Abstract
Background
The COVID-19 pandemic has led to significant concern due to its impact on human health, particularly through pneumonia-induced lung damage. Surfactant proteins A and D (SP-A and SP-D) are implicated in COVID-19 lung damage, but the role of surfactant protein B (SP-B) remains unclear.
Methods
We conducted a single-centre, prospective observational study involving 73 hospitalised COVID-19 pneumonia patients. SP-B levels were measured within 48 h of admission, alongside SP-A and SP-D in a subset. Clinical data were collected, and follow-up visits were conducted after 6 months.
Results
At hospitalisation, circulating immature SP-B levels measured in 73 patients (median 26.31 arbitrary units (AU) (interquartile range 14.27–41.31)) correlated significantly with lung involvement (r=0.447, p<0.001) and oxygen support requirement (p=0.005). SP-B levels did not predict mechanical ventilation or intensive care unit admission. SP-B decreased significantly (p<0.001) from 25.53 AU (14.36–41.46) at the acute hospitalisation to 12.73 AU (9.12–20.23) at the 6-month follow-up, whereas SP-A and SP-D did not change significantly. Immature SP-B (but not SP-A and SP-D) was confirmed to be significantly associated with the need for oxygen support (n=26, 58%) during the hospitalisation (p<0.05).
Conclusion
Immature SP-B emerges as a potential biomarker for COVID-19 pneumonia severity and prognosis. Its dynamic changes suggest utility in monitoring disease progression and long-term outcomes, despite limitations in predicting hard end-points. Larger studies are needed to validate these findings and understand the underlying mechanisms of surfactant protein dysregulation in COVID-19 pathogenesis.
Shareable abstract
Immature surfactant protein B (SP-B) emerges as a potential biomarker for assessing severity and prognosis of COVID-19 pneumonia. SP-B correlates with lung involvement and oxygen support requirement, suggesting its utility in disease monitoring. https://bit.ly/3LcdTbp
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has sparked widespread concern globally due to its severe implications for human health. One of the most frequent and occasionally severe manifestation of the disease is pneumonia, which can manifest in mild or severe forms, often leading to significant lung damage. In this context, the pulmonary surfactant proteins A and D (SP-A and SP-D) have emerged as a particularly intriguing biomarkers, as they are closely related to lung damage [1, 2] and may play a critical role in the lung's response to COVID-19 infection [3–6].
However, despite the well-known relevance of surfactant protein B (SP-B) in the context of lung damage from different aetiologies, including heart failure (HF) [7–10], its levels both during acute COVID-19 infection and in the post-acute phase have not yet been analysed. Therefore, its variation within the course of infection and its relationship with the degree of lung damage remain to be clarified. Of note, SP-B is produced only in alveolar cells. In contrast, SP-A and SP-D have several extra-thoracic production sites and have been previously associated with immunity and pathology condition of the lung [11–13], including COVID-19 infection. Indeed, both SP-A and SP-D have been reported to increase during COVID-19 [3–5].
The purpose of this study was to investigate SP-B levels during acute COVID-19 infection and at a medium-term follow-up, particularly examining the relationship of SP-B with lung damage and respiratory failure. Furthermore, we have also explored the behaviour of SP-A and SP-D in this context, to gain a more comprehensive understanding of the role of pulmonary surfactant proteins as potential biomarkers of lung damage and healing during COVID-19.
Methods
This was a single-centre, observational, prospective study. We enrolled patients hospitalised in our hospital for pneumonia with a positive swab for SARS-CoV-2 between March 2020 and April 2021. Patients were treated according to the routine clinical practice at the moment of the hospitalisation. Within 48 h from admission, we collected blood samples to measure SP-B (both mature and immature form). Additionally, we also measured SP-A and SP-D in a subpopulation. SP-B (immature form) was measured by Western blotting on plasma samples, as previously described [14]. Plasma levels of mature SP-B, SP-D and SP-A were determined using commercially available ELISA kits. The intra-assay and inter-assay coefficients of variation were <10% and <12% for the SP-B ELISA (Cloud-Clone Corp., Houston, TX, USA), <5.2% and <3%, for the SP-D ELISA (BioVendor, Heidelberg, Germany) and <8% and <10% for the SP-A ELISA (Biomatik, Kitchener, Canada).
