Abstract
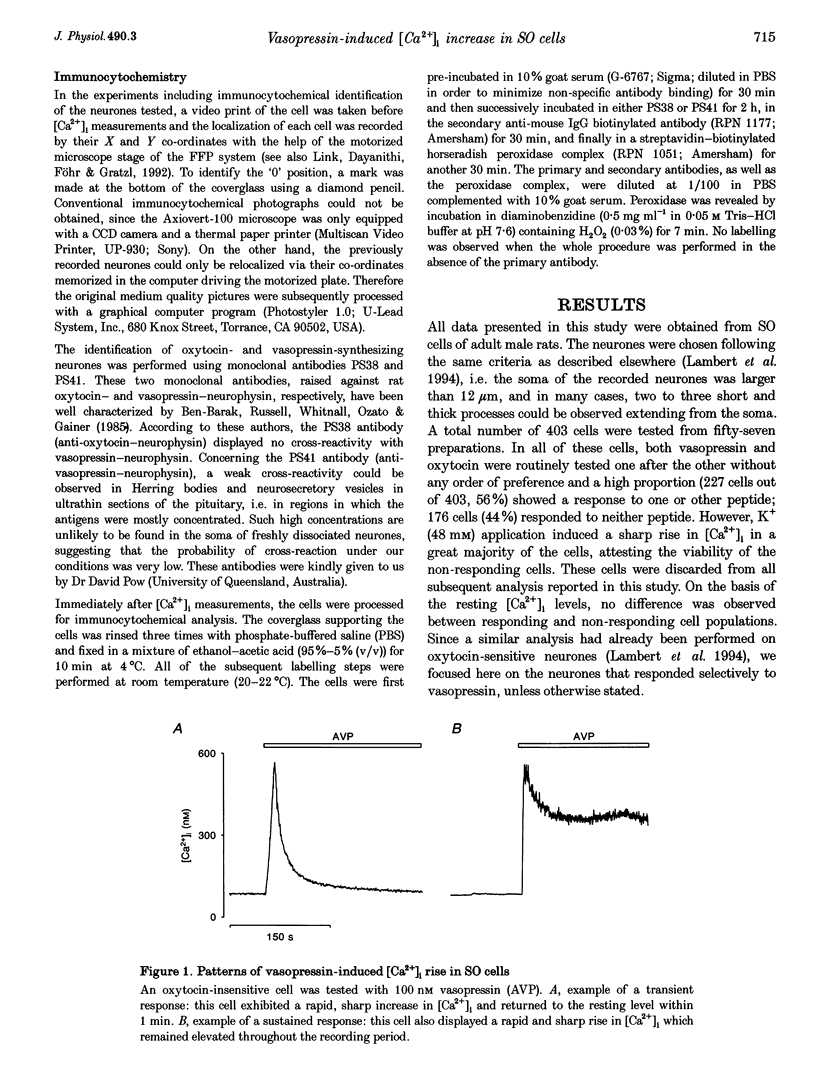
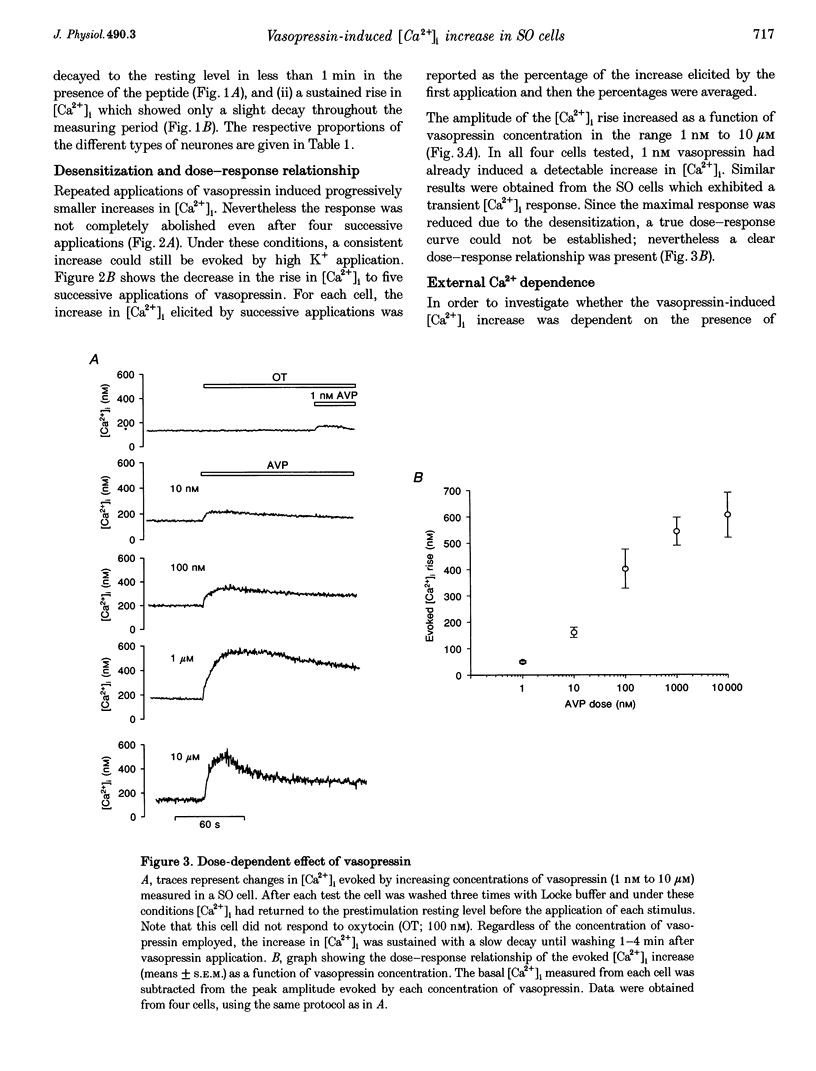
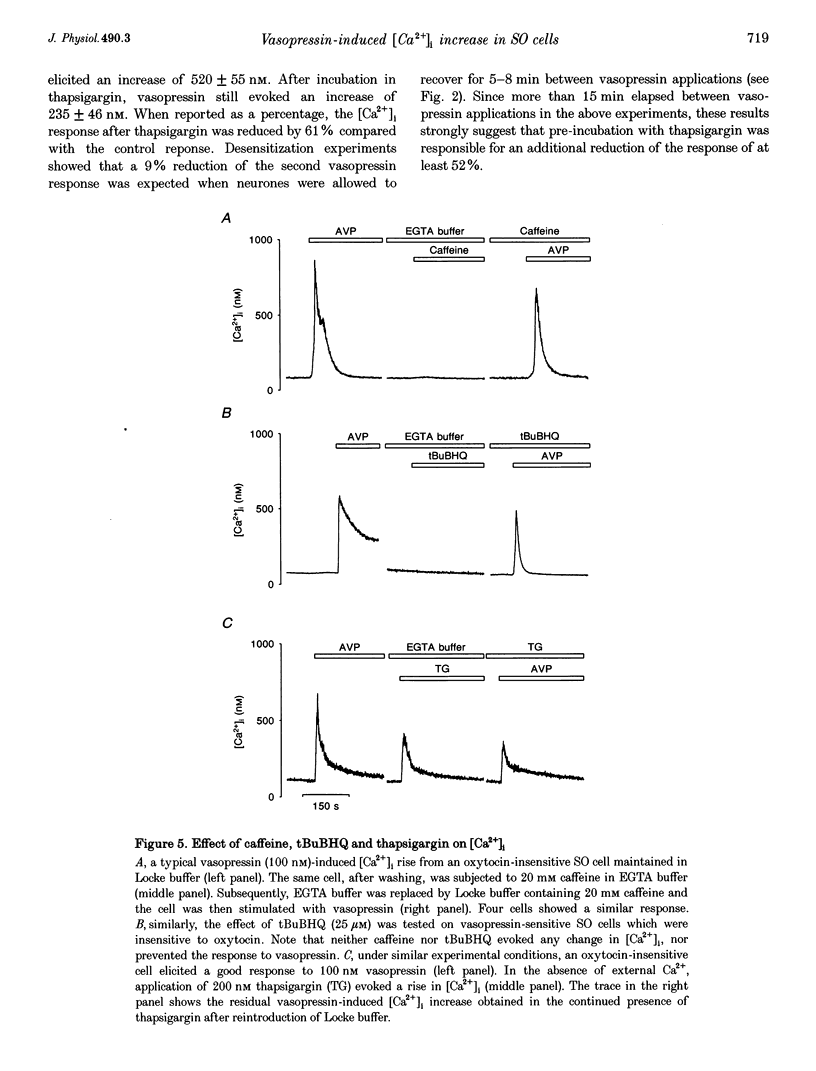
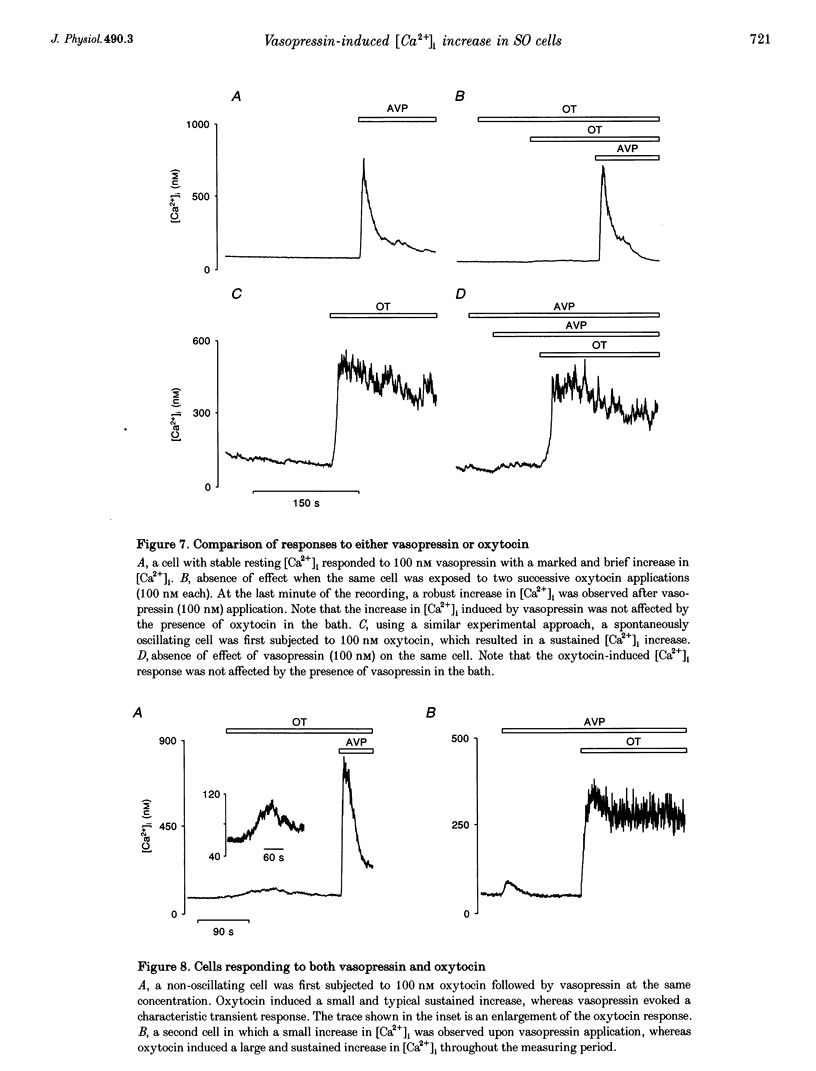
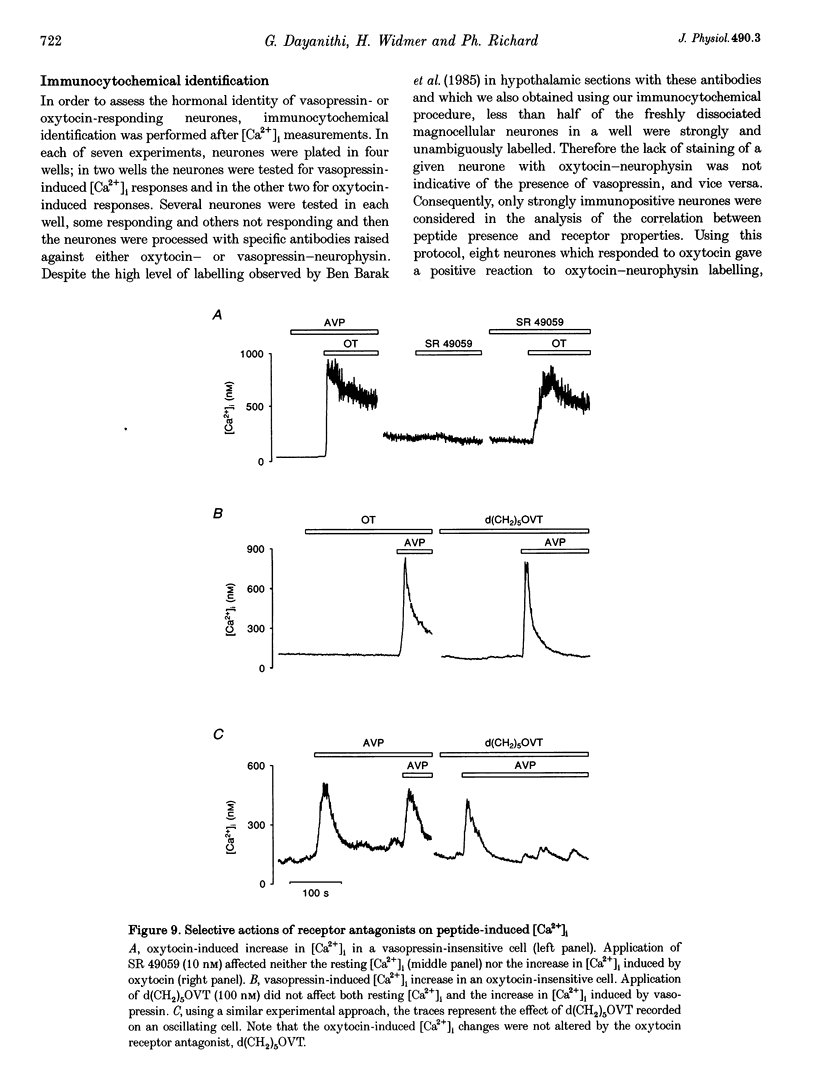
1. The intracellular Ca2+ concentration ([Ca2+]1) was monitored in single magnocellular neurones freshly isolated from rat supraoptic nucleus. Application of 100 nM vasopressin increased [Ca2+]1. Two types of [Ca2+]1 responses were observed: (i) a transient response, displayed by 86% of the vasopressin-sensitive neurones, and (ii) a sustained response displayed by 14% of the vasopressin-sensitive neurones. 2. Among responding neurones, 52% were vasopressin sensitive, 44% were oxytocin sensitive and 4% were sensitive to both peptides. 3. Responses to vasopressin were dose dependent, showed a progressive desensitization after successive applications, were specifically blocked by the V1a vasopressin receptor antagonist, SR 49059, and were unaffected by the oxytocin receptor antagonist, d(CH2)5OVT. 4. Vasopressin responses were completely suppressed by the removal of external Ca2+. 5. The intracellular Ca2+ mobilizers, caffeine and tBuBHQ, did not affect resting or vasopressin-induced [Ca2+]1 changes. Thapsigargin (200 nM) on its own evoked an increase in [Ca2+]1, and reduced the [Ca2+]1 increase evoked by vasopressin by 52%, suggesting that thapsigargin-sensitive Ca2+ stores are partially involved in the vasopressin response. 6. Immunocytochemical identification revealed that vasopressin-responding neurones synthesize vasopressin whereas oxytocin-responding neurones synthesize oxytocin. 