Abstract
Introduction
COPD is characterised by airflow obstruction, expiratory airway collapse and closure causing expiratory flow limitation (EFL) and hyperinflation. Supine posture may worsen ventilatory function in COPD, which may cause hyperinflation to persist and contribute to symptoms of orthopnoea and sleep disturbance. Our aim was to determine the impact of supine posture on hyperinflation, dynamic elastance and EFL in COPD and healthy subjects. We hypothesised that changes in hyperinflation in supine posture are influenced by EFL and gas trapping in COPD.
Methods
Clinically stable COPD patients (compatible symptoms, smoking >10 pack-years, obstructed spirometry) and healthy controls underwent oscillometry in the seated and supine positions. Hyperinflation was measured by inspiratory capacity (IC) and the ratio of IC to total lung capacity (IC/TLC) while seated and supine EFL was measured as the difference in mean inspiratory and mean expiratory oscillatory reactance at 5 Hz (Xrs5). Relationships between IC, IC/TLC and Xrs5, were examined by Spearman correlation.
Results
42 COPD patients demonstrated no change in IC/TLC from seated (0.31 L) to supine (0.32 L) position (p=0.079) compared to significant increases seen in 14 control subjects (0.37 L seated versus 0.44 L supine; p<0.001). In COPD, worse dynamic elastance (Xrs5 rs 0.499; p=0.001) and EFL (ΔXrs5 rs −0.413; p=0.007), along with increased age and lower body-mass-index were predictors of supine hyperinflation.
Conclusion
Supine persistent hyperinflation occurs in COPD and is associated with increased dynamic elastance and EFL, likely the result of increased airway closure due to gravitational redistribution of lung mass.
Shareable abstract
COPD patients demonstrate hyperinflation with failure to increase supine IC and reduced supine IC/TLC ratio compared with healthy controls. Supine persistent hyperinflation strongly correlates with increased expiratory flow limitation. https://bit.ly/45fh3nX
Introduction
COPD is a leading cause of morbidity and mortality worldwide. It is characterised by airflow obstruction and expiratory airway collapse and closure which may lead to expiratory flow limitation (EFL), hyperinflation [1, 2] and worse dyspnoea [3–5]. Worsening dyspnoea when lying down is common in COPD but under-recognised and may contribute to overall symptom burden and sleep disturbance, which is also commonly reported in COPD [6–9]. Supine posture causes changes in respiratory mechanics, which likely contribute to worse symptoms and sleep disturbance [10, 11]. Therefore, it would be useful to better understand the mechanical changes that occur in supine posture in COPD, which may be useful treatment targets in the future.
In healthy individuals, inspiratory capacity (IC) increases in the supine position associated with a decrease in functional residual capacity (FRC) [11, 12]. In COPD, there is a failure to decrease FRC, i.e. relative supine hyperinflation is present which would result in a failure to increase IC, indicating worse ventilatory impairment and greater work of breathing [11]. Respiratory mechanics are strongly determined by gravity, such that ventilation is increased in dependent lung zones and decreased in areas of airway closure, which is in turn dependent on gravity, ageing and disease. In the supine posture, ventilation and airway closure remain gravity-dependent but are additionally influenced by the decreased FRC, which itself may decrease ventilation through increased airway closure. In COPD, there are additional factors operating which may influence ventilatory function when supine. EFL worsens in the supine position in COPD and supine EFL is associated with more severe airflow obstruction, orthopnoea and nocturnal symptoms [6, 10, 13]. EFL is likely a strong driver of supine hyperinflation, with some COPD patients maintaining an elevated FRC to prevent EFL and to limit airway closure worsening. This could perhaps minimise the work of breathing needed to maintain ventilation.
Respiratory oscillometry is an effort-independent lung function test which involves superimposition of multi-frequency pressure waves over tidal breathing. Respiratory system impedance is measured which comprises two components: resistance (Rrs), reflecting overall airway calibre and reactance (Xrs), reflecting the elastic properties of the respiratory system under oscillatory (dynamic) conditions. Both Rrs and Xrs are sensitive to the uneven (heterogeneous) distribution of ventilation in COPD [14]. In addition, both, but particularly Xrs, are sensitive to severe airway narrowing, expiratory collapse and airway closure [15–17]. Xrs is particularly sensitive because of the reduction in the volume of lung that is accessible to oscillations, making the lungs appear mechanically stiffer under oscillatory conditions (including breathing) i.e. higher dynamic elastance, which reflects the increased work of breathing in COPD [15]. Therefore, more negative Xrs during tidal volume breathing indicates higher dynamic elastance, which reflects the functional consequences of heterogeneous airway narrowing, airway closure and EFL.
Expiratory airway collapse (and hence EFL) can be measured by looking at expiratory and inspiratory Xrs separately. A greater reduction in Xrs during expiration reflects increased airway closure and dynamic elastance, giving a greater differential between inspiratory and expiratory Xrs (ΔXrs) which accurately predicts EFL. Thus, respiratory oscillometry can provide a highly sensitive measure of EFL during tidal breathing in COPD [16, 18].
The effects of postural change on the interactions between hyperinflation, dynamic elastance and EFL in COPD have not been previously reported. Our aims were therefore, to compare the impacts of supine posture on hyperinflation, dynamic elastance (Xrs) and EFL, between COPD and healthy subjects. We also determined potential predictors of supine persistent hyperinflation in COPD and of supine FRC in healthy subjects. We hypothesised that increased dynamic elastance and EFL are associated with supine FRC in COPD, but not in healthy subjects.
