Abstract
In 1997, avian H5N1 influenza virus transmitted from chickens to humans resulted in 18 confirmed infections. Despite harboring lethal H5N1 influenza viruses, most chickens in the Hong Kong poultry markets showed no disease signs. At this time, H9N2 influenza viruses were cocirculating in the markets. We investigated the role of H9N2 influenza viruses in protecting chickens from lethal H5N1 influenza virus infections. Sera from chickens infected with an H9N2 influenza virus did not cross-react with an H5N1 influenza virus in neutralization or hemagglutination inhibition assays. Most chickens primed with an H9N2 influenza virus 3 to 70 days earlier survived the lethal challenge of an H5N1 influenza virus, but infected birds shed H5N1 influenza virus in their feces. Adoptive transfer of T lymphocytes or CD8+ T cells from inbred chickens (B2/B2) infected with an H9N2 influenza virus to naive inbred chickens (B2/B2) protected them from lethal H5N1 influenza virus. In vitro cytotoxicity assays showed that T lymphocytes or CD8+ T cells from chickens infected with an H9N2 influenza virus recognized target cells infected with either an H5N1 or H9N2 influenza virus in a dose-dependent manner. Our findings indicate that cross-reactive cellular immunity induced by H9N2 influenza viruses protected chickens from lethal infection with H5N1 influenza viruses in the Hong Kong markets in 1997 but permitted virus shedding in the feces. Our findings are the first to suggest that cross-reactive cellular immunity can change the outcome of avian influenza virus infection in birds in live markets and create a situation for the perpetuation of H5N1 influenza viruses.
One of the puzzling aspects of the avian influenza virus H5N1 outbreak in Hong Kong in 1997 was that most chickens in the poultry markets appeared to be healthy, despite the presence of lethal H5N1 virus in 20% of the birds (29). The available information indicated that chickens in most markets excrete H5N1 virus in their feces, yet birds in only 2 of 11 markets studied showed disease signs (29). Every H5N1 virus isolate characterized experimentally caused lethal infection in chickens (28, 33, 34).
Virologic studies showed that H9N2 influenza viruses were the second most prevalent influenza virus in the markets and were isolated from about 5% of chickens. Characterization of the H9N2 influenza virus indicated that three distinguishable lineages of H9N2 viruses were present in the markets (12). The most prevalent lineage in December 1997 was represented by A/Chicken/Hong Kong (HK)/G9/97 (H9N2), which contained PB1 and PB2 genes that were highly related to those of A/HK/156/97 (H5N1) (12). The remaining gene segments of A/Chicken/HK/G9/97 (H9N2) were most closely related to those of A/Chicken/HK/Y280/97 (H9N2) (12). A single isolate of A/Quail/HK/G1/97 (H9N2) representing a different H9N2 lineage contains genes encoding six internal proteins that are highly homologous to those of A/HK/156/97 (H5N1) (12).
Cytotoxic T lymphocytes (CTLs) lyse target cells infected with viruses in a major histocompatibility complex-restricted manner (46). Influenza virus-specific CTLs play a crucial role in clearing influenza virus from the lungs of mice (43, 20). The hemagglutinin (HA) of influenza A virus acts as a minor target antigen for subtype-specific CTLs (3, 6). Specific CTL responses to internal proteins, including nucleoprotein (NP), polymerase (PB1, PB2, and PA), matrix protein (M1), and nonstructural protein 1 (NS1), have been detected in mice and humans (2, 15, 4, 5, 11, 26). The NP of influenza A viruses is an important target antigen for both subtype-specific and cross-reactive CTLs in mice and humans (39, 45, 22). In addition, NS1 and the HA2 subunit of influenza A virus induces a protective cross-reactive CTL response in mice (17, 18). The repertoire of murine CTLs in response to influenza A viruses seems to be limited: the frequency of nonresponder alleles to influenza virus proteins in mice is very high (5), and several murine class I antigens are unable to present epitopes of a number of influenza virus proteins to CTLs. In contrast, the human memory CTL responses are targeted to a broad range of influenza A virus proteins (15, 10). Although a few studies have shown that cross-protection in chickens vaccinated with NP constructs is limited (35, 42, 16), there are no available data on CTL responses to influenza viruses in chickens, an important natural host of influenza A virus.
In this study, we tested the hypothesis that H9N2 influenza viruses in chickens provided cross-protective immunity to H5N1 infection. Our results indicate that cross-reactive immunity protected chickens from lethal H5N1 influenza virus infection and that cross-reactive CD8+ CTLs play a pivotal role in protecting chickens from lethal infection with an H5N1 influenza virus.
MATERIALS AND METHODS
Viruses.
Influenza viruses of H5N1 (A/Chicken/HK/728/97) and H9N2 (A/Chicken/HK/G9/97 and A/Quail/HK/G1/97) were propagated in the allantoic cavities of 11-day-old embryonated eggs in a U.S. Department of Agriculture-approved biosafety level 3 (BL-3) containment facility.
Animals.
Embryonated inbred eggs (B2/B2) were purchased from the Avian Disease and Oncology Laboratory (U.S. Department of Agriculture, East Lansing, Mich.). Chickens were hatched in our laboratory and were housed in a specific-pathogen-free environment at the laboratory animal and resources facility, University of Tennessee, Memphis. Outbred 3- to 4-week-old specific-pathogen-free White Leghorn chickens were purchased from SPAFAS, Inc. (Norwich, Conn.). The animal experiments were performed in a BL-3 containment facility.
Cell culture.