Clinical data, encompassing demographic details, medical background, physical manifestations, laboratory findings and radiographical images, were collected from the medical files of individuals. Standard blood assessment results were collected among those conducted for clinical reasons. Chest computed tomography (CT) scans were performed to hospitalised individuals, as prescribed by the attending physician. Specifically, CT examinations were performed using a 256-slice high-resolution CT scanner (Revolution CT; GE Healthcare, Milwaukee, WI, USA). No contrast media were administered to the patients. The percentage of extension of lung parenchyma affected by COVID-19 pneumonia was processed by a dedicated workstation (ADW4.6, GE Healthcare) using a specific reconstruction software (Thoracic-V-Car software; GE Healthcare). This quantitative approach enables an automated assessment of the pulmonary infection, depicting infection areas as high attenuation areas (HAAs) in respect of a defined threshold value ranging from 650 HU to 3071 HU. The amount of infected lung defined as the percentage of lung parenchyma above the predefined vendor-specific threshold of 650 HU (HAA%, HAA/total lung volume) was automatically calculated by the dedicated software for both lungs [15, 16].
After 6 months, patients underwent a follow-up visit and repeated blood samples. Clinical history and results of tests were collected according to the local regulation after the patient signed the appropriate informed consent. The study was approved by the Ethics Committee of Centro Cardiologico Monzino and registered as R1174/20 CCM 1237.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA). Continuous variables were expressed as mean±sd or median and (interquartile range) as appropriate, while discrete variables were expressed as absolute numbers and percentages. Comparisons between basal variables and follow-up were performed using a Wilcoxon signed-rank test. A Spearman correlation was used for non-normally distributed variables, while logistic regression was used to evaluate the association between SP-B and outcomes. A p-value ≤0.05 was considered as statistically significant.
Results
From March 2020 to April 2021, we enrolled 73 patients (mean age 72 (interquartile range 63–79) years; 62% males) admitted to Centro Cardiologico Monzino, IRCCS (Italy) due to COVID-19 pneumonia. In brief, 59% were hypertensive, 30% were active or previous smokers and 65% had a pre-existing cardiopulmonary disease, including 19% with HF and 10% with COPD. All patients were hospitalised in a clinical ward dedicated to COVID-19 patients. The median hospitalisation length was 11.0 (7.0–18.5) days; 15% of patients during hospitalisation due to clinical worsening were transferred to the intensive care unit (ICU) but only one patient needed invasive mechanical ventilation. 44 patients needed oxygen support. No deaths occurred during hospitalisation but five patients died in the following 6 months after the discharge for reasons unrelated to COVID-19.
We detected 26.31 arbitrary units (AU) (14.27–41.31) of circulating immature SP-B, whereas the mature form was undetectable in blood. Immature SP-B was significantly correlated with lung involvement at computed tomography (CT) analysis (r=0.447; p<0.001). Additionally, immature SP-B was significantly higher in those patients who required some sort of oxygen support, including nasal cannula, Venturi mask, continuous positive airway pressure (CPAP) or invasive mechanical ventilation (n=44, 60%) during hospitalisation (31.58 (17.45–48.39) versus 16.19 (10.81–19.13) p=0.003), with a significant association at logistic regression (p=0.005). Conversely, immature SP-B was not associated with the occurrence of hard end-points such as invasive mechanical ventilation, use of CPAP or ICU admission, nor it was correlated with hospitalisation duration. Finally, no association was found between immature SP-B and history of cardiopulmonary disease.