Methods
Study subjects
Current or ex-smokers, aged over 40 years with a physician diagnosis of COPD, were recruited from outpatient services at the Royal North Shore Hospital, a large tertiary referral hospital in metropolitan Sydney, Australia, and the Woolcock Institute of Medical Research patient volunteer database. Patients with a recent (<8 weeks) exacerbation of COPD or significant comorbid cardiorespiratory disease were excluded. Diagnosis of COPD was confirmed by a post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio ≤0.7 or the lower limit of normal, whichever was lower. Healthy subjects were lifelong non-smokers with no symptoms or diagnosis of respiratory disease, no use of respiratory medications and normal spirometry. Ethics approval was obtained prior to patient recruitment from the Northern Sydney Local Health District Human Research Ethics Committee (2021)/ETH01142 and The Bellberry Human Research Ethics Committee (2015-08-603). All subjects provided written informed consent.
Study design
This was a prospective observational study where subjects underwent seated and supine oscillometry measurements. Spirometry and lung volumes were measured following the completion of oscillometry testing.
Physiological measurements
Spirometry and body plethysmography were performed according to American Thoracic Society/European Respiratory Society criteria [19].
Impedance was measured using a TremoFlo oscillometry device (Thorasys Thoracic Medical Systems, Montreal, Canada) as per European Respiratory Society recommendations, using a 5-, 11- and 19-Hz multi-frequency pressure oscillation [20]. Calibration was checked daily using a test load of known resistance and reactance, supplied by the manufacturer. A minimum of three technically acceptable 30-s trials were performed in seated and supine position. Subjects were instructed to breathe normally, through an anti-bacterial filter while wearing a nose clip, holding their head in a slightly chin-up position and supporting their own cheeks. After a period of breathing stabilisation, three 30-s recordings of tidal breathing were made with the operator monitoring the subject's head position, and measurements for leaks, swallows or cough. Three maximal deep inspiratory breaths were performed in the seated position from which IC was calculated. Subjects were moved into the supine position, supported with one pillow under their head. In the supine posture the same neutral neck position was maintained with the operator holding the device in position and tidal breathing and IC breaths were again recorded.
Trials containing artifacts were excluded and measurements repeated. Measurements were assessed off-line for quality (M. Srinivasan). Any breaths containing resistances that were negative or were >5 standard deviations above the mean were excluded. Supine persistent hyperinflation was measured by the change in seated to supine IC and the supine IC/TLC ratio. There are no agreed upon criteria to define the presence of supine persistent hyperinflation. In our study, the within-subject variability when measuring IC was approximately 200 mL. We have, therefore, used this as a cut-off for defining supine persistent hyperinflation. TremoFlo (version 1.0.44.40, 2019) was used for offline analysis of data and data export.
We report only the impedance results for 5 Hz. The mean Rrs and Xrs of each acquisition was calculated, from which the mean of the three recordings were calculated (Rrsmean and Xrsmean). We also calculated Rrs and Xrs of expiration and inspiration separately and calculated the difference between the mean inspiratory and expiratory Xrs (ΔXrs), as the mean of all inspiratory Xrs in the acquisition, minus the mean of all expiratory Xrs, then calculating the mean difference of the three acquisitions. This method is slightly different to the technique described by Dellacà et al. [18] where within-breath differences in inspiratory and expiratory Xrs were calculated. Due to technical difficulties, we were unable to calculate intra-breath differences and given both methods yield similar results, we proceeded to use the threshold value of ΔXrs ≥2.8 cmH2O·L−1·s−1 during tidal volume breathing indicating that significant EFL was present [18]. This value has been demonstrated to accurately predict flow limitation in COPD in both the seated and supine position [16]. All pulmonary function and oscillometry parameters were expressed as z-scores, using Global Lung Function Initiative equations for spirometry [21] and lung volumes [22], Quanjer et al. [23] for FRC/TLC and Oostveen et al. [24] for oscillometry.
Statistical analysis
Prior research has reported a mean IC change between the seated and supine position of approximately 400 mL in healthy subjects [11]. It was therefore estimated that at least 27 COPD patients were required at 80% power to detect a change in IC of 400 mL, type 1 error 0.05.
The only published data on supine change in ΔXrs was by Milesi et al. [25] which showed a small change of 0.74 cmH2O·s−1·L−1. This was measured with the patients using a continuous positive airway pressure (CPAP) mask which influenced the results. In our pilot studies we found a mean±sd change in ΔXrs seated to supine of 2.5±3.4 cmH2O·s−1·L−1. This led to a sample size calculation of 41 patients with a power of 80%, type 1 error 0.05. There is no published data on the relationship between the change in ΔXrs and change in IC. We anticipated a linear correlation coefficient of around 0.4 to arrive at a sample size of 41 with a power of 80%, type 1 error 0.05.
Data was analysed using Jamovi 2.3.28 and SPSS statistics. Graphs were generated through GraphPad Prism version 10.2.2. Data are presented as mean±sd or median (interquartile range) as appropriate. Paired samples t-test was performed to analyse differences in oscillometry parameters and IC between the seated and supine positions. The association between supine hyperinflation and baseline anthropomorphic and spirometric parameters was measured by Spearman's correlation due to not normally distributed data. A p-value of <0.05 was considered statistically significant.
