Abstract
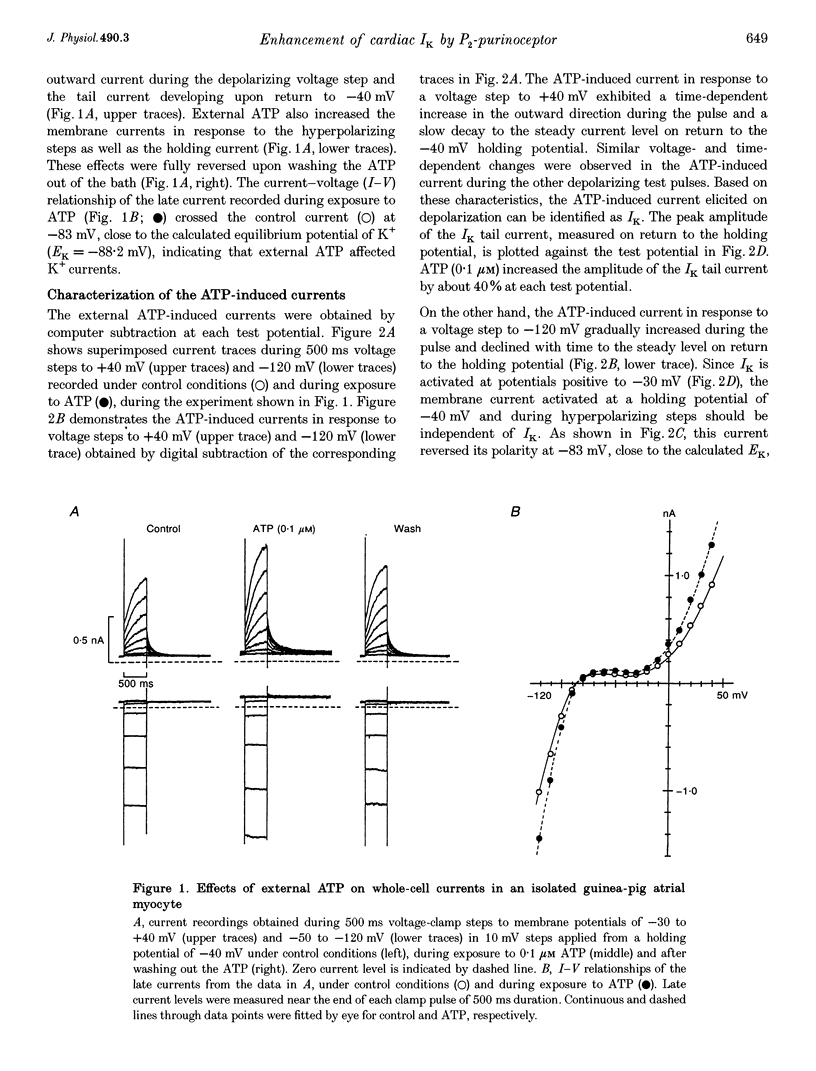
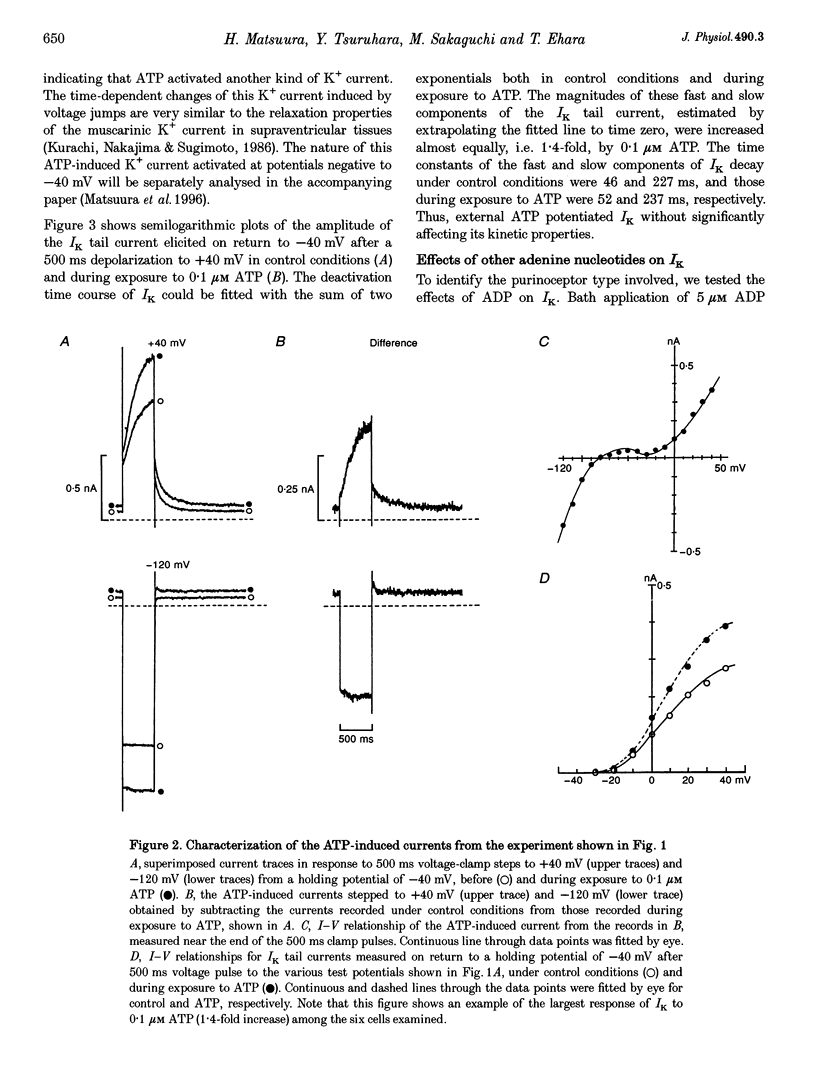
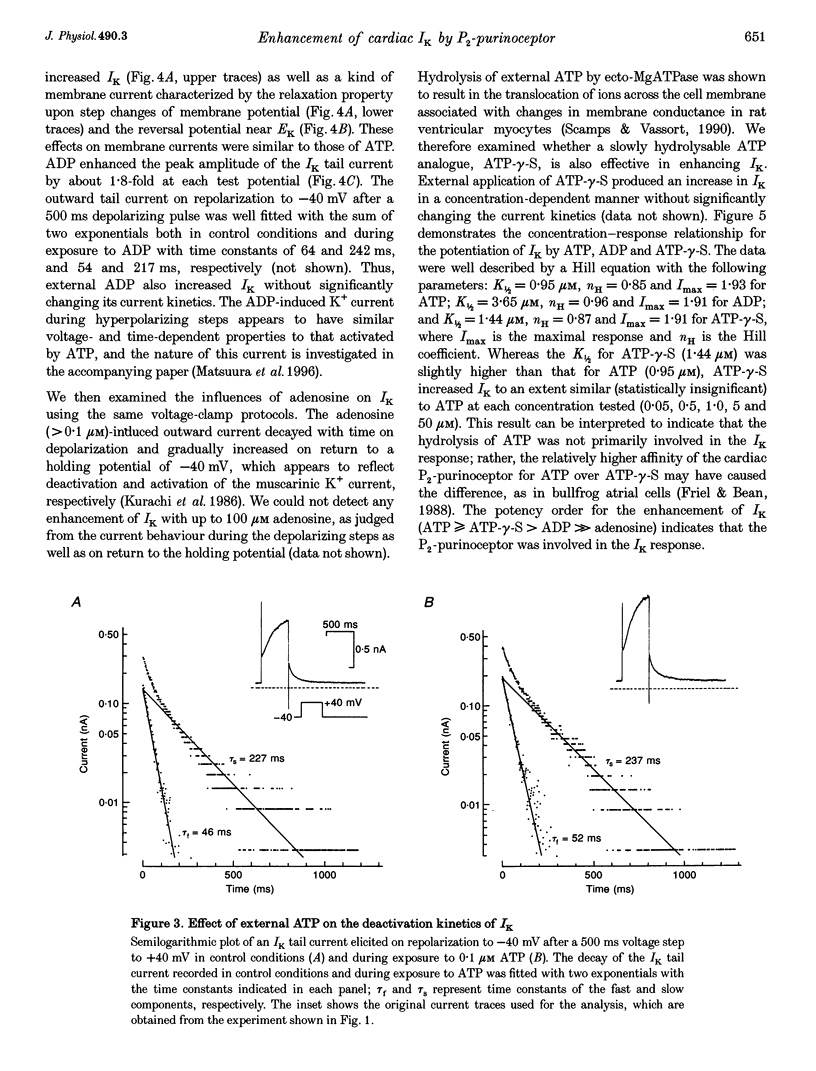
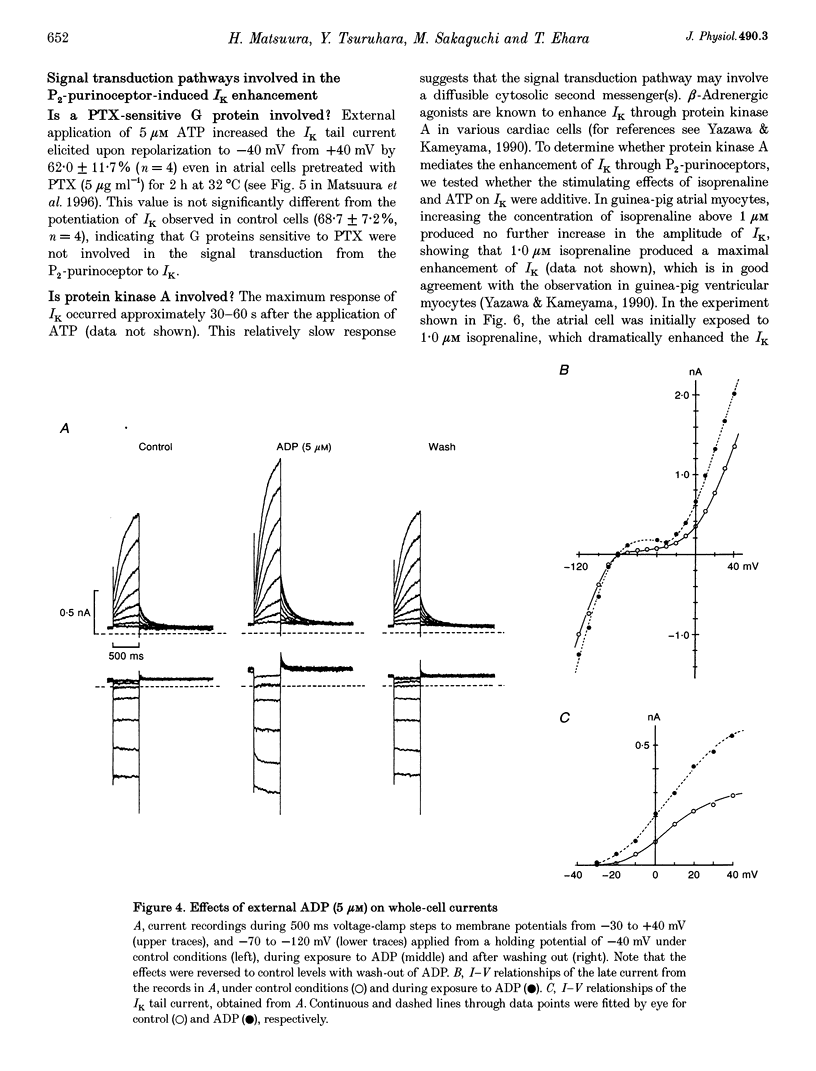
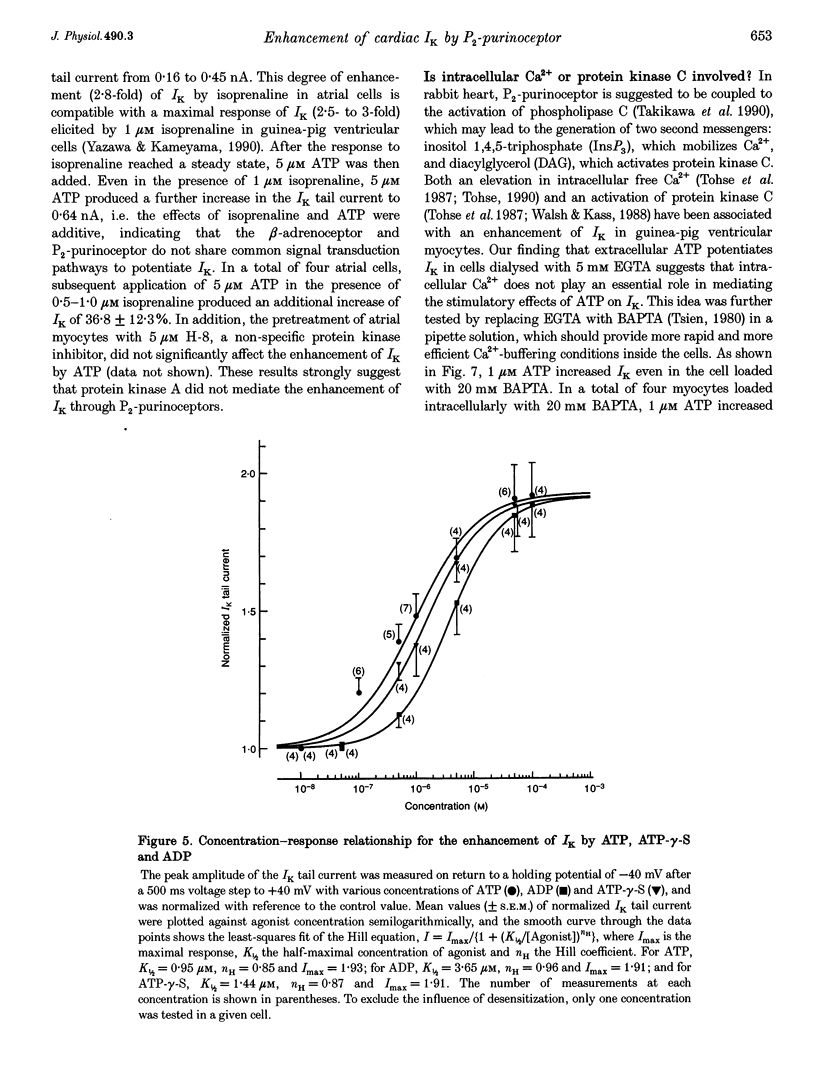
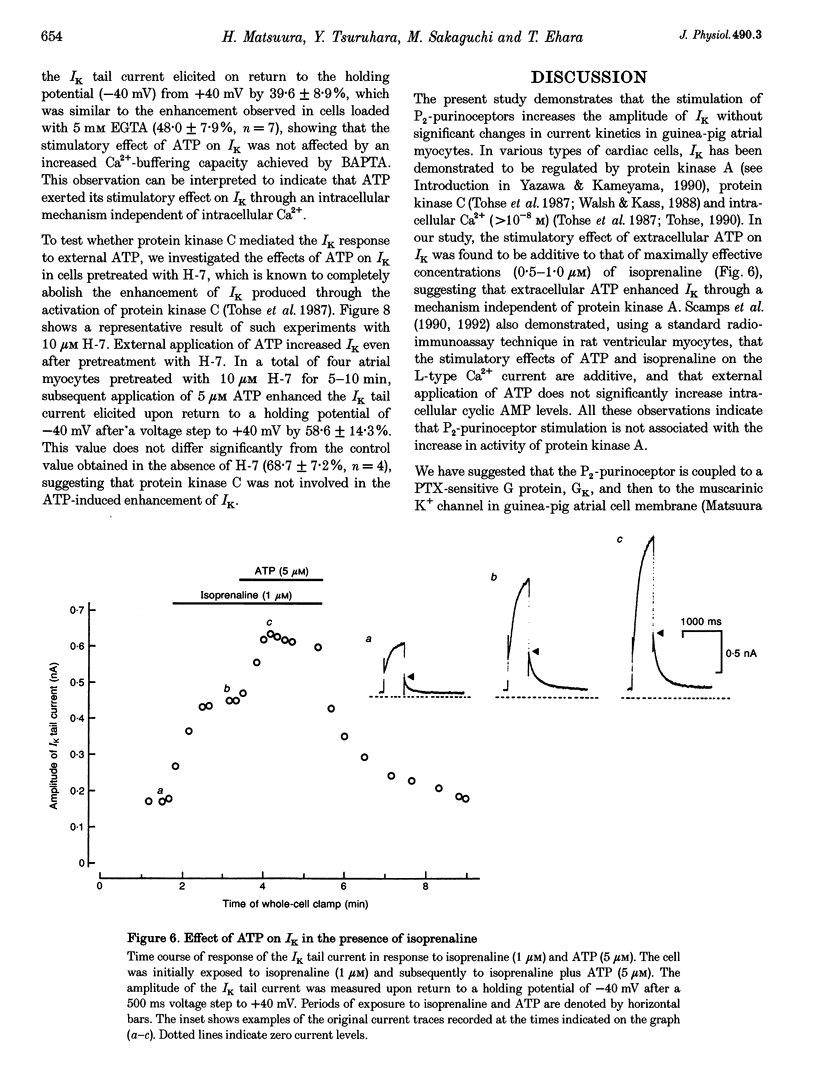
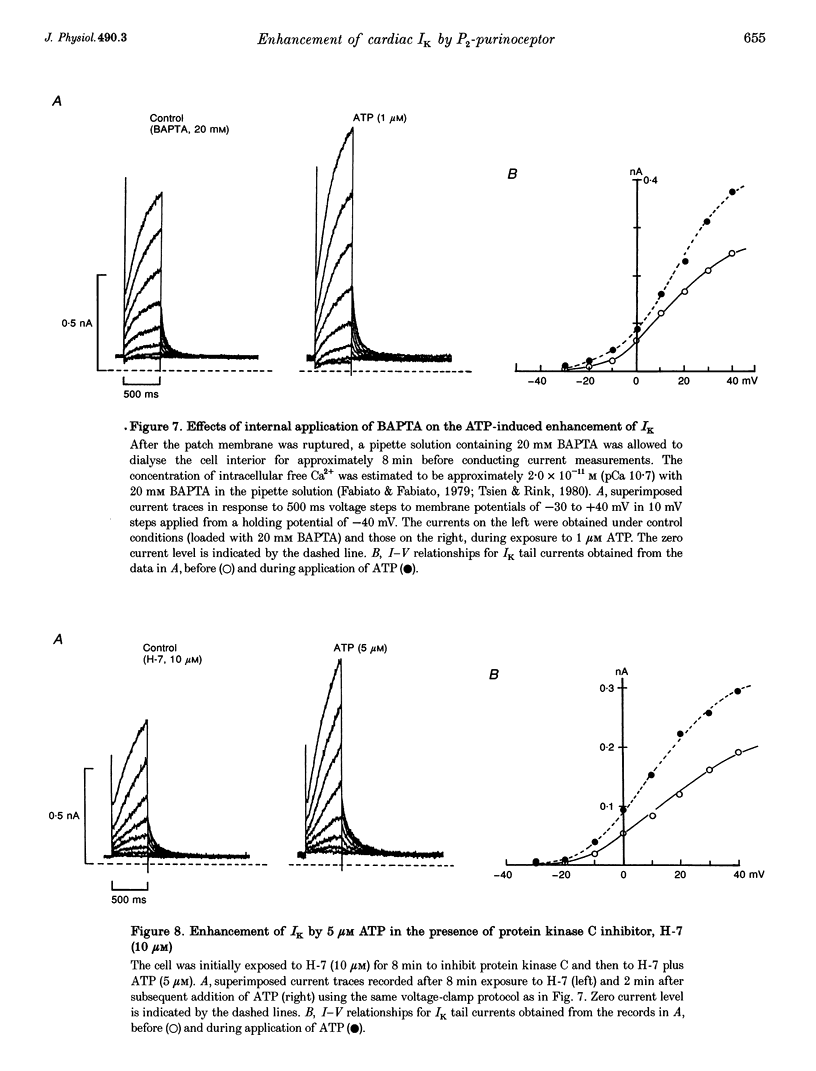
1. We studied the effects of P2-purinoceptor stimulation on the delayed rectifier K+ current (IK) in guinea-pig atrial myocytes using a whole-cell voltage-clamp technique. 2. External application of ATP increased IK, evoked by a 500 ms depolarizing pulse from a holding potential of -40 mV, under conditions in which the L-type Ca2+ channel was blocked; the effect was dose dependent with a half-maximal concentration (K1/2) of 0.95 microM. ATP (50 microM) produced a maximal increase of IK of about a factor of 2. 3. External ADP also enhanced IK in a dose-dependent manner with a K1/2 of 3.65 microM, whereas adenosine (100 microM) failed to evoke this response. Theophylline (500 microM), a blocker of the Pi-purinoceptor, did not antagonize the stimulating action of ATP on IK. These results indicate that IK was enhanced via P2-purinoceptors. 