Cell culture was performed as described previously (30). Lungs from 4-week-old inbred chickens (B2/B2) were aseptically collected and trypsinized before culturing in tissue culture flasks coated with 0.01% (wt/vol) calf skin collagen (Sigma Chemical Co., St. Louis, Mo.). We cultured approximately 105 lung cells per ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% l-glutamine, 1% sodium pyruvate, 1% MEM nonessential amino acids, 1% antibiotic-antimycotic solution (Sigma), and 10% chicken serum in tissue culture flasks (75 cm2) in a humidified incubator at 37°C. Media were changed every 3 days, and at passage 10, the inbred lung cells were used as target cells in CTL assays. Most surviving cells had an epithelial-like morphology.
Immunization and challenge infection.
We intranasally inoculated 6- to 8-week-old chickens with 103 chicken infectious doses (CID50) of A/Chicken/HK/G9/97 (H9N2) influenza virus (volume, 0.2 ml) 3 to 70 days before challenge with an H5N1 influenza virus. These chickens were challenged intranasally with 10 chicken lethal doses (CLD50) of A/Chicken/HK/728/97 (H5N1) influenza virus. Chickens were monitored to determine how many had died each day.
Serology tests.
Preimmune and postimmune chicken sera were treated with receptor-destroying enzyme before they were used in hemagglutination inhibition (HI) and viral neutralization assays. Before challenge with the H5N1 influenza virus, sera were collected on various days from 6- to 8-week-old chickens immunized with A/Chicken/HK/G9/97 (H9N2) influenza virus. The results of the HI assays were recorded as the highest serum dilutions inhibiting hemagglutination of 0.5% chicken erythrocytes.
Viral neutralization tests were performed with virus (102 tissue culture infectious doses [TCID50]) and diluted chicken sera that had been incubated together at room temperature for 1 h. To measure the residual virus infectivity, the mixture was titrated on monolayers of Madin-Darby canine kidney cells grown in 96-well tissue culture plates. Plates were incubated for 3 days at 37°C in 5% CO2. At the end of 3 days, the presence of cytopathic effects on cell monolayers and the ability of culture supernatants to induce hemagglutination were evaluated. Neutralization titers were expressed as the reciprocal of the antibody dilution that completely inhibited virus infectivity in 50% of quadruplicate cultures.
Dose-response challenge.
Eight-week-old chickens were immunized with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) 30 days before they were challenged with various doses of A/Chicken/HK/728/97 (H5N1). Chickens were monitored to determine how many died each day, and their tracheae and cloacae were swabbed to determine whether H5N1 influenza virus was shed.
Preparation of donor lymphocytes.
Lymphocytes were prepared as described previously (30). Briefly, inbred 6-week-old chickens (B2/B2, line 72) were infected with A/Chicken/HK/G9/97 (H9N2) influenza virus (103 CID50). Splenocytes were collected 7 or 10 days after infection. Splenic lymphocytes were separated with a Ficoll-Hypaque density gradient (Histopaque 1.0777; Sigma) (30). We separated the lymphocytes into different populations by passing the cells through nylon wool columns (Polysciences, Inc., Warrington, Pa.). Unbound T cells and macrophages were resuspended in RPMI 1640 with 10% chicken serum (Sigma) and incubated in tissue culture flasks for 3 h. After 3 h, the nonadherent T cells were collected. Nylon wool-bound B cells were also collected for use in an adoptive transfer study.
Depletion of T-lymphocyte subsets.
Purified T lymphocytes were further separated into CD4+ or CD8+ subtypes by using Dynabeads (Dynal, Oslo, Norway). Briefly, Dynabeads conjugated to pan-mouse immunoglobulin G (IgG) were coated with mouse anti-chicken CD4 or CD8 monoclonal antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.) at a ratio of 1 μg of antibody to 107 beads for 30 min at 4°C. Chicken T lymphocytes (2 × 107 cells) in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) were added to antibody-coated Dynabeads (8 × 107 beads) and incubated for 30 min at 4°C. The cells were separated with a Dynal MPC apparatus. The supernatants containing the cells of interest were transferred to a fresh tube for further use.
Flow cytometric analysis.
Depleted populations of uninfected T lymphocytes (107) were incubated with 1:50 dilutions of mouse anti-chicken CD4 or CD8 antibodies in PBS with 0.1% BSA on ice for 30 min. After the cells were washed three times with PBS (pH 7.2), they were incubated with diluted (1:50) fluorescein isothiocyanate (FITC) labeled goat anti-mouse IgG antibody in PBS with 0.1% BSA on ice for 30 min. The fluorescence intensity of the stained cells was analyzed with a FACSCalibur fluorospectrometer (Becton Dickinson, San Jose, Calif.).
Adoptive transfer of immune lymphocytes.
Inbred immune donor lymphocytes (4 × 107 or 2 × 107 cells in a volume of 0.3 ml) were injected into the wing veins of naive inbred chickens (B2/B2), which were then challenged with 10 CLD50 of A/Chicken/HK/728/97 (H5N1) influenza virus. As a control, sterile PBS (volume, 0.3 ml) was injected into the wing veins of chickens of the same line. Chickens were monitored to determine how many died each day.
CTL assay.
