Abstract
Background
Although smartphone application for smoking cessation was considered a promising strategy, there were scarce studies on the combination of usual interventions and apps for smoking cessation in China. Our study aimed to evaluate the efficacy of the Cigarette-Burning application combined with medication intervention for smoking cessation.
Methods
A parallel, open-label randomized clinical trial was conducted with a ratio of 1:1 allocation into the bupropion plus app group and bupropion group. All participants received bupropion intervention. Besides, participants in the bupropion plus app group were required to download and use the Cigarette-Burning app. Follow-up visits were conducted at weeks 1, 2, 4, 9, 12, and 24 after initiation of treatment.
Results
Four hundred participants were eventually included and analyzed from July 2019 to June 2021. The continuous abstinence rate at 9–12 weeks was significantly higher in the bupropion plus app group (39.5%) than in the bupropion group (27.5%) (OR = 1.64, 95% CI: 1.04–2.60, p < .05). The similar result was found for the 24-week sustained abstinence rate. The results of subgroup analysis expressed that the 9–12 weeks continuous abstinence rate in the bupropion plus app group was significantly higher than the bupropion group when the age of participants ≥ 50, the education level was college and above, FTND scores > 5, age at starting smoking ≤ 18 (p < .05).
Conclusions
Our study found that the intervention combined with the Cigarette-Burning smartphone application is more effective than medication alone, and the application for smoking cessation could be an accessible complement to smoking cessation medication treatment.
Keywords: Smoking cessation, smartphone application, medication intervention, China
Introduction
Smoking is a significant public health concern worldwide, causing numerous health and economic burdens. In China, there are more than 300 million smokers, 1 and 183.5 million adults are tobacco-dependent. 2 The annual deaths caused by tobacco will reach 3 million by 2050 unless massive smokers are able to quit. 3 This indicates that effective interventions to support smoking cessation are urgently needed.
Current treatment for smoking cessation mainly included pharmacotherapy, psychosocial intervention and behavioral support. 4 These approaches have been proven beneficial for helping individuals quit smoking.5–7 However, the long-term success rate of quitting smoking remains relatively low in real-world settings, 8 as studies have shown limited coverage and poor access to smoking cessation treatment. A survey in the United States found that only about 30% of adult smokers received smoking cessation counseling/medications. 9 Similarly, there were limited resources for smoking cessation clinics in China, especially for primary medical institutions and remote areas. 10
Smoking cessation interventions based on mobile phones might provide a new strategy to fill the gap between the need to quit smoking and the lack of accessible evidence-based services. They had a great potential impact in expanding the reach of smoking cessation interventions because of their availability at any time, low cost, multiple types of intervention forms (e.g., text, game, video, music), interactivity, etc. 11
One of the popular interventions was smoking cessation applications (apps), given the fact that the majority of adults own smartphones in China, and smartphone apps for self-management are increasingly used to promote health. 12 In this context, many smartphone apps related to smoking cessation have been developed and validated.13–15
However, the content analysis of the apps found that their adherence to clinical practice guidelines was low.16,17 So far, only a few apps are based on theory/evidence in China, 18 and there were few studies for the feasibility and acceptability of smoking cessation smartphone apps in China, 19 particularly for the long-term abstinence efficacy. In addition, there were scarce studies on the combination of usual interventions and apps for smoking cessation in China, even though studies related to apps for cardiovascular disease treatment suggested that it might be a promising strategy. 20
In China, a theory-based app for smoking cessation, the Cigarette-Burning app, has been developed based on international smoking cessation guidelines (such as the United States Clinical Practice Guideline [USCPG]) 21 and tailored to Chinese smokers under Chinese Clinical Smoking Cessation Guidelines (CCGTC), 22 which was designed to provide support to smokers who wish to quit smoking. We hypothesized that its use would increase the effectiveness of smoking cessation medications alone, and our study aimed to evaluate the effectiveness of the Cigarette-Burning app combined with medication intervention in a randomized controlled trial (RCT) conducted in China.
Methods
Trial design
The design was a parallel, open-label RCT, and participants were allocated to the bupropion plus app group and the bupropion group in a 1:1 ratio.
This study was carried out at the China-Japan Friendship Hospital in Beijing, China. Recruitment was through poster/website promotion, a hotline for smoking cessation, and local newspapers from July 2019 to June 2021. Study procedures were approved by the Institutional Review Board at the China-Japan Friendship Hospital (No. 2017–125), and registered in the Chinese Clinical Trial Registry (No.: ChiCTR1800016919, URL: http://www.chictr.org.cn). All participants provided written informed consent.
Participants and enrollment
Eligibility criteria included age 18–85; smoking for more than 5 years; the number of smoking per day at least 10 cigarettes in the past 12 months; the value of expiratory carbon monoxide (eCO) no less than 10 parts per million (ppm); having daily access to their smartphone applications; voluntarily participating in this trial and signing the informed consent form. Exclusion criteria included enrollment in other smoking cessation programs within the last 30 days; a history of allergy to bupropion; pregnancy or breastfeeding; and diseases or mental states that could not meet the requirements of the study as judged by the researchers.
Participants were recruited through posters, website promotion and referral from medical institutions. Potential participants scanned the QR code to register and filled in their personal information and phone number. Research staff then contacted respondents, confirmed eligibility, and informed them of the on-site baseline survey.
Randomization
After completing the baseline survey, participants in the trial were randomly assigned to the bupropion plus app group or the bupropion group with a ratio of 1:1. First, the study participants took the enrollment time as the block (block length of four participants) to ensure equilibrium distribution. Then, the random table was generated by the statisticians non-related to the data management and statistical analysis using SAS 9.4 statistical software and assigned the numbers to the two groups.
