Abstract
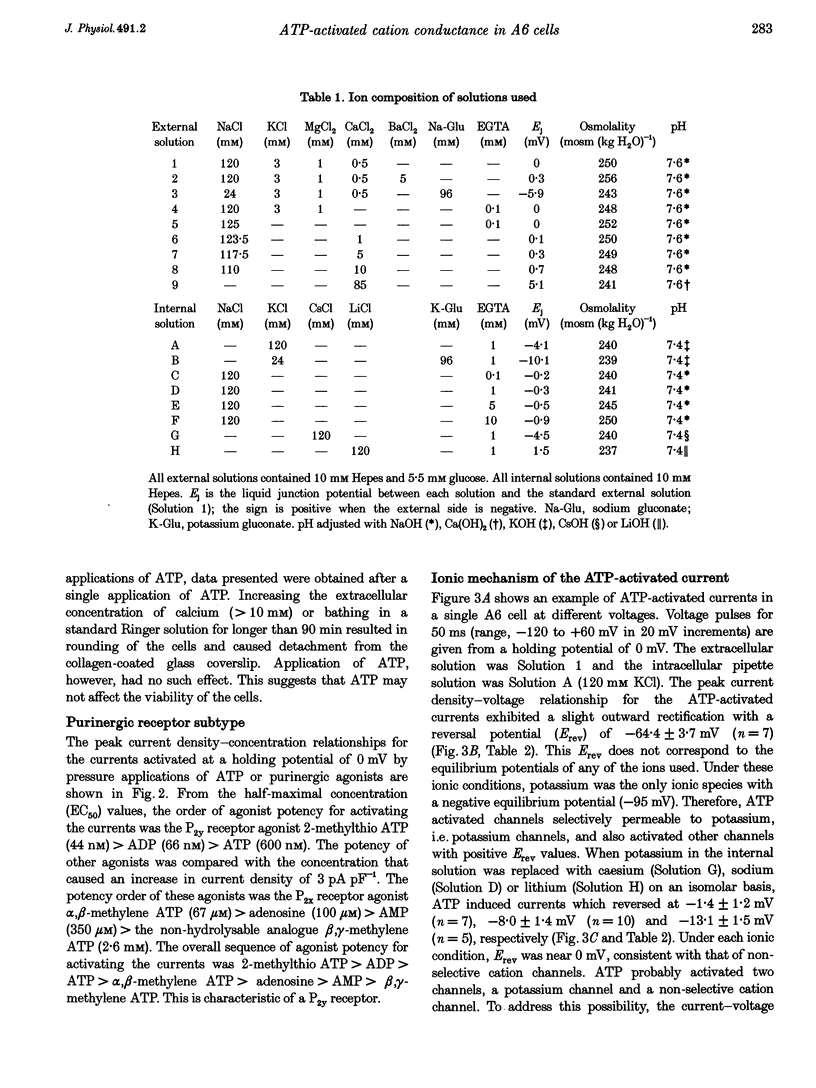
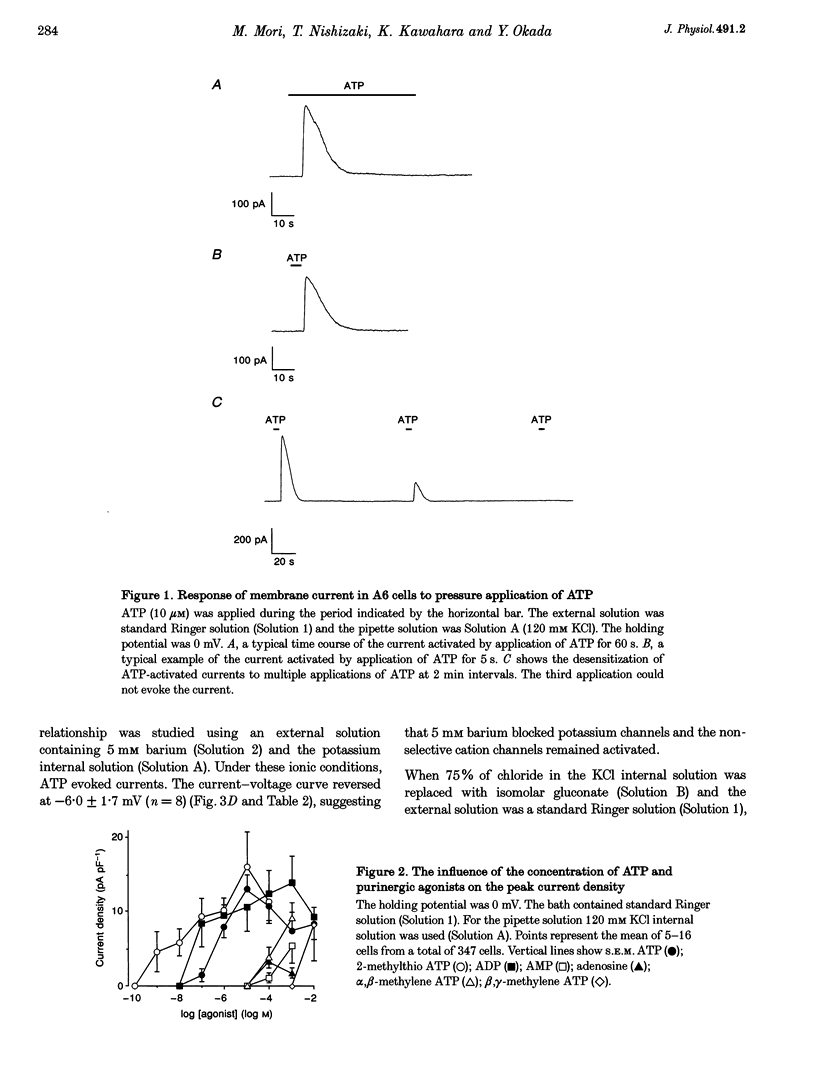
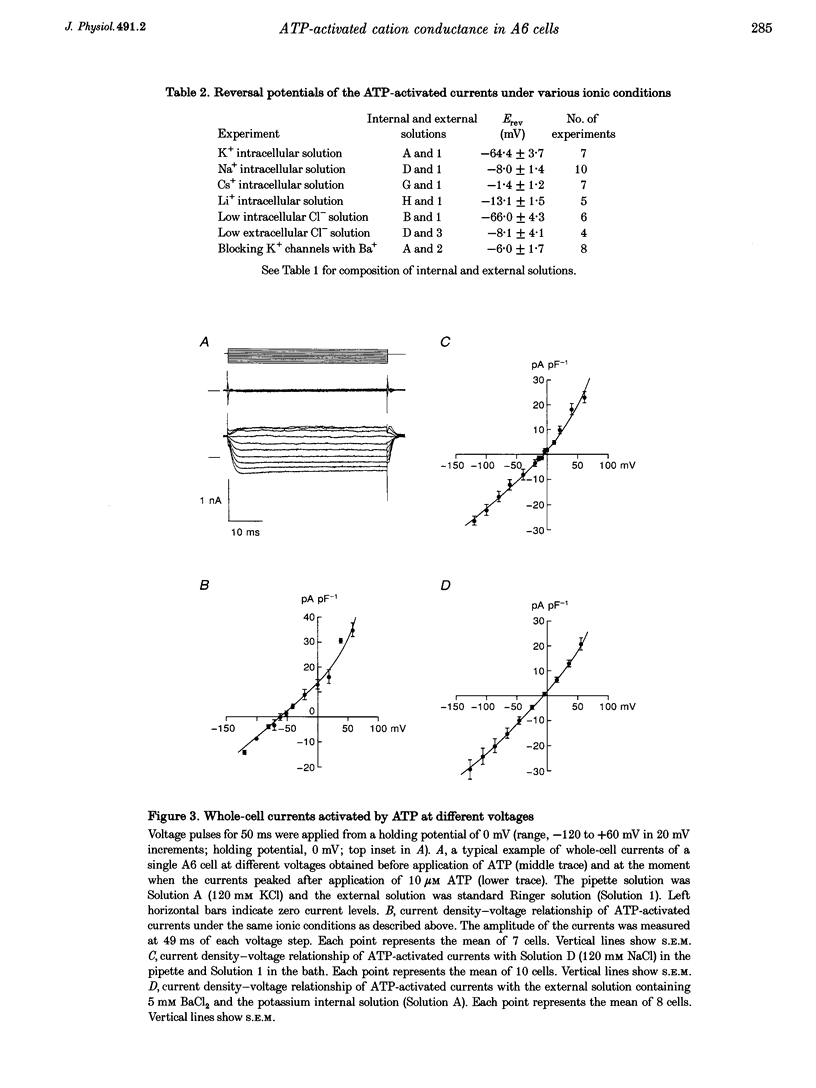
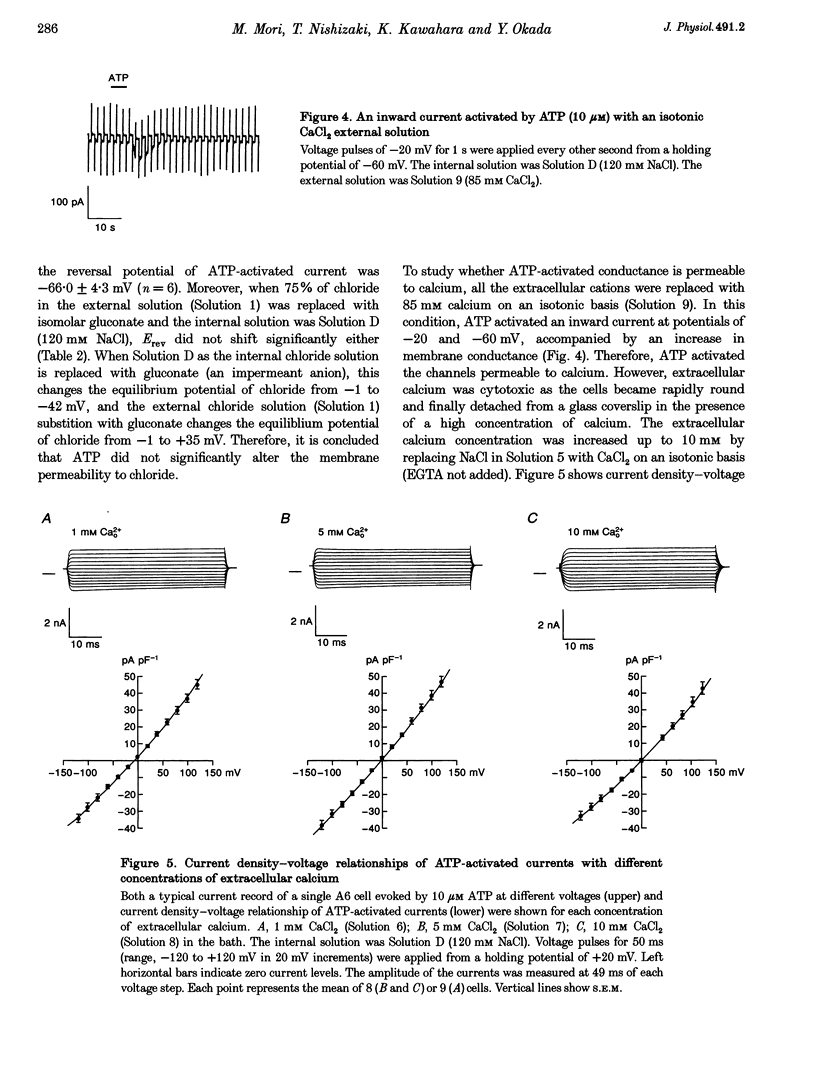
1. Using a whole-cell voltage-clamp technique and fura-2 fluorescence measurements, the actions of extracellular adenosine 5'-triphosphate (ATP) in single cells of an epithelial cell line (A6), were investigated. 2. ATP (0.1-1000 microM) induced currents in cells held under voltage clamp. The sequence of purinergic agonist potency in activating the currents (2-methylthio ATP > adenosine 5'-diphosphate (ADP) > ATP > alpha, beta-methylene ATP) was consistent with that of P2y receptors. 3. Reversal potentials (Erev) of the currents under various ionic conditions suggest that potassium channels and non-selective cation channels were responsible for the ATP-activated conductance, which was permeable to calcium. 4. ATP activated the currents in a calcium-free extracellular solution. In the presence of extracellular calcium, the currents were completely inhibited with 10 mM EGTA in the pipette. 