CTL activity was measured by the CytoTox96 nonradioactive cytotoxicity assay (Promega, Madison, Wis.), which detects the stable cytosolic enzyme lactate dehydrogenase (LDH) when it is released from lysed cells. The assay was performed as instructed by the manufacturer. Briefly, the inbred lung cells (B2/B2), which served as the target cells, were infected for 10 h with A/Chicken/HK/728/97 (H5N1) or A/Chicken/HK/G9/97 (H9N2) virus at a multiplicity of infection (MOI) of 2. Various amounts of effector T cells in 50 μl of RPMI 1640 supplemented with 1% l-glutamine, 1% sodium pyruvate, 1% MEM nonessential amino acids, and 10% chicken serum were added to each well of 96-well, round-bottom microtiter plates; 104 target cells infected or uninfected (volume, 50 μl) were also added to each well. Microtiter plates were centrifuged at 250 × g for 5 min before they were incubated for 4 h in a humidified chamber at 37°C, 5% CO2. After 4 h, the plates were centrifuged again.
Samples (50 μl) from all wells were transferred to fresh 96-well flat-bottom plates. Reconstituted substrate mix (50 μl) was added to each well of the flat-bottom plates, and the plates were incubated for 30 min at room temperature. Stop solution (50 μl) was added before the amount of released LDH was measured by spectrophotometry (A492). Specific lysis of target cells by CTLs was calculated according to the following formula: (LDH in the mixture of target and effector cells − LDH spontaneously released from target cells and effector cells/total LDH of target cells − LDH spontaneously released from target cells) × 100. All assays were performed in a quadruplicate set of wells. Individual values did not differ from the mean by more than 8%. Spontaneous release was less than 20%.
RESULTS
Absence of serologic cross-reactivity.
To determine whether sera from chickens infected with an H9N2 influenza virus can cross-react with an H5N1 influenza virus, we bled chickens infected with A/Chicken/HK/G9/97 (H9N2) influenza virus before the chickens were challenged with an H5N1 influenza virus. Sera were tested for reactivity to both H9N2 and H5N1 influenza viruses in HI assays (Table 1). Sera collected from chickens that were infected 3 days earlier or from chickens that were not infected did not inhibit hemagglutination by either H9N2 or H5N1 influenza viruses. Sera collected from chickens 6 days after H9N2 infection inhibited hemagglutination by H9N2 influenza viruses (A/Chicken/HK/G23/97, A/Duck/HK/Y280/97, A/Chicken/HK/G9/97) but did not inhibit hemagglutination by A/Quail/HK/G1/97 (H9N2) or A/Chicken/HK/728/97 (H5N1) influenza viruses. Sera collected from chickens 15, 30, or 70 days after H9N2 infection inhibited hemagglutination by H9N2 influenza viruses but did not inhibit hemagglutination by an H5N1 influenza virus. Sera from chickens given a second dose of the H9N2 virus 60 days after infection also inhibited hemagglutination by the H9N2 influenza viruses but did not inhibit hemagglutination by an H5N1 influenza virus.
TABLE 1.
Results of HI and viral neutralization assaysa
| Days after infection | Mean HI antibody titerb
|
Mean neutralization antibody titerc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H9N2
|
H5N1 | H9N2
|
H5N1 | |||||||
| G9 | G23 | Y280 | G1 | G9 | G23 | Y280 | G1 | |||
| 0 | <d | < | < | < | < | < | < | < | < | < |
| 3 | < | < | < | < | < | < | < | < | < | < |
| 6 | 20 | 20 | 20 | < | < | < | < | < | < | < |
| 10 | 160 | 160 | 160 | < | < | 1.25 | 1.0 | 1.0 | < | < |
| 15 | 320 | 160 | 160 | 40 | < | 2 | 1.0 | 1.25 | 1.0 | < |
| 30 | 320 | 320 | 320 | 40 | < | 2.25 | 1.5 | 2.0 | 1.0 | < |
| 70 | 320 | 320 | 320 | 40 | < | 2.5 | 2.0 | 2.25 | 1.0 | < |
| 70-booste | 640 | 640 | 640 | 160 | < | 3.75 | 2.75 | 3.50 | 1.5 | < |
Five chickens per group were infected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) influenza virus 3 to 70 days before sera were collected from wing veins. Abbreviations: G1, A/Quail/HK/G1/97 (H9N2); G23, A/Chicken/HK/G23/97 (H9N2); Y280, A/Duck/HK/Y280/97 (H9N2); G9, A/Chicken/HK/G9/97 (H9N2).
Expressed as the reciprocal of the highest dilution of serum that inhibits the hemagglutination of 4 HA units of virus.
Expressed as the reciprocal of the highest dilution of serum (log10) that neutralizes 100 TCID50 in 50% of virus-infected Madin-Darby canine kidney cells.
<, no reaction detected.
Chickens were reinfected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) 60 days after the initial infection, and 10 days later sera were collected.
Sera were also evaluated in viral neutralization assays using Madin-Darby canine kidney cells (Table 1). Neutralization activity against H9N2 influenza viruses was first detected in sera collected from chickens 10 days after the H9N2 infection. The activity was slightly higher in sera collected from chickens infected 15, 30, or 70 days earlier. Sera from chickens given a second dose of H9N2 influenza virus 60 days after the initial infection neutralized only H9N2 influenza viruses. Sera collected from chickens infected with A/Chicken/HK/G9/97 also distinguished between A/Chicken/HK/G9/97 and A/Quail/HK/G1/97, especially 10 days after infection. Homology of HA gene between A/Chicken/HK/G9/97 and A/Quail/HK/G1/97 is 91% (12). No sera collected from chickens infected with H9N2 influenza virus neutralized an H5N1 influenza virus.
Cross-reactive protection of chickens.
