiol. 1991 Aug;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Treistman S. N., Lemos J. R. Two types of high-threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol. 1992 Jan;445:181–199. doi: 10.1113/jphysiol.1992.sp018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. Electrophysiological properties of neuroendocrine cells of the intact rat pars intermedia: multiple calcium currents. J Neurosci. 1990 Mar;10(3):748–756. doi: 10.1523/JNEUROSCI.10-03-00748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. Synaptic modulation by dopamine of calcium currents in rat pars intermedia. J Neurosci. 1990 Mar;10(3):757–763. doi: 10.1523/JNEUROSCI.10-03-00757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. J., Pittman Q. J., MacVicar B. A. Blockade by funnel web toxin of a calcium current in the intermediate pituitary of the rat. Neurosci Lett. 1993 Jul 23;157(2):171–174. doi: 10.1016/0304-3940(93)90729-5. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Zifa E., Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992 Sep;44(3):401–458. [PubMed] [Google Scholar]