To ensure random concealment, the group information was stored in sealed opaque envelopes, and the researchers who generated and saved the random allocation sequence were not involved in selecting participants. Before randomization, neither the researchers nor the participants were aware of the random assignment.
Because of the different interventions, only statisticians were blinded to allocation.
Sample size
The sample size calculation was based on our preliminary experiment. In the preliminary experiment, we recruited 100 people and divided them equally into two groups at the beginning of the study. Nine to 12 weeks continuous abstinence rates were 38% (19/50) for the bupropion plus app group versus 24% (12/50) for the bupropion group. An allocation of 1:1 was employed with 80% power level and 5% two-sided significance level, resulting in a unilateral sample size estimate of 167 cases. Considering that the rate of lost-to-follow-up was 20%, 200 were determined as the sample size for each group, and the total sample size was 400.
Intervention
A standard data collection protocol was made by the steering committee, and all research staff was trained before the project kick-off meeting according to the CCSCG(2015 version). 22
Follow-up visits and assessments were conducted at weeks 1, 2, 4, 9, 12, and 24 after baseline assessment, and participants received up to 10 min of counseling and eCO testing at each visit. Abstinence was confirmed by eCO levels’ threshold of ≤10 ppm. The counseling content included the assessment of smoking status, emphasizing the importance of quitting smoking, prevention of relapse, coping with withdrawal symptoms, etc.
Considering that bupropion was widely available, relatively cheap and might prevent weight gain after quitting smoking, 6 so we chose it as pharmacological treatment in this study. Participants in the bupropion group received bupropion (purchased from Venturepharm, Hainan, China) for 12 weeks (150 mg, once a day) according to the CCSCG (2015 version). 22 The participants were required to set a target quit date (TQD) within 2 weeks after starting treatment. The medications used in the study were distributed free of charge, and no additional compensation fee were provided. Good medication adherence was determined if participants took more than 80% of the prescribed medication across the 12-week treatment period 23 ; otherwise, it was poor adherence. To monitor and assess adherence, the researchers used the “timeline follow-back” method 24 through self-report. With this approach, participants were asked to recall and report their medication intake over the 12-week period, and provided information about the dates and doses of medication they took.
Besides all interventions in the bupropion group, participants in the bupropion plus app group were required to download and use the Cigarette-Burning app.
The cigarette-burning app
The Cigarette-Burning app was developed based on the smoking cessation guidelines.21,22 Participants could find it either directly from app stores or via a QR code provided by research staff. It consisted of several modules, including assessment of smoking status, daily attendance, quitting achievement, popular science knowledge on smoking cessation, support on quitting, and other functions (details can be seen in Appendix Table 1 in the online supplemental materials).
We used the Adherence Index to measure its adherence/correlation to the smoking cessation guidelines, which were reported elsewhere.16,18 It was coded and scored by two independent reviewers on 21 items using a score ranging from 0 to 2. A score of 0 indicated that it was not present at all, 1 was partially present, and 2 meant fully present.16,18 The overall score of the Cigarette-Burning app was 29 (Appendix Table 2 in the online supplemental materials).
Measures
Baseline measures
A baseline assessment was performed when the participants first came to the research center. Participants were required for approximately an hour of on-site assessment, including filling out a baseline questionnaire and taking an eCO test, measured by research staff using Bedfont Micro Smokerlyzers (Bedfont Scientific). The questionnaire for screening and recording baseline characteristics of participants was as follows.
Demographic characteristics included age, sex, nationality, education level, marital status, salary (RMB), etc.
Tobacco-related characteristics included: age at starting smoking (≤18 years/>18); duration of smoking (≤20 years, 21–30, 31–40, >40); the number of cigarettes per day (10, 11–20, 21–30, >30); whether the tobacco dependence was diagnosed; the Fagerstrom Test for the Nicotine Dependence (FTND) score, which was used to evaluate the degree of tobacco dependence, and it included six items, and a total score of 0–3, 4–6, and 7–10 meant mild, moderate, and severe tobacco dependence, respectively 25 ; previous quitting attempts; etc.
The diagnosis criteria for tobacco dependence were based on international criteria (ICD-10, 26 DSM-5 27 ) and tailored to Chinese smokers according to CCGTC, 22 and it has been published elsewhere. 2 Tobacco dependence was diagnosed if a minimum of three of the following six were met: (a) craving to use tobacco; (b) unsuccessful effort to control using tobacco; (c) tobacco withdrawal symptoms after abrupt cessation or reduction of tobacco; (d) the need for markedly increased amounts of tobacco to achieve the desired effect; (e) important recreational or social activities or hobbies are given up/reduced because of tobacco use; and (f) tobacco use is continued despite recognizing the hazards of tobacco.
Outcome measures
The primary outcome was the eCO-validated rate of continuous abstinence at 9–12 weeks, which was defined as no puff of tobacco throughout the 9–12 weeks in conjunction with an eCO concentration ≤10 ppm. 5
The secondary outcome included the eCO-validated 24-week sustained abstinence rate (defined as no more than five cigarettes for 24 weeks after TQD with eCO ≤10 ppm), 28 continuous abstinence at 9–24 weeks (defined as no puff of tobacco at 9–24 weeks in conjunction with eCO ≤10 ppm), 29 7-day point prevalence abstinence (defined as no smoking in the past 7 days at the time of follow-up with eCO ≤10 ppm) 13 at each visit.
User feedback
At the 12-week follow-up visit, the user feedback was assessed by questions about the app use frequency; whether participants would recommend the app to others; the overall rating of satisfaction with the app.
Adverse events
Participants were required to record the adverse events associated with treatment (eg, headache, nausea, thirst) at each follow-up visit. The severity of adverse events assessed by trained researchers was divided into three categories: mild, moderate, and severe. For adverse events, the researchers would immediately take appropriate measures.