To investigate whether A/Chicken/HK/G9/97 (H9N2) can protect chickens from infection with an H5N1 influenza virus, we infected chickens with an H9N2 influenza virus (103 CID50) 3 to 70 days before they were challenged with an H5N1 influenza virus (10 CLD50) (Table 2). Most of the chickens survived the lethal challenge, but some of the surviving chickens shed H5N1 influenza virus from their cloacae. H5N1 influenza virus was also detected in the tracheae of many surviving chickens, but the titers of virus were lower than those of viruses isolated from the cloacae of surviving chickens. H5N1 influenza virus was detected in only undiluted tracheal samples of surviving chickens. Control chickens died within 3 days after the challenge. High titers of H5N1 virus in both tracheae and cloacae were detected in dead control chickens. Two chickens immunized 3 days earlier survived the H5N1 virus infection and shed H5N1 viruses from their cloacae (titer, 103.0 50% egg lethal doses [ELD50]) 3 to 9 days after the challenge. All five chickens immunized 6 days earlier were alive and shed no H5N1 influenza virus from their cloacae after challenge. With increasing time after immunization, more birds showed virus shedding and disease signs; by 70 days, 4 of 10 birds died after challenge with H5N1 (mean survival time of 3.5 days for four dead chickens). Unvaccinated chickens died 3 days after the lethal H5N1 virus challenge; the titer shed from their tracheae and cloacae was 106.5 ELD50. H5N1 viruses were detected in the tracheae of all surviving chickens 2 to 5 days after the challenge, but the viral titers were less than 10 ELD50. Six of 10 chickens immunized 70 days earlier with an H9N2 influenza virus survived, and four of the six survivors shed H5N1 influenza virus from their cloacae (titer, 102.75 ELD50). Most of the surviving chickens that showed signs of disease experienced minor respiratory symptoms including sneezing or nasal discharge.
TABLE 2.
Cross-reactive protection in chickensa
| Days after infection | Protection of chickens from lethal H5N1 infection (no. sick/ no. dead/total) | No. of chickens shedding H5N1/no. of survivors
|
H5N1 viral titer from surviving chickens (log10 ELD50)
|
||
|---|---|---|---|---|---|
| Trachea | Cloaca | Trachea | Cloaca | ||
| 0 (controlb) | 5/5/5 | >6.0 | >6.0 | ||
| 3 | 3/3/5 | 2/2 | 2/2 | <1 | 3.0 |
| 6 | 1/0/5 | 2/5 | 0/5 | <1 | Not detected |
| 10 | 1/1/5 | 3/4 | 1/4 | <1 | 2.5 |
| 15 | 2/0/5 | 4/5 | 1/5 | <1 | 2.0 |
| 30 | 3/1/10 | 9/9 | 3/9 | <1 | 2.6 |
| 70 | 6/4/10 | 6/6 | 4/6 | <1 | 2.75 |
| 70-boostc | 1/0/10 | 10/10 | 3/10 | <1 | 2.25 |
Chickens were infected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) influenza virus 3 to 70 days before challenge with 10 CLD50 of A/Chicken/HK/728/97 (H5N1) influenza virus. For 15 days, chickens were monitored daily for death, and their tracheae and cloacae were swabbed daily. The presence of H5N1 influenza virus was determined by candling of inoculated embryonated eggs and by the HA assay.
Control chickens, infected only with A/Chicken/HK/728/97 (H5N1), were dead 3 days after the infection, and all had H5N1 virus in their tracheae and cloacae at the time of death.
Chickens were given a second dose of A/Chicken/HK/G9/97 (H9N2) (103 CID50) 60 days after the initial infection and challenged 10 days later with 10 CLD50 of A/Chicken/HK/728/97.
To investigate whether the immunized chickens reinfected with H9N2 influenza virus are more resistant to the lethal challenge of an H5N1 influenza virus, the immunized chickens were reinfected 60 days after the initial infection with A/Chicken/HK/G9/97 (H9N2) influenza virus but before the challenge with an H5N1 influenza virus (Table 2). All chickens that had been infected with the H9N2 virus a second time survived, and only one chicken showed very mild signs of mild respiratory disease. Three of 10 chickens shed H5N1 influenza virus from their cloacae (titer, 102.25 ELD50). H5N1 influenza virus was detected in undiluted samples from the tracheae of surviving birds.
Dose-response challenge of H9N2-immunized chickens to an H5N1 influenza virus.
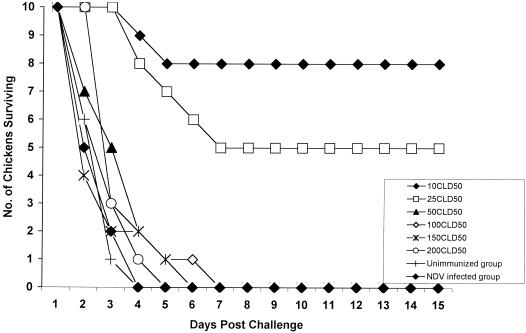
To investigate the response of H9N2-immunized chickens to increasing doses of challenge H5N1 virus, we infected chickens with an H9N2 influenza virus 30 days before they were challenged with various doses of an H5N1 influenza virus (Fig. 1). The number of chickens that died of H5N1 infection increased in a dose-dependent manner. Although three of ten chickens challenged with 10 CLD50 of an H5N1 influenza virus showed mild disease signs, eight of the chickens survived, and four of the surviving chickens shed H5N1 influenza virus from their cloacae (titer, 2.75 ELD50) (Table 3). Seven of the ten chickens challenged with 25 CLD50 of an H5N1 influenza virus showed respiratory disease signs and lost 10% of body weight, but five survived. Five surviving chickens shed H5N1 influenza virus from their cloacae (titer, 2.68 ELD50). H5N1 influenza virus was detected in the tracheae of all surviving chickens 2 to 5 days after challenge, but the titers were less than 10 ELD50. Chickens challenged with 50, 100, 150, or 200 CLD50 of an H5N1 influenza virus did not survive. The titer of virus isolated from the tracheae and cloacae of these dead birds was greater than 106 ELD50. Chickens immunized with P/Chicken/HK/QB4/99 (Newcastle disease virus) did not survive infection with lethal H5N1 influenza virus.