Results
42 stable COPD patients without relevant comorbidities and 14 healthy controls were enrolled in the study. Anthropomorphic and clinical data of the two groups is shown in tables 1 and 2. COPD subjects were slightly older but with similar body mass index (BMI) and sex distribution. COPD patients had moderately severe airflow obstruction and mild baseline gas trapping (increased residual volume/total lung capacity, RV/TLC) and hyperinflation (FRC/TLC) compared with controls. There was mild impairment of respiratory resistance at 5 Hz (Rrs5) but severely reduced respiratory reactance at 5 Hz (Xrs5) while values in the control group were within normal limits (see table 2).
TABLE 1.
Baseline characteristics
| COPD (n=42) | Control (n=14) | p-value | |
|---|---|---|---|
| Anthropometric data | |||
| Age (years) | 68.5±9.1 | 60.8±9.5 | 0.009 |
| Female sex (%) | 45.24 | 57.14 | 0.44 |
| BMI (kg·m−2) | 26.3±4.9 | 25.9±3.6 | 0.76 |
| Spirometry and lung volumes | |||
| FEV1 (L) | 1.46±0.58 | 3.10±0.97 | <0.001 |
| FEV1 (Z-score) | −2.67±1.17 | 0.23±1.10 | <0.001 |
| FVC (L) | 3.07±0.85 | 4.10±1.19 | <0.001 |
| FVC (Z-score) | −0.72±1.14 | 0.44±1.04 | 0.002 |
| FEV1/FVC | 47.1±12.5 | 75.5±5.05 | <0.001 |
| FEV1/FVC (Z-score) | −3.28±1.07 | −0.39±0.68 | <0.001 |
| TLC (L) | 6.26±1.17 | 6.39±1.35 | 0.728 |
| TLC (Z-score) | 0.58±1.03# | −0.37±1.04 | 0.52 |
| FRC (Z-score) | 1.68±1.15# | 0.56±0.95 | 0.002 |
| FRC/TLC (Z-score) | 1.29±1.45# | −0.05±1.52 | 0.005 |
| RV (Z-score) | 1.53±1.01# | 0.42±1.02 | 0.001 |
| RV/TLC (Z-score) | 1.86±1.15# | 0.48±1.19 | <0.001 |
Values are presented as mean±sd, unless otherwise stated. Bold indicates statistical significance. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; FRC: forced residual capacity; RV: residual volume. #: data missing for 1 patient.
TABLE 2.
Baseline oscillometry characteristics
| COPD (n=42) | Control (n=14) | p-value | |
|---|---|---|---|
| Seated Rrs5 (cmH2O·L−1·s−1) | 4.88 (3.77–6.67) | 2.71 (2.42–3.67) | <0.001 |
| Seated Rrs5 (Z-score) | 1.59±1.73 | −0.01±0.87 | <0.001 |
| Supine Rrs5 (cmH2O·L−1·s−1) | 6.4 (4.62–8.28) | 3.82 (3.35–5.25) | 0.005 |
| Supine Rrs5 (Z-score) | 2.54±1.35 | 1.39±0.7 | 0.003 |
| Seated Xrs5 (cmH2O·L−1·s−1) | −3.27 (−5.21– −2.32) | −1.26 (−1.59– −0.84) | <0.001 |
| Seated Xrs5 (Z-score) | −4.62±3.77 | −0.04±1.15 | <0.001 |
| Supine Xrs5 (cmH2O·L−1·s−1) | −6.04 (−8.12– −3.75) | −1.61 (−3.06– −1.37) | <0.001 |
| Supine Xrs5 (Z-score) | −7.72±3.91 | −2.17±1.77 | <0.001 |
| Seated ΔXrs5 (cmH2O·L−1·s−1) | 1.59 (0.42–3.59) | −0.14 (−0.30–0.04) | 0.001 |
Values are mean±sd or median (interquartile range). Bold indicates statistical significance. Rrs5: respiratory resistance at 5 Hz; Xrs5: respiratory reactance at 5 Hz; ΔXrs5: difference between inspiratory and expiratory reactance.
The changes in IC/TLC, Rrs and Xrs with supine posture are shown in table 3. COPD subjects demonstrated supine persistent hyperinflation with no change in IC/TLC from seated to supine posture (p=0.08). In contrast, the control subjects showed a significant increase in IC (p<0.001) and in IC/TLC (p=0.0005) (see figure 1). Dynamic elastance (Xrs5) became more negative in supine posture in both cohorts (p<0.001 for both healthy and COPD, see table 3) with no difference between groups in the change (p=0.152). There was also a significant increase in ΔXrs5 between seated and supine posture in COPD patients (p<0.001), such that the number of subjects with ΔXrs5 >2.8 cmH2O·L−1·s−1 (the cut-off used for indicating the presence of airways closure) increased from 15 out of 42 (35.7%) to 27 out of 42 (64.3%). Although a statistically significant increase in the ΔXrs5 was also seen in controls in supine posture (p=0.039), only one healthy subject had a ΔXrs5 of >2.8 cmH2O·L−1·s−1 in the supine position (see figure 2). Using the cut-off value of an increase in supine IC of 200 mL, 31/41 (75.6%) patients and 5/14 (35.7%) controls demonstrated supine persistent hyperinflation. There is a possible sex difference as shown in supplementary tables 1 and 2 but strong conclusions could not be drawn.
TABLE 3.