Statistical analysis
SPSS 26.0 software was used for statistical description and analysis. Categorical measures were expressed as frequency and percentage, and chi-square tests were used to compare the baseline characteristics of participants between the two groups. The intention-to-treat (ITT) approach was used for comparing smoking cessation outcomes between groups, and participants who were lost to follow-up were recognized as failure in quitting.
Considering its suitability for modeling binary outcomes and ability to control for multiple covariates, we used logistic regression analysis to compare the smoking cessation efficacy between groups and analyze the factors of smoking cessation efficacy, which could effectively address our research questions and strengthen the overall rigor of our analytical approach. The dependent variable was the smoking cessation efficacy at 9–12 weeks. Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, nationality, education, marital status, salary, age at starting smoking, duration of smoking, the number of cigarettes per day, whether tobacco dependence was diagnosed, FTND score and previous quitting attempts. The chi-square test was used to compare the results of the subgroup. The results were expressed by the odds ratio (OR) and the 95% confidence interval (CI). A two-sided p < .05 was statistically significant. Sensitivity analyses were used to evaluate the robustness of the effect size after removing the data from missing individuals.
Results
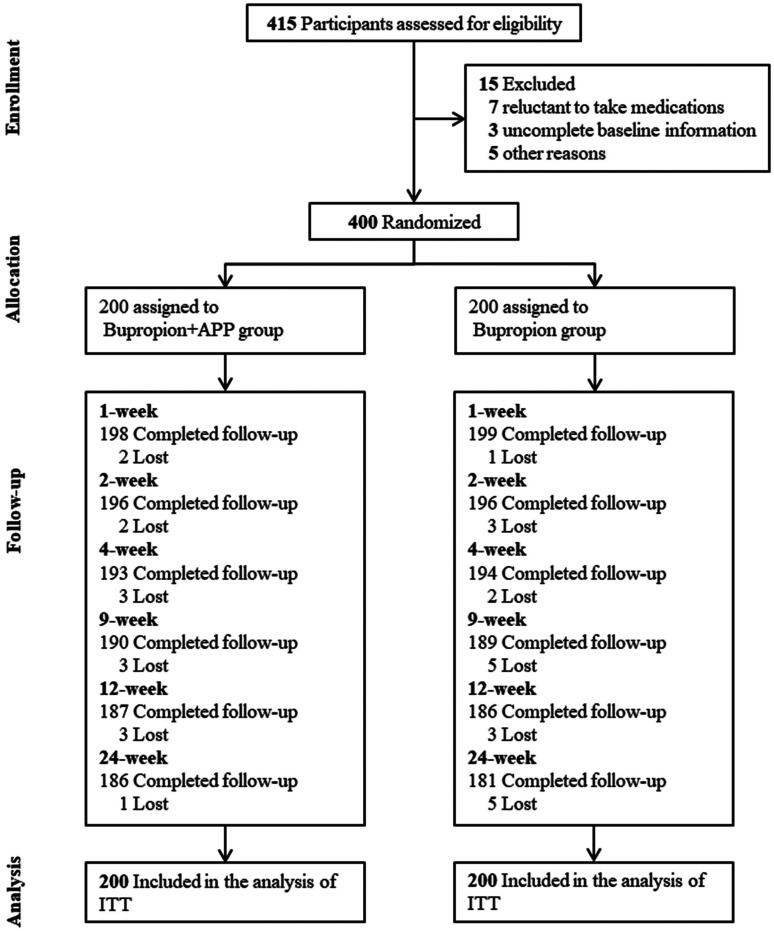
A total of 415 participants were assessed for eligibility from July 2019 to June 2021, of which 15 did not meet the inclusion and exclusion criteria, and 400 participants were eventually randomized and included in the ITT analysis. At the end of the study, 7.0% (14/200) participants in the bupropion plus app group and 9.5% (19/200) participants in the bupropion group were lost, and the overall retention rates were 91.7% (Figure 1).
Figure 1.
Flowchart of participants.
Note. ITT = intention to treatment.
The median age of participants was 48.0 years (IQR = 38.0–59.0), and 93.5% (374/400) of participants were men. 95.3% (381/400) participants were Han Chinese, and 64.8% (259/400) participants had a college education level and above. Three hundred and twenty-seven participants (81.8%) smoked no more than 20 cigarettes per day, and 263 participants (65.8%) smoked for 20 years or more. There was no significant difference in baseline characteristics between the bupropion plus app and bupropion groups (p > .05). The results are shown in Table 1.
Table 1.
Characteristics of participants.