7. In conclusion, vasopressin- (partially external Ca2+ dependent) and oxytocin (totally external Ca2+ independent)-induced [Ca2+]1 changes are mediated by specific receptors. In addition, vasopressin and oxytocin neurones are specifically autoregulated by their own peptides.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audigier S., Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO J. 1985 Jun;4(6):1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Barak Y., Russell J. T., Whitnall M. H., Ozato K., Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985 Jan;5(1):81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette B., Poulain P. Vasopressin-sensitive neurons in the lateral paraventricular nucleus area in a guinea pig slice preparation. Brain Res Bull. 1989 Jun;22(6):969–974. doi: 10.1016/0361-9230(89)90008-7. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. The caffeine-sensitive Ca2+ store in bovine adrenal chromaffin cells; an examination of its role in triggering secretion and Ca2+ homeostasis. FEBS Lett. 1990 Jun 18;266(1-2):91–95. doi: 10.1016/0014-5793(90)81514-o. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Rage F., Richard P., Tapia-Arancibia L. Characterization of spontaneous and N-methyl-D-aspartate-induced calcium rise in rat cultured hypothalamic neurons. Neuroendocrinology. 1995 Mar;61(3):243–255. doi: 10.1159/000126846. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Tribollet E., Dubois-Dauphin M., Raggenbass M. Neurohypophysial hormones: neuronal effects in autonomic and limbic areas of the rat brain. Arch Histol Cytol. 