FIG. 1.
Dose-response challenge of chickens. Ten chickens per group primed 30 days earlier by infection with 103 CID50 of A/Chicken/HK/G9/97(H9N2) influenza virus were challenged with various doses of an H5N1 influenza virus. Chickens were monitored to determine how many died each day until 15 days after the challenge. Chickens immunized 30 days earlier with P/Chicken/HK/QB4/99 (Newcastle disease virus) were challenged with 10 CLD50 of H5N1 influenza virus.
TABLE 3.
Dose of H5N1 virus required to overcome protective immunity provided by H9N2 infectiona
| Dose (CLD50) of H5N1 challenge virus | Protection of chickens (no. sick/no. dead) | H5N1 virus in surviving chickensb
|
|
|---|---|---|---|
| Trachea | Cloaca | ||
| 10 | 3/2 | + (8/8c) | 2.75d (4/8) |
| 25 | 7/5 | + (5/5) | 2.68d (5/5) |
| 50 | 10/10 | +++ | +++ |
| 100 | 10/10 | +++ | +++ |
| 150 | 10/10 | +++ | +++ |
| 200 | 10/10 | +++ | +++ |
Ten chickens per group were infected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) 30 days prior to challenge with various doses of A/Chicken/HK/728/97 (H5N1). Challenged chickens were daily monitored for death and tracheae and cloacae were swabbed for detection of H5N1 influenza viruses. The presence of H5N1 influenza viruses was determined by inoculating samples into four eggs. Results were evaluated by embryo death and HA test.
H5N1 viruses were detectable only in the undiluted samples (less than 10 ELD50). H5N1 influenza viruses were detectable 2 to 5 days postchallenge. +++, H5N1 titers over 106 ELD50; chicken did not survive.
Number of chickens positive for H5N1 influenza viruses/number that survived.
Average log ELD50. H5N1 influenza viruses were detectable from 3 to 10 days postchallenge.
Adoptive transfer of immune lymphocytes.
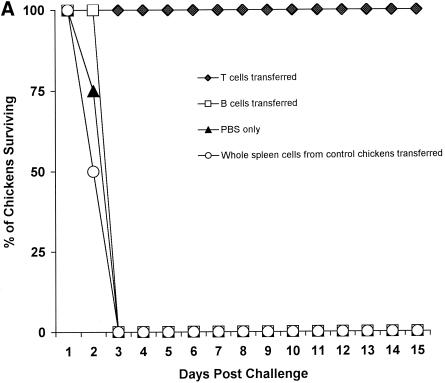
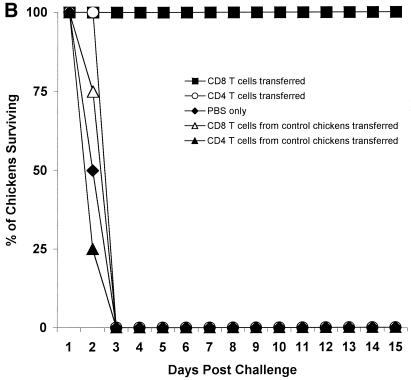
To study the in vivo role of immune lymphocytes in protecting chickens, we infected inbred chickens (B2/B2) with 103 CID50 of A/Chicken/HK/G9/97(H9N2) influenza virus, and 10 days later we collected and purified splenocytes from these animals. Purified mixed lymphocytes (4 × 107 cells per chicken) were injected into the wing veins of naive inbred chickens (B2/B2), and 1 day later the chickens were challenged with 10 ELD50 of an H5N1 influenza virus (Fig. 2A). Chickens receiving both CD4+ and CD8+ T lymphocytes survived, and none of them shed H5N1 influenza virus from their cloacae. H5N1 virus was detected in the tracheae 2 to 4 days after challenge, but the viral titers were less than 10 ELD50 (Table 4). All chickens in each group receiving B lymphocytes alone, whole splenic cells from control chickens, or PBS died 3 days after challenge, and the titers of H5N1 virus in the tracheae and cloacae ranged from 105.5 to 106.5 ELD50 (Table 4).
FIG. 2.
Adoptive transfer of immune splenocytes and subtypes of T lymphocytes. (A) Splenic lymphocytes (4 × 107) collected 10 days postinfection from four inbred chickens (B2/B2) immunized by infection with 103 CID50 of A/Chicken/HK/G9/97(H9N2) influenza virus were adoptively transferred through the wing veins to four naive inbred chickens (B2/B2), and 1 day later chickens were challenged with 10 LD50 of an H5N1 influenza virus. As a control, whole splenic cells from unimmunized chickens were transferred to naive chickens prior to challenge. Results were evaluated for deaths of chickens 15 days postchallenge. (B) Splenic T cells were collected from four inbred chickens (B2/B2) immunized 7 days earlier with 103 CID50 of A/Chicken/HK/G9/97(H9N2) influenza virus. T lymphocytes were depleted of CD4+ or CD8+ subtype of T cells using pan-mouse IgG-coated Dynabeads. Subtypes of T cells 2 × 107 were transferred to four naive inbred chickens (B2/B2) through the wing veins, and 1 day later chickens were challenged with 10 LD50 of an H5N1 influenza virus. As a control, CD4+ or CD8+ T cells from unimmunized chickens were transferred to naive chickens prior to challenge. Results were evaluated for deaths of chickens 15 days postchallenge.