| Characteristics | Bupropion plus app (n = 200) | Bupropion (n = 200) | Total (N = 400) | p |
|---|---|---|---|---|
| Age, years | .58 | |||
| <40 | 61 (30.5%) | 60 (30.0%) | 121 (30.3%) | |
| 40–49 | 45 (22.5%) | 47 (23.5%) | 92 (23.0%) | |
| 50–59 | 56 (28.0%) | 42 (21.0%) | 98 (24.5%) | |
| ≥60 | 38 (19.0%) | 51 (25.5%) | 89 (22.3%) | |
| Sex | .42 | |||
| Male | 189 (94.5%) | 185 (92.5%) | 374 (93.5%) | |
| Female | 11 (5.5%) | 15 (7.5%) | 26 (6.5%) | |
| Nationality | .48 | |||
| Han | 192 (96.0%) | 189 (94.5%) | 381 (95.3%) | |
| Minorities | 8 (4.0%) | 11 (5.5%) | 19 (4.8%) | |
| Education level | .66 | |||
| High school and below | 73 (36.5%) | 68 (34.0%) | 141(35.3%) | |
| College and above | 127 (63.5%) | 132 (66.0%) | 259 (64.8%) | |
| Marital status | .12 | |||
| Unmarried | 22 (11.0%) | 24 (12.0%) | 46 (11.5%) | |
| Married | 172 (86.0%) | 154 (77.0%) | 326 (81.5%) | |
| Divorced, widowed, and separated | 6 (3.0%) | 22 (11.0%) | 28 (7.0%) | |
| Salary (RMB) | .46 | |||
| <3000 | 17 (8.5%) | 24 (12.0%) | 41 (10.3%) | |
| 3000–5999 | 66 (33.0%) | 64 (32.0%) | 130 (32.5%) | |
| ≥6000 | 117 (58.5%) | 112 (56.0%) | 229 (57.3%) | |
| Age at starting smoking, years | .76 | |||
| ≤18 | 85 (42.5%) | 88 (44.0%) | 173 (43.3%) | |
| >18 | 115 (57.5%) | 112 (56.0%) | 227 (56.8%) | |
| Duration of smoking, years | .55 | |||
| ≤20 | 72 (36.0%) | 65 (32.0%) | 137 (34.3%) | |
| 21–30 | 50 (25.0%) | 56 (28.0%) | 106 (26.5%) | |
| 31–40 | 48 (24.0%) | 44 (22.0%) | 92 (23.0%) | |
| >40 | 30 (15.0%) | 35 (17.5%) | 65 (16.3%) | |
| The number of cigarettes per day | .50 | |||
| 10 | 45 (22.5%) | 43 (21.5%) | 88 (22.0%) | |
| 11–20 | 122 (61.0%) | 117 (58.5%) | 239 (59.8%) | |
| 21–30 | 20 (10.0%) | 26 (13.0%) | 46 (11.5%) | |
| >30 | 13 (6.5%) | 14 (7.0%) | 27 (6.8%) | |
| Whether tobacco dependence was diagnosed | .07 | |||
| Yes | 148 (74.0%) | 131 (65.5%) | 279 (69.8%) | |
| No | 52 (26.0%) | 69 (34.5%) | 121 (30.3%) | |
| FTND score | .78 | |||
| 0–3 | 63 (31.5%) | 57 (28.5%) | 120 (30.0%) | |
| 4–6 | 84 (42.0%) | 92 (46.0%) | 176 (44.0%) | |
| 7–10 | 53 (26.5%) | 51 (25.5%) | 104 (26.0%) | |
| Previous quitting attempts | .63 | |||
| None | 64 (32.0%) | 61 (30.5%) | 125 (31.3%) | |
| 1 | 59 (29.5%) | 57 (28.5%) | 116 (29.0%) | |
| ≥2 | 77 (38.5%) | 82 (41.0%) | 159 (39.8%) |
Note. FTND = Fagerstrom Test for Nicotine Dependence.
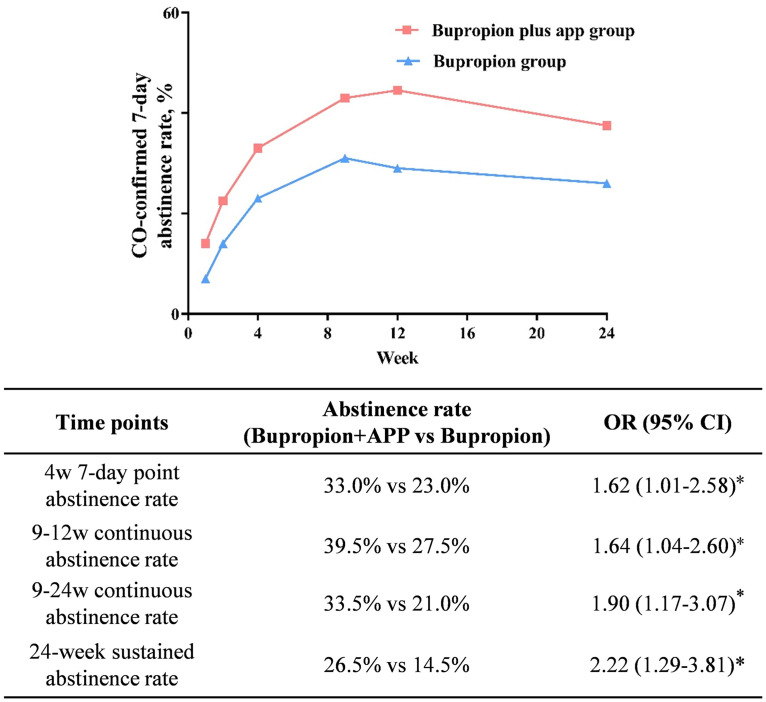
The continuous abstinence rate at 9–12 weeks was significantly higher in the bupropion plus app group (39.5%) than in the bupropion group (27.5%) (OR = 1.64, 95% CI: 1.04–2.60, p < .05). Effect sizes were statistically significant for the 24-week sustained abstinence rate (OR = 2.22, 95% CI: 1.29–3.81, p < .05). The comparison of abstinence rates at other time points is shown in Figure 2. A similar result was found after removing the data of missing individuals (Appendix Table 3 in the online supplemental materials) or participants (age ≥ 60) (Appendix Table 4 in the online supplemental materials).
Figure 2.
Comparison of abstinence rate between groups at different time points.
Note. OR was adjusted for age, sex, nationality, education, marital status and salary, age at starting smoking, duration of smoking, the number of cigarettes per day, tobacco dependence, FTND score, previous quitting attempts; CO = carbon monoxide; or = odds ratio; *p < .05.