Seated versus supine parameters
| COPD (n=42) | p-value | Control (n=14) | p-value | |||
|---|---|---|---|---|---|---|
| Seated | Supine | Seated | Supine | |||
| IC (L) | 1.94±0.67# | 1.99±0.68# | 0.12 | 2.42±0.91 | 2.89±1.1 | <0.001 |
| IC/TLC | 0.31±0.09# | 0.32±0.09# | 0.079 | 0.37±0.07 | 0.44±0.09 | <0.001 |
| ΔXrs (cmH2O·L−1·s−1) | 2.28±2.55 | 4.91±4.10 | <0.001 | −0.12±0.46 | 0.51±1.27 | 0.039 |
| Rrs5 (Z-score) | 1.59±1.73 | 2.54±1.35 | <0.001 | −0.1±0.86 | 1.39±0.70 | <0.001 |
| Xrs5 (Z-score) | −4.62±3.77 | −7.72±3.91 | <0.001 | −0.04±1.15 | −2.17±1.77 | <0.001 |
Values are mean±sd. Bold indicates statistical significance. IC: inspiratory capacity; TLC: total lung capacity; Rrs5: respiratory resistance at 5 Hz; Xrs5: respiratory reactance at 5 Hz; ΔXrs5: difference between inspiratory and expiratory reactance. #: data missing for 1 patient.
FIGURE 1.
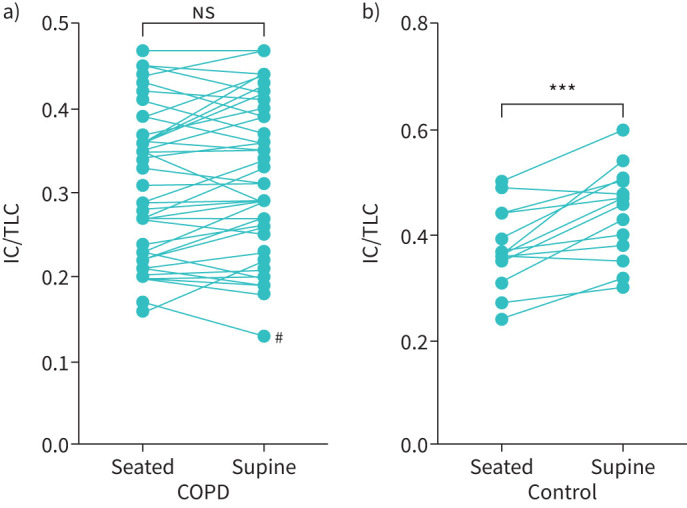
Inspiratory capacity/total lung capacity ratios (IC/TLC), in upright and supine postures for (a) COPD cohort (n=41, p=0.0759) and (b) control subjects (n=14, ***: p=0.0005). #: Technical difficulties with exporting this subject's data may have affected results obtained. Analysis was run with and without this patient included in the dataset with no change to results. ns: not statistically significant.
FIGURE 2.
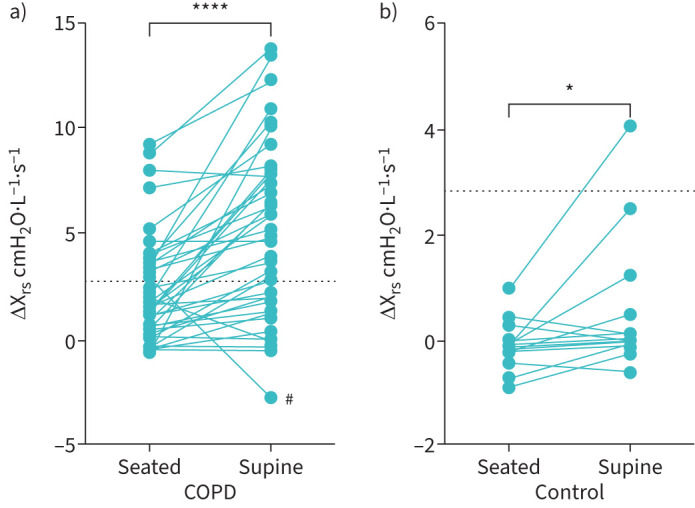
Difference between inspiratory and expiratory reactance (ΔXrs) in the seated and supine position for COPD patients (panel a, n=42, p<0.0001) and control subjects (panel b, n=14, p=0.0388). Dotted line at ΔXrs5 2.8 cmH2O·L−1·s−1, signifying significant expiratory flow limitation. #: Technical difficulties with exporting this subject's data may have affected results obtained. Analysis was run with and without this patient included in the dataset with no change to results.
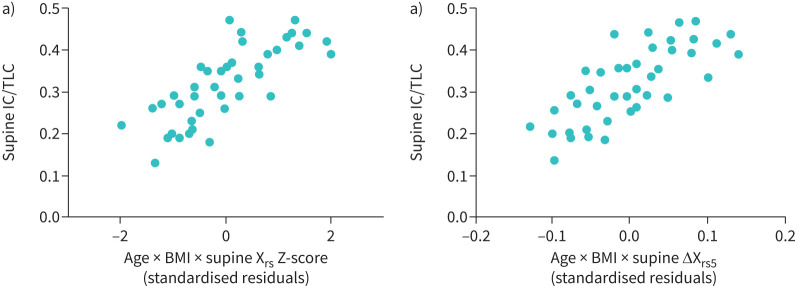
The univariate predictors of supine IC/TLC (hyperinflation) are shown in table 4. In control subjects, RV/TLC Z-score was the sole predictor of supine IC/TLC. In the COPD cohort, the predictors of worse supine hyperinflation were older age, lower BMI, worse airflow obstruction and closure, according to FEV1/FVC, RV/TLC, Xrs5 and ΔXrs5. In multivariate analyses examining the effects of oscillometry parameters (see figure 3), age, BMI and Xrs5 Z-score were independent predictors of supine IC/TLC accounting for 39% of the variability (p<0.001, standardised β coefficients −0.226, 0.457, 0.464, respectively). Using ΔXrs instead of Xrs Z-score produced similar results and accounted for 29% of the variability in IC/TLC ratio (p=0.001, standardised β coefficients −0.282, 0.311 and −0.321, respectively).