TABLE 4.
Shedding of H5N1 virus by chickens that received immune lymphocytesa
| Days after challenge | Virus titer (log10 ELD50) after:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Splenocyte transfer
|
B-cell transfer
|
CD8+ T-cell transfer
|
CD4+ T-cell transfer
|
Control
|
||||||
| Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | |
| 1 | —b | — | 3.0 | — | — | — | — | — | 3.5 | — |
| 2 | <1c | — | 4.5 | 4.0 | <1d | — | 5.0 | 5.0 | 5.0 | 4.5 |
| 3 | <1c | — | 5.5 | 6.0 | <1d | — | 6.5 | 6.5 | 6.5 | 6.25 |
| 4 | — | — | NAe | NA | <1d | — | NA | NA | NA | NA |
Groups of inbred chickens (four per group) received CD4+ and CD8+ cells, B, cells, CD4+ cells, or CD8+ cells obtained from inbred chickens infected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) influenza virus. One day later, the chickens were challenged with 10 CLD50 of an H5N1 influenza virus. The tracheae and cloacae were swabbed daily, and examined for the presence of H5N1 influenza virus as described for Table 2.
—, no virus isolation.
On day 2, three of the chickens shed virus. On day 3, one chicken shed virus.
On day 2 and 3, four chickens shed virus. On day 4, two chickens shed virus.
NA, not applicable (all dead).
To identify the subtypes of T lymphocytes involved in protecting chickens from H5N1 influenza virus infection, we used Dynabeads to remove specific T-cell subtypes from populations isolated from inbred chickens immunized 7 days earlier. The T-cell populations depleted of particular subsets were transferred to naive inbred chickens, which were challenged 1 day later with 10 CLD50 of an H5N1 influenza virus (Fig. 2B). Chickens receiving a T-cell population (2 × 107 cells) depleted of CD4+ cells survived the lethal challenge of the H5N1 influenza virus. Chickens receiving an immunized T-cell population (2 × 107 cells) depleted of CD8+ cells, CD4+ or CD8+ cells from control chickens, or PBS alone did not survive the lethal challenge: all had died by day 3. The tracheae and cloacae of chickens receiving different subtypes of T cells were swabbed daily until 15 days after the challenge infection (Table 4). The presence of virus in the samples was determined in chicken embryos. One day after challenge, no influenza virus was detected in tracheal or cloacal swabs from chickens receiving either CD4+ or CD8+ T cells. H5N1 influenza virus was detected in the tracheal swabs, but not in the cloacal swabs, of control chickens (titer, 103.5 ELD50). On day 2, H5N1 influenza virus was detected in the tracheae and cloacae of control chickens and of chickens receiving 2 × 107 CD4+ T cells (range, 104.5 to 105 ELD50). On day 3, H5N1 viral titers in the tracheae and cloacae of control chickens and of chickens receiving 2 × 107 CD4+ T cells ranged from 106.25 to 106.5 ELD50, while titers in chickens receiving CD8+ T cells were detected only in the tracheae (titer, less than 10 ELD50) 2 to 4 days after challenge.
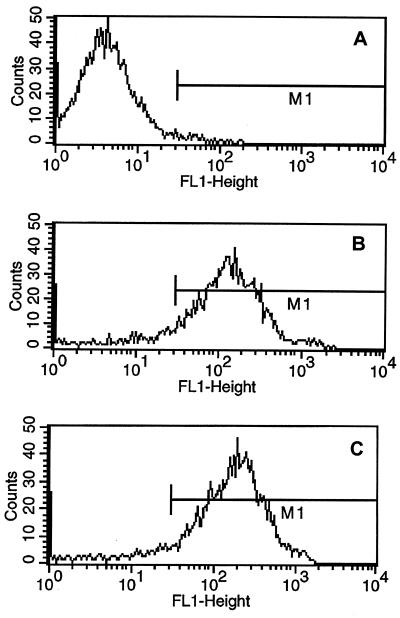
We wanted to determine the purity of depleted populations of T cells by performing flow cytometric analysis with mouse anti-chicken CD4+ or CD8+ monoclonal antibodies. However, the experiments involving the viruses were performed in a BL-3 facility that lacks a flow cytometer. Therefore, we used cells from the spleens of normal chickens to confirm the purity of the depleted populations. (Fig. 3). By using mouse anti-chicken CD4 or CD8, flow cytometric analysis indicated that more than 90% of the cells in the population depleted of CD4+ cells were CD8+; similarly, more than 90% of the cells in the population depleted of CD8+ T cells were CD4+. The percentage of T cells depleted of CD4+ T cells stained with CD4 monoclonal antibody or T cells depleted of CD8+ T cells stained with CD8 monoclonal antibody was less than 1% (data not shown).
FIG. 3.
Identification of the T-lymphocyte phenotypes of splenic T cells depleted of CD4+ or CD8+ T cells by flow cytometry. Splenic T cells depleted of CD4+ or CD8+ T cells were stained with mouse anti-chicken CD4 or CD8 monoclonal antibody and FITC-labeled goat anti-mouse IgG. Control T cells were stained with FITC-labeled goat anti-mouse IgG (A), splenic T cells depleted of CD4+ T cells were stained with mouse anti-chicken CD8 antibody (B), and splenic T cells depleted of CD8+ T cells were stained with mouse anti-chicken CD4 antibody (C).