5. ATP (10 microM) increased the intracellular calcium concentration ([Ca2+]i) whether cells were bathed in a solution containing calcium or not. 6. These results indicate that ATP evoked a calcium-dependent cation conductance, permeable to calcium, through P2y receptors by releasing calcium from intracellular stores in A6 cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai B. J., Friedman P. A. Activation of latent Ca2+ channels in renal epithelial cells by parathyroid hormone. Nature. 1990 Sep 27;347(6291):388–391. doi: 10.1038/347388a0. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Ausiello D. A. G protein subunit, alpha i-3, activates a pertussis toxin-sensitive Na+ channel from the epithelial cell line, A6. J Biol Chem. 1989 Dec 15;264(35):20867–20870. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Ellis V. R. Purinergic P2y receptors stimulate renin secretion by rat renal cortical slices. J Pharmacol Exp Ther. 1993 Jul;266(1):160–163. [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara N., Ichinose M., Sawada M., Imai K., Maeno T. Activation of single Ca2(+)-dependent K+ channel by external ATP in mouse macrophages. FEBS Lett. 1990 Jul 16;267(2):281–284. doi: 10.1016/0014-5793(90)80945-f. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Okajima F., Tomura H., Majid M. A., Takeuchi T., Kondo Y. Change of intracellular calcium of neural cells induced by extracellular ATP. FEBS Lett. 1991 Jun 24;284(2):235–237. doi: 10.1016/0014-5793(91)80693-w. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Song L., Powers R. [3H]PtdIns hydrolysis in postmortem human brain membranes is mediated by the G-proteins Gq/11 and phospholipase C-beta. Biochem J. 1994 Dec 1;304(Pt 2):655–659. doi: 10.1042/bj3040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. P., Mangel A. W., Basavappa S., Fitz J. G. Nucleotide receptors regulate membrane ion transport in renal epithelial cells. Am J Physiol. 1993 May;264(5 Pt 2):F867–F873. doi: 10.1152/ajprenal.1993.264.5.F867. [DOI] [PubMed] [Google Scholar]
- Mitchell K. D., Navar L. G. Modulation of tubuloglomerular feedback responsiveness by extracellular ATP. Am J Physiol. 1993 Mar;264(3 Pt 2):F458–F466. doi: 10.1152/ajprenal.1993.264.3.F458. [DOI] [PubMed] [Google Scholar]
- Nanoff C., Freissmuth M., Tuisl E., Schütz W. P2-, but not P1-purinoceptors mediate formation of 1, 4, 5-inositol trisphosphate and its metabolites via a pertussis toxin-insensitive pathway in the rat renal cortex. Br J Pharmacol. 1990 May;100(1):63–68. doi: 10.1111/j.1476-5381.1990.tb12052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Sasakawa N., Nakaki T., Yamamoto S., Kato R. Stimulation by ATP of inositol trisphosphate accumulation and calcium mobilization in cultured adrenal chromaffin cells. J Neurochem. 1989 Feb;52(2):441–447. doi: 10.1111/j.1471-4159.1989.tb09140.x. [DOI] [PubMed] [Google Scholar]
- Schneider P., Hopp H. H., Isenberg G. Ca2+ influx through ATP-gated channels increments [Ca2+]i and inactivates ICa in myocytes from guinea-pig urinary bladder. J Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela D., Ram E., Atlas D. ATP receptor. A putative receptor-operated channel in PC-12 cells. J Biol Chem. 1991 Sep 25;266(27):17990–17994. [PubMed] [Google Scholar]