After adjusting Model 1, the logistic regression analysis of smoking cessation efficacy at 9–12 weeks showed that salary (RMB), the number of cigarettes per day, the FTND score, previous quitting attempts, and smoking cessation inventions were correlated with the efficacy of smoking cessation (p < .05). After adjusting Model 2, the result showed that salary (RMB), age at starting smoking, duration of smoking, the number of cigarettes per day, and smoking cessation inventions were correlated with the smoking cessation efficacy. Among them, the use of apps significantly increased the smoking quitting rate of bupropion (OR = 1.64, 95% CI: 1.04–2.60, p = .035) (Table 2).
Table 2.
Logistic regression analyses to the efficacy of smoking cessation at 9–12w.
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| (OR, 95% CI) | p | (OR, 95% CI) | p | |
| Age, years | ||||
| <40 | Ref | Ref | ||
| 40–49 | 1.15 (0.64–2.06) | .650 | 0.56 (0.21–1.47) | .236 |
| 50–59 | 1.37 (0.78–2.41) | .277 | 0.64 (0.21–1.91) | .419 |
| ≥60 | 1.32 (0.74–2.37) | .345 | 0.44 (0.12–1.65) | .221 |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.88 (0.38–2.11) | .788 | 1.39 (0.52–3.74) | .516 |
| Nationality | ||||
| Han | Ref | Ref | ||
| Minorities | 1.19 (0.46–3.11) | .723 | 1.12 (0.39–3.21) | .829 |
| Educational level | ||||
| High school and below | Ref | Ref | ||
| College and above | 0.00 (0.00–0.00) | .999 | 1.23 (0.71–2.14) | .462 |
| Marital status | ||||
| Unmarried | Ref | Ref | ||
| Married | 1.02 (0.49–2.14) | .957 | 0.95 (0.43–2.10) | .903 |
| Divorced, widowed, and separated | 0.29 (0.08–1.06) | .060 | 0.27 (0.07–1.06) | .061 |
| Salary (RMB) | ||||
| <3000 | Ref | Ref | ||
| 3000–5999 | 1.20 (0.52–2.81) | .667 | 1.01 (0.41–2.47) | .979 |
| ≥6000 | 3.05 (1.35–6.87) | .007 | 2.56 (1.08–6.12) | .034 |
| Age at starting smoking, years | ||||
| ≤18 | Ref | Ref | ||
| >18 | 1.43 (0.93–2.20) | .109 | 1.67 (1.01–2.76) | .047 |
| Duration of smoking, years | ||||
| ≤20 | Ref | Ref | ||
| 21–30 | 1.93 (0.81–4.59) | .137 | 3.61 (1.42–9.20) | .007 |
| 31–40 | 1.86 (0.68–5.10) | .226 | 4.80 (1.51–15.27) | .008 |
| >40 | 1.76 (0.56–5.53) | .334 | 7.19 (1.80–28.79) | .005 |
| The number of cigarettes per day | ||||
| 10 | Ref | Ref | ||
| 11–20 | 0.60 (0.36–0.99) | .046 | 0.61 (0.34–1.09) | .094 |
| 21–30 | 0.31 (0.14–0.72) | .006 | 0.29 (0.11–0.78) | .014 |
| >30 | 0.25 (0.08–0.73) | .011 | 0.26 (0.07–0.90) | .033 |
| Whether tobacco dependence was diagnosed | ||||
| Yes | Ref | Ref | ||
| No | 0.96 (0.61–1.52) | .867 | 0.96 (0.57–1.63) | .890 |
| FTND score | ||||
| 0–3 | Ref | Ref | ||
| 4–6 | 0.63 (0.39–1.03) | .064 | 0.82 (0.47–1.43) | .486 |
| 7–10 | 0.56 (0.32–0.99) | .047 | 0.95 (0.45–2.00) | .883 |
| Previous quitting attempts | ||||
| None | Ref | Ref | ||
| 1 | 1.51 (0.87–2.63) | .143 | 1.44 (0.78–2.61) | .237 |
| ≥2 | 1.68 (1.01–2.81) | .047 | 1.61 (0.92–2.80) | .095 |
| Intervention | ||||
| Bupropion | Ref | Ref | ||
| Comprehensive bupropion and app | 1.73 (1.13–2.64) | .011 | 1.64 (1.04–2.60) | .035 |
Note. Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, nationality, education, marital status, salary (RMB), age at starting smoking, duration of smoking, the number of cigarettes per day, whether tobacco dependence was diagnosed, FTND score and previous quitting attempts; FTND = Fagerstrom Test for Nicotine Dependence; OR = odds ratio.
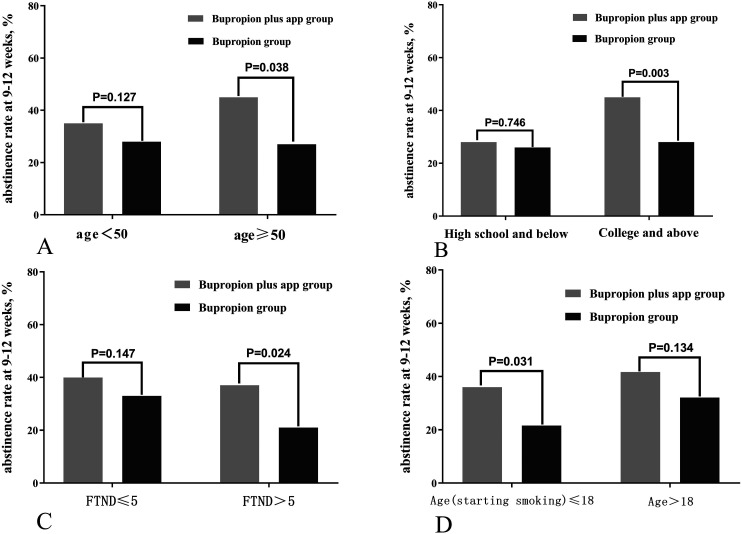
The results of the subgroup analysis are shown in Figure 3. The 9–12 weeks continuous abstinence rate was significantly higher in the bupropion plus app group (43.6%) than in the bupropion group (29.0%) (χ2 = 4.297, p = .038) when the age of participants was more than 50, while there was no significant difference when the age of participants was less than 50 (Figure 3a). The efficacy in the bupropion plus app group (46.5%) was superior to the bupropion group (28.8%) when the education level was college and above (χ2 = 8.626, p = .003), however, there was no statistical significance when the education level was high school and below (Figure 3b). There was also statistical significance when the score of FTND > 5 (p = .024)/age at starting smoking ≤ 18 (p = .031), and no significant difference between them (FTND ≤ 5/age > 18) (Figure 3c and d).