1989;52 (Suppl):129–138. doi: 10.1679/aohc.52.suppl_129. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Stoeckel M. E., Klein M. J. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994 Oct 1;480(Pt 1):155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hatton G. I., Bicknell R. J., Hoyland J., Bunting R., Mason W. T. Arginine vasopressin mobilises intracellular calcium via V1-receptor activation in astrocytes (pituicytes) cultured from adult rat neural lobes. Brain Res. 1992 Aug 14;588(1):75–83. doi: 10.1016/0006-8993(92)91346-g. [DOI] [PubMed] [Google Scholar]
- Inenaga K., Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 1986 Jan;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Lambert R. C., Dayanithi G., Moos F. C., Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994 Jul 15;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R. C., Moos F. C., Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993 Dec;57(4):1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Ludwig M. Vasopressin release within the supraoptic and paraventricular nuclei of the rat brain: osmotic stimulation via microdialysis. Brain Res. 1991 Sep 6;558(2):191–196. doi: 10.1016/0006-8993(91)90768-q. [DOI] [PubMed] [Google Scholar]
- Leng G., Mason W. T. Influence of vasopressin upon firing patterns of supraoptic neurons: a comparison of normal and Brattleboro rats. Ann N Y Acad Sci. 1982;394:153–158. doi: 10.1111/j.1749-6632.1982.tb37422.x. [DOI] [PubMed] [Google Scholar]