In vitro CTL response.
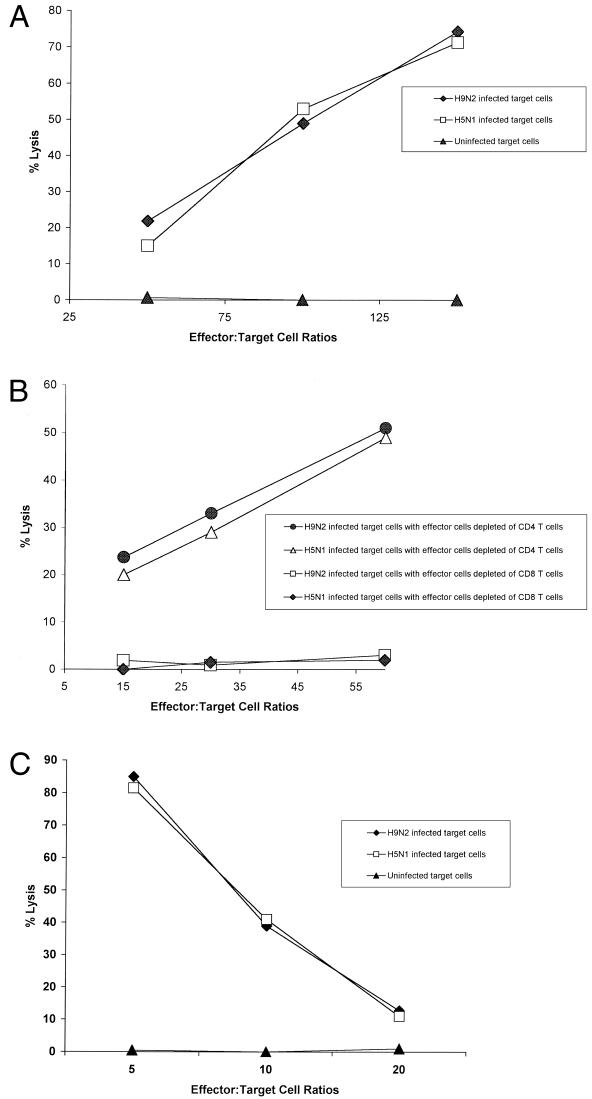
To examine the mechanism of protection mediated by cross-reactive T cells, we evaluated the in vitro CTL activity of whole populations or populations depleted of particular T-cell subsets against H9N2 and H5N1 influenza viruses. The populations of effector cells were collected from inbred chickens infected 7 days earlier with 103 CID50 of A/Chicken/HK/G9/97 (H9N2). Target cells were prepared from lungs of inbred chickens (B2/B2), because inbred chicken lung cells support the replication of H9N2 and H5N1 influenza viruses, as indicated by the presence of cytopathic effects and by the results of HA assays (data not shown). Splenic T cells from inbred chickens infected with an H9N2 influenza virus lysed target cells infected with either an H9N2 or H5N1 influenza virus (Fig. 4A). The splenic T cells did not lyse uninfected target cells. At an effector-to-target cell ratio of 150:1, 74% of target cells infected with an H9N2 influenza virus were lysed; 71% of target cells infected with an H5N1 virus were lysed. The population of effector cells depleted of CD4+ T cells lysed target cells infected with either an H9N2 or H5N1 influenza virus in a similar pattern (Fig. 4B). CTL activity was stronger against target cells infected with an H9N2 influenza virus than against those infected with an H5N1 influenza virus. Depletion of CD8+ T cells abolished CTL activity against target cells infected with either an H9N2 or an H5N1 influenza virus.
FIG. 4.
Cytotoxicity of splenic T cells from chickens previously infected with an H9N2 virus. (A) The effector cells were splenocytes collected from four inbred chickens (B2/B2) infected with A/Chicken/HK/G9/97 (H9N2) influenza virus 7 days earlier. Lung target cells infected with either an H9N2 or H5N1 influenza virus served as the target cells. CTL activity was measured with the nonradioactive CTL assay that detects LDH release. (B) Purified T cells were isolated from inbred chickens (B2/B2) that had been infected 7 days earlier with A/Chicken/HK/G9/97 (H9N2) influenza virus. Before the CTL assays were performed, the populations were depleted of CD4+ or CD8+ T cells using pan anti-mouse IgG-coated Dynabeads and mouse anti-chicken CD4 or CD8 monoclonal antibodies. Inbred lung cells (B2/B2) infected with either an H9N2 or H5N1 influenza virus were used as target cells. (C) Splenic T cells were isolated from inbred chickens (B2/B2) that had been infected 60 days earlier with A/Chicken/HK/G9/97 (H9N2) influenza virus (MOI of 2). Before CTL assays were performed, the splenic T cells were stimulated with inbred splenic cells infected with A/Chicken/HK/G9/97 (H9N2) for 7 days. Inbred chicken lung cells (B2/B2) infected with an H9N2 or H5N1 influenza virus were used as target cells.
Memory CTL responses were also determined using splenic T cells from the inbred chickens (B2/B2) infected with 103 CID50 of A/Chicken/HK/G9/97 (H9N2) 60 days earlier. The splenic T cells were stimulated with inbred splenic cells infected with A/Chicken/HK/G9/97 (H9N2) (MOI of 2) with a 1:5 ratio of effector to stimulator cells for 7 days prior to CTL assay. The stimulated memory CTL recognized target cells infected with either A/Chicken/HK/G9/97 (H9N2) or A/Chicken/HK/728/97 (H5N1) in a dose-dependent manner (Fig. 4C).