TABLE 4.
Univariate correlations between anthropomorphic, spirometric and oscillometry parameters and supine hyperinflation as measured by supine IC/TLC ratio and the change in IC/TLC from the seated to supine position
| COPD (n=41) | Control (n=14) | |||||||
|---|---|---|---|---|---|---|---|---|
| Supine IC/TLC# | Change in IC/TLC | Supine IC/TLC | Change in IC/TLC | |||||
| rs | p-value | rs | p-value | rs | p-value | rs | p-value | |
| Age (years) | 0.321 | 0.041 | 0.096 | 0.545 | −0.17 | 0.562 | −0.099 | 0.736 |
| BMI (kg·m−2) | 0.341 | 0.029 | 0.061 | 0.704 | 0.420 | 0.137 | 0.127 | 0.664 |
| FEV1 (L) | 0.674 | <0.001 | 0.254 | 0.104 | 0.429 | 0.126 | 0.597 | 0.048 |
| FEV1 (Z-score) | 0.587 | <0.001 | 0.090 | 0.575 | 0.437 | 0.118 | 0.358 | 0.208 |
| FEV1/FVC | 0.603 | <0.001 | 0.266 | 0.08 | 0.178 | 0.542 | 0.180 | 0.537 |
| FEV1/FVC (Z-score) | 0.504 | 0.001 | 0.012 | 0.939 | 0.0284 | 0.326 | 0.442 | 0.114 |
| RV/TLC | −0.803 | <0.001 | −0.11 | 0.495 | −0.899 | <0.001 | −0.614 | 0.02 |
| RV/TLC (Z-score) | −0.557 | <0.001 | −0.201 | 0.221 | −0.824 | <0.001 | −0.424 | 0.131 |
| Rrs5 (cmH2O·L−1·s−1) | −0.227 | 0.153 | −0.245 | 0.118 | 0.407 | 0.15 | −0.042 | 0.887 |
| Rrs5 (Z-score) | −0.282 | 0.074 | 0.014 | 0.930 | −0.169 | 0.563 | −0.367 | 0.197 |
| Xrs5 (cmH2O·L−1·s−1) | 0.499 | 0.001 | 0.268 | 0.086 | 0.0857 | 0.773 | 0.460 | 0.098 |
| Xrs5 (Z-score) | 0.405 | 0.009 | −0.090 | 0.578 | 0.424 | 0.131 | 0.073 | 0.805 |
| ΔXrs5 (cmH2O·L−1·s−1) | −0.413 | 0.007 | −0.18 | 0.252 | −0.275 | 0.341 | 0.398 | 0.158 |
| Supine ΔXrs5 (cmH2O·L−1·s−1) | −0.465 | 0.002 | −0.239 | 0.127 | −0.279 | 0.333 | 0.0484 | 0.869 |
| Change in Rrs5 | −0.091 | 0.574 | 0.17 | 0.274 | 0.35 | 0.22 | 0.396 | 0.16 |
| Change in Xrs5 | 0.107 | 0.505 | 0.02 | 0.899 | −0.231 | 0.427 | 0.022 | 0.94 |
Bold indicates statistical significance. rs: Spearman correlation coefficient; Rrs5: respiratory resistance at 5 Hz; Xrs5: respiratory reactance at 5 Hz; ΔXrs5: difference between inspiratory and expiratory reactance during tidal volume breathing; change in Rrs5 and Xrs5: changes in Z-scores between upright and supine posture; IC: inspiratory capacity; TLC: total lung capacity; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; RV: residual volume. #: in the COPD cohort, there was little difference between mean seated and supine IC/TLC and therefore similar relationships were seen in the seated parameter.
FIGURE 3.
Predictors of supine hyperinflation (supine inspiratory capacity/total lung capacity ratios; IC/TLC). a) COPD cohort; x-axis shows standardised residuals of regression with age (years), body mass index (BMI, kg·m−2) and supine respiratory reactance (Xrs, Z-score) as predictors (n=41, adjusted r2=0.39, p<0.001, standardised β coefficients −0.226, 0.457, 0.464, respectively). b) COPD cohort; x-axis shows standardised residuals of regression with age (years), BMI (kg·m−2) and the supine difference between inspiratory and expiratory reactance (ΔXrs5 cm H2O·L−1·s−1) as predictors (n=41, adjusted r2=0.39, p=0.001, standardised β coefficients −0.229, 0.404 and −0.459, respectively).
In both control subjects and COPD patients, there were no associations between changes in any oscillatory impedance or spirometric parameters; and the changes in IC/TLC between seated to supine postures (see table 4).