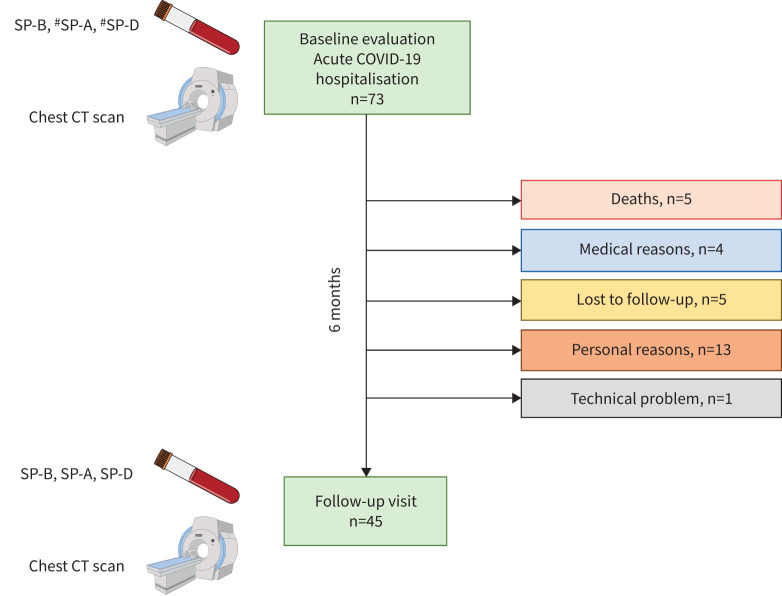
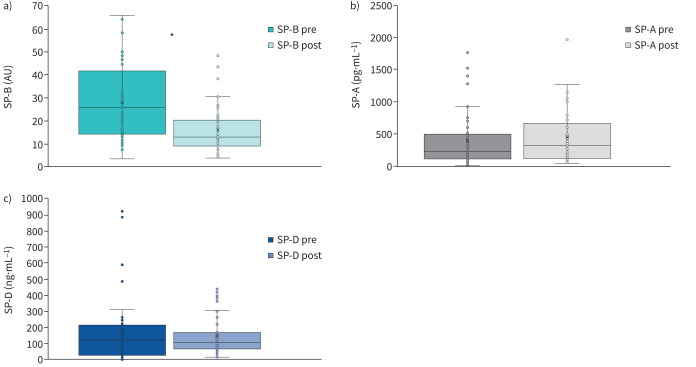
45 patients (62%) were re-evaluated at 6 months after hospital discharge at a follow-up visit. The remaining 28 patients did not attend the scheduled follow-up appointment for various reasons (figure 1). The main characteristics of this population with acute and medium-term evaluation are reported in table 1, presenting the data collected at enrolment (hospitalisation). These patients had an SP-B (mature and immature form), SP-A and SP-D assay twice, during hospitalisation (within 48 h of hospital admission) and at the follow-up visit. Figure 2 shows that immature SP-B significantly reduced at 6 months, whereas SP-A and SP-D did not change. Notably, also in this subgroup, immature SP-B (but not SP-A and SP-D) was confirmed to be significantly associated with the need for oxygen support (n=26, 58%) during the hospitalisation (p<0.05) with a lower value of immature SP-B of 20.60 (13.41–29.46) versus 30.67 (16.44–48.39); p<0.05) in patients who did not need oxygen supplementation (figure 3).
FIGURE 1.
Flow chart presents the two main steps of the study with the reasons why some patients did not attend the follow-up visit. SP: surfactant protein; CT: computed tomography. #: n=45.
TABLE 1.
Basal (hospitalisation) characteristics of the 45 patients with acute and 6-month follow-up
| n (%) | Median (IQR) | |
|---|---|---|
| Age, years | 45 | 71.50 (64.50–78.00) |
| Sex, male | 29 (64.4) | |
| Medical history | ||
| Previous history of CP disease | 28 (62.2) | |
| Active smokers | 4 (8.9) | |
| Previous smokers | 10 (22.2) | |
| Hypertension | 29 (64.4) | |
| Diabetes | 14 (31.1) | |
| Dyslipidaemia | 20 (44.4) | |
| Atrial fibrillation | 17 (37.8) | |
| Arrythmia | 4 (8.9) | |
| Ischaemic cardiomyopathy | 15 (33.3) | |
| Previous myocardial infarction | 12 (26.7) | |
| Heart failure | 8 (17.8) | |
| Valvulopathy | 6 (13.3) | |
| Stroke | 2 (4.4) | |
| Previous CV hospitalisations | 17 (37.8) | |
| COPD | 4 (8.9) | |
| Hospitalisation data | ||
| Hospitalisation length, days | 45 | 10.00 (5.75–16.00) |
| Intensive care | 5 (11.1) | |
| Deaths during hospitalisation | 0 (0) | |
| Need of any oxygen therapy | 26 (58) | |
| Highest level of O2 support | ||
| O2 low flow | 13 (28.9) | |
| CPAP | 12 (26.7) | |
| Venturi | 0 (0) | |
| Intubation | 1 (2.2) | |
| CT lung involvement, % | 43 | 11.23 (7.51–18.43) |
| hsTnI at admission, ng·L−1 | 32 | 20.80 (6.25–64.54) |
| hsTnI highest, ng·L−1 | 28 | 65.00 (15.01–1694.32) |
| BNP, pg·mL−1 | 42 | 243 (74.00–379.00) |
| CRP at admission, mg·L−1 | 45 | 23.30 (9.05–52.85) |
| CRP highest, mg·L−1 | 45 | 53.95 (25.20–139.45) |
| Leukocytes, 103·μL−1 | 45 | 7.40 (5.85–9.53) |
| Leukocytes highest, 103·μL−1 | 45 | 10.30 (8.28–13.93) |
| ST2, ng·mL−1 | 25 | 70.80 (38.55–100.55) |
| D-dimer peak, ng·mL−1 | 39 | 821.21 (575.45–1411.18) |
Data are presented as n (%) or median (interquartile range (IQR)). CP: cardiopulmonary; CV: cardiovascular; CPAP: continuous positive airway pressure; CT: computed tomography; hsTnI: high sensitive troponin-I; BNP: B-type natriuretic peptide; CRP: C-reactive protein; ST2: soluble interleukin 1 receptor-like 1.