Figure 3.
Subgroup analysis to the comparison of abstinence rate at 9–12 weeks follow-up.
Note. Comparison of abstinence rate in (A) age < 50 / age ≥ 50 group; (B) high school and below / college and above group; (C) FTND ≤ 5 / FTND > 5 group; (D) age (starting smoking) ≤18 / age > 18 group.
User feedback
As shown in Appendix Table 5 in the online supplemental materials, 72.2% of the users were satisfied with the Cigarette-Burning app, and 56.7% would recommend it to other smokers. For the frequency of use, 82.9% accessed the app weekly, and 20.9% used it daily.
Medication adherence and adverse events
In terms of medication adherence, 59.5% (119/200) of participants in the bupropion plus app group had better adherence to smoking cessation medications than those (43.5%) in the bupropion group. At the 2-week follow-up visit, the most common adverse events were thirst (n = 15), dreaminess/abnormal dreams (n = 11), and headache (n = 9), other adverse events were shown in Appendix Table 6 in the online supplemental materials.
Discussion
To the best of our knowledge, this was the first randomized controlled trial to evaluate the efficacy of the smartphone application combined with medication intervention for smoking cessation in China. Our study filled several knowledge gaps. First, bupropion plus the app showed the better efficacy of smoking cessation than bupropion; second, besides the use of bupropion and the app, salary and smoking-related characteristics were also significant factors in the efficacy of smoking cessation; third, the use of the Cigarette-Burning app significantly increased the smoking cessation rate of medications, especially for smokers with older age, higher education level, heaver tobacco dependence, and younger age at starting smoking. A rigorous study design and stringent quality control ensured the reliability and validity of our study findings.
To date, despite the great availability of smartphone apps in the health field, only a few have reported their quality and reliability in accordance with recommendations of clinical smoking cessation guidelines. 17 For example, the results of previous studies showed that a majority of apps in China did not align with smoking cessation guidelines, and their average adherence scores were low (scores for iPhone and Android were 14.6 and 11.1, respectively).16,18 In response to this issue, the Cigarette-Burning app was developed and extensively refined, with several modules modified according to the content of smoking cessation guidelines. This resulted in relatively high adherence to the guidelines and increased user satisfaction with the app.
Previous studies have reported the efficacy of smoking cessation apps in different populations. In an RCT of 584 adults, 29 the CureApp Smoking Cessation (CASC) system showed better efficacy than standard smoking cessation treatment alone. The study by Bricker et al. 14 also found that there was still potential for evidence-based apps to achieve better smoking cessation outcomes. These were consistent with our findings. However, our result was different from several previous studies. For example, a later RCT of 5293 smokers 13 did not find associations between smartphone apps and cessation outcomes. A trial by Schwaninger et al. 15 also showed that smoking cessation apps did not predict the efficacy of smoking cessation. This discrepancy might be due to differences in study type, treatment disparities, characteristics of the study population and the study area. 30
Meanwhile, our study was the first study to integrate the use of the app into medication interventions among Chinese smokers, which found that the app could increase the efficacy of smoking cessation medications. Like behavioral interventions, the use of the app could help smokers understand the knowledge of tobacco dependence and the benefits of medication, thereby improving the adherence with medication.31,32 In addition, to the best of our knowledge, our study was the first study to assess the long-term abstinence rate of smartphone apps in China. Liao et al. 19 recently conducted a smoking cessation app program based on cognitive behavioral theory (N = 180). Compared to that, we noted that they focused on short-term abstinence rates at 33 days after TQD, while our investigation aimed to evaluate the long-term efficacy (24 weeks) of the app on smoking cessation. Despite the distinctions, both studies underscored the potential of smartphone apps as a viable tool to promote smoking cessation in China. Therefore, adding the Cigarette-Burning app as a complement to medication treatment holds promise as a way to improve smoking cessation outcomes in the future.
In addition, we identified several predictors of smoking cessation, including demographic factors such as salary. Our study found that smokers with higher salaries were more likely to successfully quit, which was consistent with the study by Hiscock et al., 33 possibly due to smokers of higher socioeconomic status generally having more access to smoking cessation resources.33,34 Smoking-related characteristics were also significant factors affecting the efficacy of smoking cessation. For example, those who were older at starting smoking, 35 and with fewer daily cigarettes 36 were significantly more likely to successfully quit smoking.
Furthermore, our study yielded several noteworthy findings from subgroup analysis. Firstly, our study showed that, when utilizing the app and medication, participants with the education level of college and above had an increased abstinence rate at 9–12 weeks compared with those with the education level of high school and below. This finding was consistent with a prior study of 756 participants in the United States, 37 suggesting that educational level may play a role in the effectiveness of smoking cessation medication interventions combined with the app. Secondly, our research revealed that individuals aged 50 and above had a higher likelihood of quitting than using medications alone. This might be attributed to the fact that older individuals often experience significant health consequences due to smoking, which could serve as a strong motivation to make the decision to quit. 38 Additionally, our study showed that for smokers with moderate-to-heavy tobacco dependence (FTND > 5 or starting smoking age ≤ 18), bupropion alone showed poor efficacy, while bupropion plus app greatly increased the abstinence rate at 9–12 weeks, suggesting that app-based interventions have the potential to provide effective smoking cessation support to “hard-to-quit” populations. However, while our exploratory findings are promising, there is still a need for further research to delve into the underlying factors that moderate the app's effectiveness for different subgroups of smokers. Identifying these moderators will allow us to tailor app-based interventions more effectively and provide targeted support to individuals with varying characteristics.