Discussion
This is the first prospective, observational study to determine the relationship between supine persistent hyperinflation in COPD and dynamic elastance and tidal expiratory flow limitation. Patients with COPD exhibit supine persistent hyperinflation whereas healthy controls do not. In healthy subjects there was an increase in IC/TLC ratio when supine due to the aforementioned reduction in supine FRC with no change to TLC, resulting in an increase in IC. In healthy individuals there were also increases in Rrs5 and ΔXrs5, and a decrease in Xrs5 (worsening dynamic elastance). The changes in Rrs5, ΔXrs5 and Xrs5 were, however, independent of volume changes occurring due to an increase in IC/TLC. In contrast in the COPD patients, IC/TLC did not change between seated and supine posture, strongly suggesting that they exhibited relative supine hyperinflation. Despite this, there were also increases in Rrs5 and ΔXrs5, and Xrs5 became more negative. The independent predictors of greater hyperinflation (IC/TLC) in COPD patients, were older age, lower BMI and worse ventilatory impairment as indicated by either upright spirometry or Xrs5. Lastly, as is shown in table 4, the changes in oscillatory impedance due to supine posture were unrelated to IC and therefore independent of changes in operating lung volume in both healthy controls and COPD patients and are presumably due to changes in the distribution and overall magnitude of regional time constants.
In healthy subjects, FRC and therefore end-expiratory lung volume (EELV) decrease with supine posture, which has been shown to partially explain increases in Rrs and decreases in Xrs [26]. Changes in EELV with supine posture, as measured by ERV (since RV and TLC change minimally [26–28], are less with older age [29] and obesity [30]. In upright posture, Rrs and Xrs are both strongly volume dependent [31–33] Airway closure causes reactance to suddenly decrease. In the supine position, the fall in FRC means that airway closure may occur during tidal volume breathing due to FRC falling below closing capacity.
The location of closure also changes, being in dependent lung regions in healthy subjects [34] and so in supine posture, will change from caudal regions to being along the posterior regions of the lung. The distribution of airway calibres will also change. Hence the effects of supine posture on Rrs and Xrs are a complex interaction of changes in lung volume, distributions of compliances and airway calibres, airway closure, age and BMI. Emphysema is a heterogenous disease, and the size and location of bullous disease may further impact on changes in respiratory mechanics when patients are moved into the supine position. Assessing the impact of the heterogenous distribution of emphysema was outside the scope of this study but would be an interesting direction for further research.
In the current study, the changes in supine oscillometry parameters were not correlated with changes in EELV, again suggesting that the supine increase in Rrs and ΔXrs and decrease in Xrs cannot be attributed to a change in volume alone. The strong relationship between supine IC/TLC and RV/TLC in healthy subjects is expected given that for IC to increase, EELV gets closer to RV i.e. if RV/TLC is high, it would inhibit any increase in IC/TLC, given RV/TLC does not change in supine posture.
Elbehairy et al. [11] previously demonstrated the lack of supine IC change in patients with severe COPD. Worsening of Rrs and Xrs in the supine posture [11, 25], has also previously been demonstrated but the relationship between supine persistent hyperinflation with upright and supine oscillatory mechanics and anthropomorphic factors, has not been previously reported. These findings suggest that the drivers of supine EELV in COPD are predominantly airway closure and expiratory flow limitation. We found ΔXrs5 to be the strongest oscillometric driver of supine IC/TLC, while Rrs5 was not, which supports this proposed mechanism of supine persistent hyperinflation in COPD. We postulate that the overall worsening of ventilatory function while supine is a consequence of the gravitational effects on the lungs in COPD, which may lead to a reduction in mean airway calibre, increased airway closure and more EFL and that the consequence of this is maintenance of hyperinflation. Maintenance of hyperinflation may mitigate these effects on the work of breathing and perhaps also on gas exchange. Higher ΔXrs5 indicates a larger increase in dynamic elastance in expiration, due to increasing airway collapse, to the point of expiratory flow limitation. Dellacà et al. showed that when ΔXrs5 exceeded 2.8 cmH2O·L−1·s−1, this predicted a flow-limited breath [18]. The linear relationship between ΔXrs5 and supine IC/TLC (see figure 3b) suggests that even values which do not reach this threshold may have a physiological effect on EELV.
In addition to ΔXrs, BMI was also an independent predictor of supine persistent hyperinflation in COPD. While larger ΔXrs was associated with more supine hyperinflation, higher BMI was associated with less supine hyperinflation. Higher BMI is associated with less resting hyperinflation in COPD, greater maximal oxygen consumption and less breathlessness at any given minute of ventilation [35]. Thus, the effects of obesity in reducing static hyperinflation may explain part of the “obesity paradox”. Our data also suggest that higher BMI mitigates to some extent, the effects of ventilatory impairment (ΔXrs5) on persistent supine hyperinflation. We note that the effects of BMI on supine hyperinflation in our COPD participants, only apply to mild obesity, since the maximum BMI was 36.6 kg·m−2.
Understanding the physiologic changes that occur in COPD patients in the supine position is clinically important since nocturnal symptoms are common in COPD [7, 10, 11]. Clinicians are aware that supine breathlessness, nocturnal symptoms and sleep disturbance may be due to comorbidities such as heart failure and obstructive sleep apnoea. Ironically there is much less appreciation of how those symptoms may be due to an increase in hyperinflation, airflow obstruction and expiratory flow limitation. Supine persistent hyperinflation is likely driven by expiratory flow limitation, airway closure and intrinsic positive end expiratory pressure. It is hypothesised that COPD patients maintain hyperinflation to minimise the effects of airway collapse and closure, thereby reducing the resistive work of breathing. The disadvantage to this is the resulting, higher mean trans-respiratory pressures and the requirement of larger pressure swings to generate the same change in volume, and thus greater elastic cost to the work of breathing and perhaps greater symptoms. Supine EFL and hyperinflation are likely to impact sleep quality and strategies to optimise respiratory mechanics during sleep might provide clinical benefit.