4. External ATP or ADP did not produce a significant change in the current kinetics of IK. 5. Pre-incubation of the atrial myocytes with pertussis toxin (PTX, 5 micrograms ml-1) did not affect the stimulating action of ATP on IK, indicating that PTX-sensitive G proteins did not mediate the ATP action. 6. The enhancement of IK by ATP developed slowly; the effects usually reached a maximum approximately 30-60 s after the application of ATP. This suggests the involvement of a diffusible cytosolic second messenger(s) in the response. ATP could further increase IK after maximal enhancement by isoprenaline (0.5-1.0 microM), suggesting that the intermediate steps were independent of cyclic AMP-dependent protein kinase (protein kinase A). 7. Potentiation of IK by ATP was not attenuated by either (i) pretreatment of the cells with 5 microM 1-(5-isoquinolinylsulphonyl)-2-methylpiperazine dihydrochloride (H-7) or (ii) intracellular perfusion of 20 mM 1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), suggesting that protein kinase C and intracellular Ca2+ did not mediate the response. 8. It is concluded that the activation of P2-purinoceptors increases IK through intracellular mechanisms independent of protein kinase A, protein kinase C or intracellular free Ca2+ in guinea-pig atrial myocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Born G. V., Kratzer M. A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984 Sep;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst M. M., Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res. 1991 Mar;68(3):797–806. doi: 10.1161/01.res.68.3.797. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Meghji P. The effect of adenyl compounds on the rat heart. Br J Pharmacol. 1983 May;79(1):211–218. doi: 10.1111/j.1476-5381.1983.tb10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurones. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Dual control by ATP and acetylcholine of inwardly rectifying K+ channels in bovine atrial cells. Pflugers Arch. 1990 Mar;415(6):651–657. doi: 10.1007/BF02584001. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. External ATP-induced changes in [Ca2+]i and membrane currents in mammalian atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 1):C673–C680. doi: 10.1152/ajpcell.1991.260.4.C673. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986 May 27;124(3):285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Nairn A. C., Gadsby D. C. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J Gen Physiol. 1992 Apr;99(4):465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Sakaguchi M., Tsuruhara Y., Ehara T. Activation of the muscarinic K+ channel by P2-purinoceptors via pertussis toxin-sensitive G proteins in guinea-pig atrial cells. J Physiol. 1996 Feb 1;490(Pt 3):659–671. doi: 10.1113/jphysiol.1996.sp021175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Vassort G. Extracellular MgATP activates the Cl-/HCO3- exchanger in single rat cardiac cells. J Physiol. 1991 Dec;444:241–256. doi: 10.1113/jphysiol.1991.sp018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin A. C., Sitsapesan R., Kane K. A. Antagonism by adenosine and ATP of an isoprenaline-induced background current in guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1990 Dec;22(12):1371–1378. doi: 10.1016/0022-2828(90)90982-8. [DOI] [PubMed] [Google Scholar]
- Scamps F., Legssyer A., Mayoux E., Vassort G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res. 1990 Oct;67(4):1007–1016. doi: 10.1161/01.res.67.4.1007. [DOI] [PubMed] [Google Scholar]
- Scamps F., Rybin V., Puceat M., Tkachuk V., Vassort G. A Gs protein couples P2-purinergic stimulation to cardiac Ca channels without cyclic AMP production. J Gen Physiol. 1992 Oct;100(4):675–701. doi: 10.1085/jgp.100.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Vassort G. Mechanism of extracellular ATP-induced depolarization in rat isolated ventricular cardiomyocytes. Pflugers Arch. 1990 Nov;417(3):309–316. doi: 10.1007/BF00370997. [DOI] [PubMed] [Google Scholar]
- Takikawa R., Kurachi Y., Mashima S., Sugimoto T. Adenosine-5'-triphosphate-induced sinus tachycardia mediated by prostaglandin synthesis via phospholipase C in the rabbit heart. Pflugers Arch. 1990 Sep;417(1):13–20. doi: 10.1007/BF00370763. [DOI] [PubMed] [Google Scholar]
- Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990 Apr;258(4 Pt 2):H1200–H1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Tohse N., Nakaya H., Kanno M. Alpha 1-adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circ Res. 1992 Dec;71(6):1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. S., Christie A., Levy M. N., Scarpa A. Modulation by extracellular ATP of two distinct currents in rat myocytes. Am J Physiol. 1993 Jun;264(6 Pt 1):C1411–C1417. doi: 10.1152/ajpcell.1993.264.6.C1411. [DOI] [PubMed] [Google Scholar]