Our study has important clinical implications for smoking cessation treatment. As mentioned above, smartphone apps have long been considered as a promising way to quit smoking due to their flexibility and wide reach. Our study contributes valuable insights into the potential of the Cigarette-Burning app as a smoking cessation tool, and the app could be a useful supplementary method for those who are treated with pharmacotherapy, which could increase the smoking cessation rate by more than 50%. A notable finding was that the use of apps might have an important beneficial effect on smokers with higher levels of tobacco dependence, increasing the likelihood of successful quitting in this group. Therefore, providing smoking cessation support with apps to smokers who have difficulty quitting would be a viable strategy. Furthermore, we conducted an analysis of the association between app usage frequency and cessation rate, and we found that participants with a higher frequency of APP use had a higher likelihood of quitting smoking, which highlighted the importance of increasing the frequency of APP use. 39 Later, we developed a mobile system including a WeChat public account, a mini-program and a smoking cessation website based on this app for in-depth research and spreading it into clinical practice.
This study had several strengths, including being the first study to evaluate the efficacy of the smartphone application combined with medication intervention for smoking cessation in China, stringent procedure design, assessment of medication uses at multiple time points and abstinence with biochemical verification. In addition, assessment at multiple time points might improve adherence to smoking cessation treatment, 23 so the overall rate of lost-to-follow-up was low (8.3%).
Several limitations should be considered. First, considering that many factors (such as culture, 40 healthcare differences, 41 policy 42 ) could influence the generalizability, participants were restricted to people having access to their smartphone applications, which may not be generalizable to those without a smartphone or unable to use apps; and our study excluded individuals with major psychiatric and severe diseases considering contraindications, so the results may not be directly extrapolated to these populations. Further research was needed to determine the efficacy of combined interventions for smoking cessation in these groups; Second, our study only analyzed the efficacy of bupropion, although bupropion has been proven to be applicable for the majority of the population. 5 Further studies are needed to determine the efficacy when integrated into other widely used medications, such as nicotine replacement therapy products; Third, the lack of blinding for participants and researchers may introduce potential bias in the assessment of outcomes and participant behavior. However, we have addressed this concern by incorporating strict quality control measures and statistical analyses to mitigate potential bias and ensure the reliability of our findings. In addition, we did not collect participants’ complications or personality traits, which were associated with smoking cessation success,34,43 and future studies should explore the relationship between them. Last, although the use of the app such as frequency has been self-reported by users in the feedback questionnaire, the app did not track user behaviors, such as logins, duration of use, and module use frequency. Future research should try to refine the app module, and examined the specific characteristics and theoretical principles of app intervention in order to identify and develop the functional modules that play a role in it.
Conclusions
This trial provides evidence that the Cigarette-Burning smartphone application significantly increased smoking abstinence when combined with medication intervention, especially for smokers with older age, high education level, heavier tobacco dependence, and younger age at starting smoking. Our study contributes valuable insights into the potential of the Cigarette-Burning app as a useful supplementary method for those who are treated with pharmacotherapy.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH
Acknowledgment
The authors thank all the patients for their participation in this study.
Contributorship: All authors were involved in the planning of the study, literature review, interpretation of the findings, and manuscript preparation. Chen Wang and Dan Xiao conceived and designed the study. Dan Xiao supervised the study. Dan Xiao, Xiao-wen Wei, Rui Qin and Yong-zhuang Liu drafted the report. Xiao-wen Wei and Rui Qin did the statistical analysis. All authors contributed to data collection, analysis, and interpretation. All authors revised the report and approved the final version before submission.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Institutional Review Board at China-Japan Friendship Hospital (No. 2017-125), and registered in the Chinese Clinical Trial Registry (No.: ChiCTR1800016919, URL: http://www.chictr.org.cn). Written informed consent was obtained from all participants.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0506400).
Guarantor: Dan Xiao
ORCID iDs: Dan Xiao https://orcid.org/0000-0002-3691-7852
Supplemental material: Supplemental material for this article is available online.