This study has some limitations. Firstly, TLC was not measured in the supine position and some studies have demonstrated small but statistically significant reductions in TLC in the supine position. Thus, the assumption that changes in IC reflect changes in EELV may not be entirely valid [26, 27, 36]. Secondly, the control group was small and on average, 8 years younger than the patient group. This made interpreting the effect of anthropomorphic factors in this group difficult. A larger study of healthy subjects of wider range of BMI and age, would be useful to better understand what represents “normal” supine oscillometry. Similarly, the mean age of patients from the the reference dataset used by Oostveen et al. [24] was approximately 50 years which may affect the standardisation of data in both the COPD and control population.
In conclusion, supine persistent hyperinflation occurs in COPD but not in healthy participants and is associated with increased dynamic elastance and EFL, likely the result of increased airway closure due to gravitational redistribution of lung mass in the supine posture. This effect is modified by age and BMI. While healthy participants demonstrate both a decrease in supine EELV and worsening of oscillometric parameters, they are dissociated, suggesting that there is a complex interplay of factors including weight, age, changes in lung volume and airway closure. Further studies assessing the relationships between supine hyperinflation and EFL; and daytime and nocturnal symptoms and polysomnographic parameters in COPD are warranted to determine if there may be clinical benefit from targeting supine hyperinflation and EFL.
Acknowledgements
We would like to acknowledge the support of Ryan Wallis, Fei Ni Hau and Shawna Reesor as Respiratory Scientists at the Royal North Shore Respiratory Investigation Unit, and all the patients and control subjects participating in the study.
Provenance: Submitted article, peer reviewed.
Ethics statement: Ethics approval was obtained prior to patient recruitment from the Northern Sydney Local Health District Human Research Ethics Committee (2021/ETH01142) and The Bellberry Human Research Ethics Committee (2015-08-603). All subjects provided written informed consent.
Author contributions: M. Srinivasan and G.G. King conceived and designed the research; M. Srinivasan, K. Patel, K.Blokland, D.G. Chapman and D. Touma performed the experiments; M. Srinivasan, H. Pollard and G.G. King analysed the data; M. Srinivasan and G.G. King interpreted the results of experiments. M. Srinivasan and G.G. King prepared figures and drafted the manuscript. All authors contributed to revising the article, gave final approval for the version to be published and agree to be accountable to all aspects of the work.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00620-2024
Conflict of interest: G.G. King is a nonexecutive board member of Cyclomedica Ltd, Australia. He has received honoraria for consultancy services to AstraZeneca, Chiesi, GSK, Sanofi. He receives grants from Cyclomedica and philanthropic trusts. All other authors have nothing to disclose.
Support statement: M. Srinivasan was supported by the Faculty of Medicine and Health Executive Dean Stipend Scholarship through the University of Sydney. K. Blokland was supported by a grant from the Berg Family Trust. D. Touma was supported by a grant from Mr John Notaras.
References
- 1.Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med 2022; 10: 447–458. doi: 10.1016/S2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donnell DE Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exercise 2001; 33: 7; Suppl., S647–S655. doi: 10.1097/00005768-200107001-00002 [DOI] [PubMed] [Google Scholar]
- 3.Dean J, Kolsum U, Hitchen P, et al. Clinical characteristics of COPD patients with tidal expiratory flow limitation. Int J Chron Obstruct Pulmon Dis 2017; 12: 1503–1506. doi: 10.2147/COPD.S137865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boni E, Corda L, Franchini D, et al. Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at rest. Thorax 2002; 57: 528–532. doi: 10.1136/thorax.57.6.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltayara L, Becklake MR, Volta CA, et al. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 154: 1726–1734. doi: 10.1164/ajrccm.154.6.8970362 [DOI] [PubMed] [Google Scholar]
- 6.Eltayara L, Ghezzo H, Milic-Emili J. Orthopnea and tidal expiratory flow limitation in patients with stable COPD. Chest 2001; 119: 99–104. doi: 10.1378/chest.119.1.99 [DOI] [PubMed] [Google Scholar]
- 7.Price D, Small M, Milligan G, et al. Impact of night-time symptoms in COPD: a real-world study in five European countries. Int J Chron Obstruct Pulmon Dis 2013; 8: 595–603. doi: 10.2147/COPD.S48570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding B, Small M, Bergstrom G, et al. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 589–599. doi: 10.2147/COPD.S122485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson JJ, Cai Q, Mocarski M, et al. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 577–586. doi: 10.2147/COPD.S76157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uccelli S, Pini L, Bottone D, et al. Dyspnea during night-time and at early morning in patients with stable copd is associated with supine tidal expiratory flow limitation. Int J Chron Obstruct Pulmon Dis 2020; 15: 2549–2558. doi: 10.2147/COPD.S269346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbehairy AF, Faisal A, McIsaac H, et al. Mechanisms of orthopnoea in patients with advanced COPD. Eur Respir J 2021; 57: 2000754. doi: 10.1183/13993003.00754-2020 [DOI] [PubMed] [Google Scholar]