- Link H., Dayanithi G., Föhr K. J., Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992 Apr;130(4):2183–2191. doi: 10.1210/endo.130.4.1312449. [DOI] [PubMed] [Google Scholar]
- Mezey E., Kiss J. Z. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinology. 1991 Oct;129(4):1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- Moos F. C., Ingram C. D. Electrical recordings of magnocellular supraoptic and paraventricular neurons displaying both oxytocin- and vasopressin-related activity. Brain Res. 1995 Jan 16;669(2):309–314. doi: 10.1016/0006-8993(94)01296-t. [DOI] [PubMed] [Google Scholar]
- Moos F., Poulain D. A., Rodriguez F., Guerné Y., Vincent J. D., Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76(3):593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989 Jan;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Neumann I., Russell J. A., Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993 Mar;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Ostrowski N. L., Lolait S. J., Bradley D. J., O'Carroll A. M., Brownstein M. J., Young W. S., 3rd Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992 Jul;131(1):533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- Ostrowski N. L., Lolait S. J., Young W. S., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994 Oct;135(4):1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Pow D. V., Morris J. F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32(2):435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Raggenbass M., Tribollet E., Dubois-Dauphin M., Dreifuss J. J. Vasopressin receptors of the vasopressor (V1) type in the nucleus of the solitary tract of the rat mediate direct neuronal excitation. J Neurosci. 