FIGURE 2.
Evaluation of a) surfactant binding protein (SP)-B (immature form), b) SP-A and c) SP-D at enrolment (hospitalisation due to COVID-19) and at follow-up (6 months). AU: arbitrary units. *: p<0.01.
FIGURE 3.
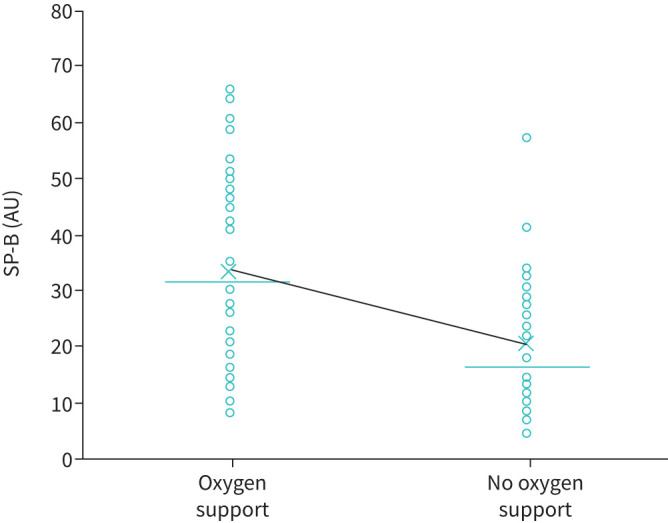
Association between immature surfactant binding protein (SP)-B and oxygen support during hospitalisation. Dots represent individual measurements, crosses represent the median value and lines represent the means. AU: arbitrary units.
Excluding patients with known HF (n=8), the results confirmed a significant decrease of immature SP-B at the 6-month follow-up (24.35 (14.24–44.27) versus 12.54 (8.28–20.0), p<0.001) and a persistent significant correlation of in-hospital immature SP-B value with oxygen need (p=0.038 at logistic regression).
All the tested haematological variables reported in table 1 were evaluated against surfactant proteins but there were no significant correlations.
Discussion
Our investigation, conducted during the most severe phase of the COVID-19 pandemic [17], revealed for the first time, a significant association between circulating immature SP-B levels and the extent of lung involvement assessed by CT analysis [18–20]. Furthermore, higher immature SP-B levels were observed in patients requiring oxygen support, suggesting a potential role of immature SP-B as a biomarker for respiratory distress in COVID-19 pneumonia.
The population we studied was characterised by acute COVID-19 infection associated with pneumonia. Of note, patients directly admitted to the ICU were not considered in the present analysis, which focuses on patients with only moderate respiratory distress severity, who need hospitalisation but were not considered in need of intensive care at presentation. Indeed, the clinical profile of our patients revealed a high prevalence of comorbidities, with hypertension being the most common, followed by a history of smoking and pre-existing cardiopulmonary diseases such as HF and COPD (table 1). These findings may reflect the peculiar nature of the hospital where the trial was conducted (a centre specialised in cardiovascular care), but also align with previous studies [21, 22] highlighting the association between COVID-19 severity and underlying health conditions.