- 12.Brody JS, Glazier JB. The effect of position on pulmonary function in chronic obstructive lung disease. Am Rev Respir Dis 1965; 92: 579–588. [DOI] [PubMed] [Google Scholar]
- 13.Aarli BB, Calverley PM, Jensen RL, et al. The association of tidal EFL with exercise performance, exacerbations, and death in COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2179–2188. doi: 10.2147/COPD.S138720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeichi N, Yamazaki H, Fujimoto K. Comparison of impedance measured by the forced oscillation technique and pulmonary functions, including static lung compliance, in obstructive and interstitial lung disease. Int J Chron Obstruct Pulmon Dis 2019; 14: 1109–1118. doi: 10.2147/COPD.S198030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milne S, Jetmalani K, Chapman DG, et al. Respiratory system reactance reflects communicating lung volume in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2019; 126: 1223–1231. doi: 10.1152/japplphysiol.00503.2018 [DOI] [PubMed] [Google Scholar]
- 16.Dellacà RL, Rotger M, Aliverti A, et al. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J 2006; 27: 983–991. doi: 10.1183/09031936.06.00080005 [DOI] [PubMed] [Google Scholar]
- 17.Otis AB, McKerrow CB, Bartlett RA, et al. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 1956; 8: 427–443. doi: 10.1152/jappl.1956.8.4.427 [DOI] [PubMed] [Google Scholar]
- 18.Dellacà RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 2004; 23: 232–240. doi: 10.1183/09031936.04.00046804 [DOI] [PubMed] [Google Scholar]
- 19.Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. an official American Thoracic Society technical statement. Am J Respir Crit Care Med 2017; 196: 1463–1472. doi: 10.1164/rccm.201710-1981ST [DOI] [PubMed] [Google Scholar]
- 20.King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020; 55: 1900753. doi: 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021; 57: 2000289. doi: 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6: Suppl. 16, 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 24.Oostveen E, Boda K, van der Grinten CP, et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J 2013; 42: 1513–1523. doi: 10.1183/09031936.00126212 [DOI] [PubMed] [Google Scholar]
- 25.Milesi I, Porta R, Barbano L, et al. Automatic tailoring of the lowest PEEP to abolish tidal expiratory flow limitation in seated and supine COPD patients. Respir Med 2019; 155: 13–18. doi: 10.1016/j.rmed.2019.06.022 [DOI] [PubMed] [Google Scholar]
- 26.Navajas D, Farre R, Rotger MM, et al. Effect of body posture on respiratory impedance. J Appl Physiol (1985) 1988; 64: 194–199. doi: 10.1152/jappl.1988.64.1.194 [DOI] [PubMed] [Google Scholar]
- 27.Bae J, Ting E, Giuffrida J. The effect of changes in the body position obsese patients on pulmonary volume and ventilatory function. Bull N Y Acad Med 1976; 52: 830–837. [PMC free article] [PubMed] [Google Scholar]
- 28.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol (1985) 2005; 98: 512–517. doi: 10.1152/japplphysiol.00430.2004 [DOI] [PubMed] [Google Scholar]
- 29.Michels A, Decoster K, Derde L, et al. Influence of posture on lung volumes and impedance of respiratory system in healthy smokers and nonsmokers. J Appl Physiol (1985) 1991; 71: 294–299. doi: 10.1152/jappl.1991.71.1.294 [DOI] [PubMed] [Google Scholar]
- 30.Steier J, Lunt A, Hart N, et al. Observational study of the effect of obesity on lung volumes. Thorax 2014; 69: 752–759. doi: 10.1136/thoraxjnl-2014-205148 [DOI] [PubMed] [Google Scholar]
- 31.Kelly VJ, Brown NJ, Sands SA, et al. Effect of airway smooth muscle tone on airway distensibility measured by the forced oscillation technique in adults with asthma. J Appl Physiol (1985) 2012; 112: 1494–1503. doi: 10.1152/japplphysiol.01259.2011 [DOI] [PubMed] [Google Scholar]
- 32.Baldi S, Dellacà R, Govoni L, et al. Airway distensibility and volume recruitment with lung inflation in COPD. J Appl Physiol (1985) 2010; 109: 1019–1026. doi: 10.1152/japplphysiol.00147.2010 [DOI] [PubMed] [Google Scholar]
- 33.Nagels J, Làndsér FJ, van der Linden L, et al. Mechanical properties of lungs and chest wall during spontaneous breathing. J Appl Physiol Respir Environ Exerc Physiol 1980; 49: 408–416. [DOI] [PubMed] [Google Scholar]
- 34.King GG, Eberl S, Salome CM, et al. Airway closure measured by a technegas bolus and SPECT. Am J Respir Crit Care Med 1997; 155: 682–688. doi: 10.1164/ajrccm.155.2.9032213 [DOI] [PubMed] [Google Scholar]
- 35.Ora J, Laveneziana P, Ofir D, et al. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med 2009; 180: 964–971. doi: 10.1164/rccm.200904-0530OC [DOI] [PubMed] [Google Scholar]
- 36.Elliott AR, Prisk GK, Guy HJ, et al. Lung volumes during sustained microgravity on Spacelab SLS-1. J Appl Physiol (1985) 1994; 77: 2005–2014. doi: 10.1152/jappl.1994.77.4.2005 [DOI] [PubMed] [Google Scholar]