1989 Nov;9(11):3929–3936. doi: 10.1523/JNEUROSCI.09-11-03929.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard P., Moos F., Freund-Mercier M. J. Central effects of oxytocin. Physiol Rev. 1991 Apr;71(2):331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C., Wagnon J., Garcia C., Lacour C., Guiraudou P., Christophe B., Villanova G., Nisato D., Maffrand J. P., Le Fur G. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993 Jul;92(1):224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Goumaz M., Raggenbass M., Dubois-Dauphin M., Dreifuss J. J. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res. 1991 Jan 15;58(1):13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]
- Wotjak C. T., Ludwig M., Landgraf R. Vasopressin facilitates its own release within the rat supraoptic nucleus in vivo. Neuroreport. 1994 Jun 2;5(10):1181–1184. doi: 10.1097/00001756-199406020-00005. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Kiyama H., Kimura T., Araki T., Maeno H., Tanizawa O., Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993 Sep;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. W., van der Beek E. M., van Heerikhuize J. J., Wolters P., van der Meulen G., Wan Y. P. Quantitative light microscopic autoradiographic localization of binding sites labelled with [3H]vasopressin antagonist d(CH2)5Tyr(Me)VP in the rat brain, pituitary and kidney. Neurosci Lett. 1987 Sep 23;80(2):121–126. doi: 10.1016/0304-3940(87)90640-9. [DOI] [PubMed] [Google Scholar]