The role of SP-B, both mature and immature forms, as markers of alveolar cells distress is already known in different clinical contexts such as HF and respiratory diseases [7–9, 14]. As a protein contained only within the alveolar cells, the release of immature SP-B in the blood signifies established alveolar damage, regardless of the cause (HF, COPD, acute respiratory distress syndrome, and physical stressors such as diving and smoke) [14, 23–27]. Indeed, in chronic HF a strong correlation was previously reported between circulating immature SP-B and alveolar–capillary membrane gas diffusion [14]. In contrast, to the best of our knowledge no data exist on SP-B behaviour, both in the mature and immature form, in cases of acute non-COVID-19 pneumonia in adults. Of note, our results were confirmed when we excluded patients with HF, confirming the role of SP-B as a marker of alveolar damage, which can occur from both sides of the alveolar–capillary membrane: the circulatory side (HF) and the pulmonary side (COVID-19). While altogether these findings make SP-B less specific from a purely diagnostic point of view compared with other biomarkers, its importance in assessing lung involvement in several diseases remains crucial.
COVID-19 has proven to be a systemic illness, affecting numerous organs and tissues [28–32]. Yet, also considering the virus's point of entry (the respiratory system), the lung has consistently emerged as the primary target of the disease, with the hallmark of interstitial pneumonia described since the earliest documented cases in China [33]. In recent years, many prognostic biomarkers have been associated with COVID-19 severity and prognosis [29, 30, 34–39]; however, most of these are not lung-specific. Given its peculiar biology, SP-B can play a crucial role in this context, identifying significant lung involvement from the early stages of the disease with possible clinical and therapeutic repercussions. However, despite the association between immature SP-B levels and lung damage on a CT scan and between immature SP-B levels and oxygen requirement during hospitalisation, we did not observe significant correlations with hard end-points such as intubation, need of CPAP and/or ICU admission. This could be related to the small sample considered and due to a relevant number of nonsevere COVID-19 cases, as confirmed by the absence of in-hospital deaths. Additionally, immature SP-B levels were not predictive of hospitalisation length. The demographic characteristics of our cohort, summarised in table 1, depict a predominantly older male population with a median age of 72 years, consistent with the demographics of COVID-19 patients reported in other studies [39, 40].
Interestingly, while immature SP-B levels exhibited a significant reduction at the 6-month follow-up visit, SP-A and SP-D levels remained unchanged. This finding underscores the unique dynamics of immature SP-B in the context of COVID-19 recovery. Indeed, differently from previous studies we did not find a correlation between SP-A/SP-D values and clinical condition at hospitalisation or clinical improvement with time. This is likely related to the low prevalence in our population of severe COVID-19 pneumonias in the acute phase, reinforcing the clinical role of SP-B (immature form), which is associated with clinical condition at hospitalisation and after recovery. Altogether these findings highlight the potential utility of immature SP-B as a prognostic marker for assessing acute lung damage as well as long-term respiratory outcomes, even in subjects with moderate lung disfunction. These findings, however, deserve to be validated in larger studies as well as in patients with more severe COVID-19 pneumonia.
Limitations
This is a monocentric study with a relatively small sample size; thus, the results would need validation in a larger cohort as well as in cohorts with patients with more severe COVID-19 pneumonia lung involvement. This limitation is relevant for several possible subanalyses.
Moreover, the patients were affected by the early forms of the SARS-CoV-2 virus, which has since undergone significant mutations (patients were enrolled during the so-called “first and second waves” in Italy). It is crucial to determine whether a more attenuated virus can elicit the same or different responses. Similarly, we lack information on surfactant proteins response in vaccinated individuals as we do not know whether the values of the various surfactant proteins obtained at enrolment were the highest. Indeed, we had only two measurements: at the time of hospitalisation and 6 months after. Therefore, the time-related changes of surfactant proteins are unknown. Finally, a limited number of patients returned for follow-up visits, resulting in insufficient data for robust correlations with long-term clinical outcomes.
Conclusions
In conclusion, our study provides valuable insights into the clinical characteristics and biomarker profiles of COVID-19 pneumonia patients, highlighting the potential utility of immature SP-B as both a marker of pneumonia severity and a prognostic indicator for respiratory outcomes. Future research should focus on validating these findings in larger cohorts and exploring the underlying mechanisms driving surfactant protein dysregulation in COVID-19 pathogenesis.
Footnotes
Provenance: Submitted article, peer reviewed.
Ethics statement: The study was approved by the Ethics Committee of Centro Cardiologico Monzino and registered as R1174/20 CCM 1237. Each subject provided written consent to the study.