References
- 1.Chinese Center for Disease Control and Prevention. Global adult tobacco survey in China in 2018. Beijing: People 's medical Publishing House, 2019. [Google Scholar]
- 2.Liu Z, Li YH, Cui ZY, et al. Prevalence of tobacco dependence and associated factors in China: findings from nationwide China Health Literacy Survey during 2018-19. Lancet Reg Health West Pac 2022; 24: 100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 2015; 386: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigotti NA, Kruse GR, Livingstone-Banks Jet al. et al. Treatment of tobacco smoking: a review. JAMA 2022; 327: 566–577. [DOI] [PubMed] [Google Scholar]
- 5.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016; 387: 2507–2520. [DOI] [PubMed] [Google Scholar]
- 6.Howes S, Hartmann-Boyce J, Livingstone-Banks J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2020; 4: CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnode CD, Henderson JT, Coppola EL, et al. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021; 325: 280–298. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99: 29–38. [DOI] [PubMed] [Google Scholar]
- 9.Caraballo RS, Shafer PR, Patel Det al. et al. Quit methods used by US adult cigarette smokers, 2014–2016. Prev Chronic Dis 2017; 14: E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H, Xiao D, Liu Z, et al. National survey of smoking cessation provision in China. Tob Induc Dis 2019; 17: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Be He@lthy, be mobile annual report 2018. Geneva: World Health Organization and International Telecommunication Union, 2019. [Google Scholar]
- 12.China Internet Network Information Center. The 51th statistical report on China's internet development. Beijing: China Internet Network Information Center, 2023. https://cnnic.cn/n4/2023/0302/c199-10755.html . [Google Scholar]
- 13.Etter J-F, Khazaal Y. The Stop-tabac smartphone application for smoking cessation: a randomized controlled trial. Addiction 2022; 117: 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bricker JB, Watson NL, Mull KE, et al. Efficacy of smartphone applications for smoking cessation: a randomized clinical trial. JAMA Intern Med 2020; 180: 1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwaninger P, Berli C, Scholz Uet al. et al. Effectiveness of a dyadic buddy app for smoking cessation: randomized controlled trial. J Med Internet Res 2021; 23: e27162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abroms LC, Lee Westmaas J, Bontemps-Jones J, et al. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med 2013; 45: 732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bold KW, Garrison KA, DeLucia A, et al. Smartphone apps for smoking cessation: systematic framework for app review and analysis. J Med Internet Res 2023; 25: e45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng F, Xu J, Su C, et al. Content analysis of smartphone apps for smoking cessation in China: empirical study. JMIR Mhealth Uhealth 2017; 5: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Tang J. Feasibility and acceptability of a cognitive behavioral therapy-based smartphone app for smoking cessation in China: a single-group cohort study. Front Psychiatry 2022; 12: 759896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Arkee S, Mason J, Lane DA, et al. Mobile apps to improve medication adherence in cardiovascular disease: systematic review and meta-analysis. J Med Internet Res 2021; 23: e24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiore MC, Jaen CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update - A US public health service report. Am J Prev Med 2008; 35: 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.China National Health and Family Planning Commission. China clinical guidelines for tobacco cessation. Beijing: People 's Medical Publishing House, 2015. [Google Scholar]
- 23.Pacek LR, McClernon FJ, Bosworth HB. Adherence to pharmacological smoking cessation interventions: a literature review and synthesis of correlates and barriers. Nicotine Tob Res 2018; 20: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances–systematic review and meta-analysis. Addict Behav 2012; 37: 225–233. [DOI] [PubMed] [Google Scholar]
- 25.Heatherton TF, Kozlowski LT, Frecker RCet al. et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Switzerland: World Health Organization, 1992. [Google Scholar]
- 27.American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 28.West R, Hajek P, Stead Let al. et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005; 100: 299–303. [DOI] [PubMed] [Google Scholar]
- 29.Masaki K, Tateno H, Nomura A, et al. A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. NPJ Digit Med 2020; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittaker R, McRobbie H, Bullen C, et al. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev 2019; 10: CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann-Boyce J, Hong B, Livingstone-Banks J, et al. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2019; 6: CD009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cokkinides VE, Ward E, Jemal Aet al. et al. Under-use of smoking-cessation treatments: results from the national health interview survey, 2000. Am J Prev Med 2005; 28: 119–122. [DOI] [PubMed] [Google Scholar]
- 33.Hiscock R, Judge K, Bauld L. Social inequalities in quitting smoking: what factors mediate the relationship between socioeconomic position and smoking cessation? J Public Health (Oxf) 2011; 33: 39–47. [DOI] [PubMed] [Google Scholar]
- 34.Tøttenborg SS, Thomsen RW, Johnsen SP, et al. Determinants of smoking cessation in patients with COPD treated in the outpatient setting. Chest 2016; 150: 554–562. [DOI] [PubMed] [Google Scholar]
- 35.Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addict Behav 1999; 24: 673–677. [DOI] [PubMed] [Google Scholar]
- 36.Lin C-J, Huang W-H, Hsu C-Y, et al. Smoking cessation rate and its predictors among heavy smokers in a smoking-free hospital in Taiwan. Int J Environ Res Public Health 2021; 18(24): 12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetter DW, Cofta-Gunn L, Irvin JE, et al. What accounts for the association of education and smoking cessation? Prev Med 2005; 40: 452–460. [DOI] [PubMed] [Google Scholar]
- 38.Sachs-Ericsson N, Schmidt NB, Zvolensky MJ, et al. Smoking cessation behavior in older adults by race and gender: the role of health problems and psychological distress. Nicotine Tob Res 2009; 11: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei X, Qin R, Cheng A, et al. Association of the APP usage frequency and the effect in smoking cessation clinic intervention. Chin J Health Manage 2023; 17: 661–667. [Google Scholar]
- 40.Xiang Y-T, Chiu HFK, Ungvari GS. Quality of life and mental health in Chinese culture. Curr Opin Psychiatry 2010; 23: 43–47. [DOI] [PubMed] [Google Scholar]
- 41.Burciaga Valdez R, Encinosa W. Racial and ethnic disparities in healthcare costs and outcomes of cigarette smoking in USA: 2008-2019. Tob Control: tc-2023-058136. [DOI] [PubMed] [Google Scholar]
- 42.Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health 2017; 38: 165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin W, Xian B, Zhao Let al. et al. Association between personality traits and smoking cessation among Chinese adults. BMC Psychol 2023; 11: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076241297732 for Efficacy of the cigarette-burning application combined with medication intervention for smoking cessation in China: A randomized controlled trial by Xiao-Wen Wei, Rui Qin, Yong-Zhuang Liu, Zhao Liu, An-Qi Cheng, Xin-Mei Zhou, Zheng Su, Zi-Yang Cui, Jin-Xuan Li, Liang Zhao, Dan Xiao and Chen Wang in DIGITAL HEALTH