Author contributions: P. Agostoni, M. Mapelli, E. Salvioni, I. Mattavelli and S. Harari wrote the main manuscript text. C. Banfi, A. Greco and S. Ghilardi performed the surfactant binding protein analysis. M.L. Biondi performed all other laboratory assessments. E. Salvioni performed statistical analysis. S. Rovai collected data. E. Mancini analysed thoracic computer tomography data. All authors reviewed and approved the manuscript.
Conflict of interests: None declared.
Support statement: This research was supported by the Italian Ministry of Health (ricerca corrente, CUP=B43C24000090001).
Data availability
Raw data will be available upon request at https://zenodo.org/badge/DOI/10.5281/zenodo.13885724.svg.
References
- 1.Han S, Mallampalli RK. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc 2015; 12: 765–774. doi: 10.1513/AnnalsATS.201411-507FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouch E, Hartshorn K, Ofek I. Collectins and pulmonary innate immunity. Immunol Rev 2000; 173: 52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- 3.Salvioni L, Testa F, Sulejmani A, et al. Surfactant protein D (SP-D) as a biomarker of SARS-CoV-2 infection. Clin Chim Acta 2022; 537: 140–145. doi: 10.1016/j.cca.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong M, Xiong Y, Zhu C, et al. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect Dis 2021; 21: 737. doi: 10.1186/s12879-021-06447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alay H, Laloglu E. The role of angiopoietin-2 and surfactant protein-D levels in SARS-CoV-2-related lung injury: a prospective, observational, cohort study. J Med Virol 2021; 93: 6008–6015. doi: 10.1002/jmv.27184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghati A, Dam P, Tasdemir D, et al. Exogenous pulmonary surfactant: a review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SP-A and SP-D as added clinical marker. Curr Opin Colloid Interface Sci 2021; 51: 101413. doi: 10.1016/j.cocis.2020.101413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi C, Agostoni P. Surfactant protein B: From biochemistry to its potential role as diagnostic and prognostic marker in heart failure. Int J Cardiol 2016; 221: 456–462. doi: 10.1016/j.ijcard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Gargiulo P, Banfi C, Ghilardi S, et al. Surfactant-derived proteins as markers of alveolar membrane damage in heart failure. PLoS One 2014; 9: e115030. doi: 10.1371/journal.pone.0115030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Pasquale CG, Arnolda LF, Doyle IR, et al. Plasma surfactant protein-B: a novel biomarker in chronic heart failure. Circulation 2004; 110: 1091–1096. doi: 10.1161/01.CIR.0000140260.73611.FA [DOI] [PubMed] [Google Scholar]
- 10.Hamvas A. Inherited surfactant protein-B deficiency and surfactant protein-C associated disease: clinical features and evaluation. Semin Perinatol 2006; 30: 316–326. doi: 10.1053/j.semperi.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med 2000; 162: 258–263. doi: 10.1164/ajrccm.162.1.9903014 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Fujishima T, Koba H, et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med 2000; 162: 1109–1114. doi: 10.1164/ajrccm.162.3.9910080 [DOI] [PubMed] [Google Scholar]
- 13.Eisner MD, Parsons P, Matthay MA, et al. Acute respiratory distress syndrome N. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003; 58: 983–988. doi: 10.1136/thorax.58.11.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magri D, Brioschi M, Banfi C, et al. Circulating plasma surfactant protein type B as biological marker of alveolar-capillary barrier damage in chronic heart failure. Circ Heart Fail 2009; 2: 175–180. doi: 10.1161/CIRCHEARTFAILURE.108.819607 [DOI] [PubMed] [Google Scholar]
- 15.Andreini D, Conte E, Mushtaq S, et al. Extent of lung involvement over severity of cardiac disease for the prediction of adverse outcome in COVID-19 patients with cardiovascular disease. Int J Cardiol 2021; 323: 292–294. doi: 10.1016/j.ijcard.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annoni AD, Conte E, Mancini ME, et al. Quantitative evaluation of COVID-19 pneumonia lung extension by specific software and correlation with patient clinical outcome. Diagnostics (Basel) 2021; 11: 265. doi: 10.3390/diagnostics11020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagnesi M, Inciardi RM, Lombardi CM, et al. Determinants of the protective effect of glucocorticoids on mortality in hospitalized patients with COVID-19: Insights from the Cardio-COVID-Italy multicenter study. Int J Infect Dis 2021; 108: 270–273. doi: 10.1016/j.ijid.2021.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostoni P, Mapelli M, Conte E, et al. Cardiac patient care during a pandemic: how to reorganise a heart failure unit at the time of COVID-19. Eur J Prev Cardiol 2020; 27: 1127–1132. doi: 10.1177/2047487320925632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mapelli M. Getting lost in the fog of the pandemic: insights from the ‘second wave' of COVID-19. Eur Heart J 2021; 42: 2323–2325. doi: 10.1093/eurheartj/ehab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mapelli M, Buzzi S, Maggioni P. Waiting for the light at the end of the COVID-19 tunnel: best and worst moments 1 year into the pandemic. Eur Heart J 2021; 42: 4783–4786. doi: 10.1093/eurheartj/ehab367. [DOI] [PubMed] [Google Scholar]
- 21.Bajgain KT, Badal S, Bajgain BB, et al. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control 2021; 49: 238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020; 94: 91–95. doi: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susilovic-Grabovac Z, Banfi C, Brusoni D, et al. Diving and pulmonary physiology: Surfactant binding protein, lung fluid and cardiopulmonary test changes in professional divers. Respir Physiol Neurobiol 2017; 243: 27–31. doi: 10.1016/j.resp.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 24.Agostoni P, Banfi C, Brioschi M, et al. Surfactant protein B and RAGE increases in the plasma during cardiopulmonary bypass: a pilot study. Eur Respir J 2011; 37: 841–847. doi: 10.1183/09031936.00045910 [DOI] [PubMed] [Google Scholar]
- 25.Papaioannou AI, Papiris S, Papadaki G, et al. Surfactant proteins in smoking-related lung disease. Curr Top Med Chem 2016; 16: 1574–1581. doi: 10.2174/1568026616666150930120640 [DOI] [PubMed] [Google Scholar]
- 26.D'Ascanio M, Viccaro F, Pizzirusso D, et al. Surfactant protein B plasma levels: reliability as a biomarker in COPD patients. Biomedicines 2023; 11: 124. doi: 10.3390/biomedicines11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banfi C, Brioschi M, Mapelli M, et al. Immature circulating SP-B, bound to HDL, represents an early sign of smoke-induced pathophysiological alterations. Biomolecules 2021; 11: 551. doi: 10.3390/biom11040551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firouzabadi N, Ghasemiyeh P, Moradishooli F, et al. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int Immunopharmacol 2023; 117: 109968. doi: 10.1016/j.intimp.2023.109968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agostoni P, Mapelli M, Salvioni E, et al. Symptomatic post COVID patients have impaired alveolar capillary membrane function and high VE/VCO2. Respir Res 2024; 25: 82. doi: 10.1186/s12931-023-02602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ameri P, Inciardi RM, Di Pasquale M, et al. Pulmonary embolism in patients with COVID-19: characteristics and outcomes in the Cardio-COVID Italy multicenter study. Clin Res Cardiol 2021; 110: 1020–1028. doi: 10.1007/s00392-020-01766-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020; 116: 1666–1687. doi: 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuzzi V, Merlo M, Specchia C, et al. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Fail 2021; 8: 3504–3511. doi: 10.1002/ehf2.13462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Castelnuovo A, Bonaccio M, Costanzo S, et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis 2020; 30: 1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halasz G, Sperti M, Villani M, et al. A machine learning approach for mortality prediction in COVID-19 pneumonia: development and evaluation of the Piacenza score. J Med Internet Res 2021; 23: e29058. doi: 10.2196/29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iorio A, Lombardi CM, Specchia C, et al. Combined role of troponin and natriuretic peptides measurements in patients with COVID-19 (from the Cardio-COVID-Italy Multicenter study). Am J Cardiol 2022; 167: 125–132. doi: 10.1016/j.amjcard.2021.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardi CM, Specchia C, Conforti F, et al. Sex-related differences in patients with coronavirus disease 2019: results of the Cardio-COVID-Italy multicentre study. J Cardiovasc Med (Hagerstown) 2022; 23: 254–263. doi: 10.2459/JCM.0000000000001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokolski M, Trenson S, Sokolska JM, et al. Heart failure in COVID-19: the multicentre, multinational PCHF-COVICAV registry. ESC Heart Fail 2021; 8: 4955–4967. doi: 10.1002/ehf2.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation 2021; 143: 2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data will be available upon request at https://zenodo.org/badge/DOI/10.5281/zenodo.13885724.svg.