Protection of chickens by another H9N2 virus (A/Quail/HK/G1/97). In addition to the isolation of A/Chicken/HK/G9/97 (H9N2) from chickens in the poultry markets in 1997, A/Quail/HK/G1/97(H9N2) was also isolated from one quail. We determined whether this H9N2 virus can protect chickens from a lethal H5N1 influenza virus challenge. Initial studies showed that the dose of A/Quail/HK/G1/97 (H9N2) required to infect chickens is much higher than that of A/Chicken/HK/G9/97 (H9N2) (Table 5): the CID50 of A/Quail/HK/G1/97 (H9N2) was 104.5 EID50, whereas the CID50 for A/CK/HK/G9/97 (H9N2) was 10 EID50. All five chickens infected 15 days earlier with 105 EID50 (10 CID50) of A/Quail/HK/G1/97 (H9N2) influenza virus survived after lethal challenge with an H5N1 virus, but one chicken shed H5N1 virus from its cloaca (titer, 102.5 ELD50). One of the five chickens infected with 104 EID50 (1 CID50) of A/Quail/HK/G1/97(H9N2) influenza virus survived and shed H5N1 virus from its cloaca (titer, 102.25 ELD50) (Table 5). All five chickens infected initially with 102 EID50 (10 CID50) of A/Chicken/HK/G9/97 (H9N2) influenza virus survived and shed H5N1 influenza virus from their cloacae (titer, 102.5 ELD50). Control chickens that were not infected with A/Quail/HK/G1/97 (H9N2) virus had died by day 3 after the challenge. Two to 5 days after the challenge, H5N1 influenza virus was detected in the tracheae of surviving chickens (titer, less than 10 ELD50) (Table 5). Thus, A/Quail/HK/G1/97 (H9N2) virus provided cross-protective immunity; however, the high dose of this virus required to infect chickens makes it unlikely that this virus would infect chickens in the live poultry markets.
TABLE 5.
High doses of A/Quail/HK/G1/97 (H9N2) are required to provide cross-protection in chickensa
| Virus used in priming | Dose (EID50) | No. of survivors | Titer of H5N1 virus isolated from survivors (log10 ELD50) (no. of survivors shedding/no. of survivors)
|
|
|---|---|---|---|---|
| Trachea | Cloaca | |||
| A/Quail/HK/G1/97 | 105 (10 CID50) | 5 | <1 (5/5) | 2.5 (1/5) |
| A/Quail/HK/G1/97 | 104 (1 CID50) | 1 | <1 (1/1) | 2.25 (1/1) |
| A/Chicken/HK/G9/97 | 102 (10 CID50) | 5 | <1 (5/5) | 2.5 (1/5) |
| None | None | 0 | 6.0 | 6.0 |
Chickens (five per group) were primed with an H9N2 (G1) or an H9N2 (G9) influenza virus and challenged 15 days later with 10 CLD50 of an H5N1 influenza virus. Chickens were observed daily for death. Virus detection was done as described for Table 2.
DISCUSSION
Our findings show that prior infection with the A/Chicken/HK/G9/97 (H9N2) virus provided protection from signs of disease caused by the highly pathogenic avian H5N1 influenza virus. In experimentally infected chickens, this H5N1 virus typically causes generalized hemorrhage, paralysis, and rapid death. In our study, the signs of disease were usually mild and included ruffled feathers, sneezing, or nasal discharge. Despite the normal appearance of most chickens challenged with the H5N1 virus, the birds shed virus. Viruses were detected in the tracheae of the most chickens that survived H5N1 infection (Table 2), but the titers were low (less than 10 ELD50). Higher levels of virus were shed in the feces (102 to 103 ELD50) of 34% of the surviving chickens. We therefore postulate that the presence of H9N2 influenza viruses in the chicken population of southeastern China was an important contributing factor in the transmission of H5N1 to humans in 1997. The absence of disease signs in most Hong Kong poultry markets and the shedding of virus by approximately 20% of the birds (29) can now be explained by our findings of cross-protective immunity. This cross-protective cell-mediated immunity permitted the penetration of the highly pathogenic H5N1 influenza viruses into the live poultry market and the creation of conditions that masked the lethal effects of the virus and permitted transmission to incoming poultry and to humans. The H5N1 influenza viruses transmitted to humans appear to have originated in the live poultry markets and were not imported from mainland China, because no serologic evidence of H5N1 infection of chickens has been detected despite daily testing since 1998.
The precursors of the H5N1 viruses, including A/Quail/HK/G1/97 (H9N2)-like (13) and A/Goose/Guandong/1/96-like viruses (H5N1), continue to circulate in China (7). Most chickens in the Hong Kong bird markets are shipped daily from mainland China. Serologic surveillance from April 1999 to March 2000 at the port of entry to Hong Kong, Special Administrative Region, showed that up to 60% of poultry tested had antibodies that reacted with both A/Quail/HK/G1/97 and A/Chicken/HK/G9/97 (13). Although serologic tests were not done in the Hong Kong poultry markets in 1997, it seems likely that chickens were preinfected with A/Chicken/HK/G9/97 (H9N2) influenza virus before they were transferred to the Hong Kong markets.
The absence of disease signs in most chickens in the Hong Kong markets during the H5N1 outbreak was not the result of protective humoral immune responses. Sera collected from chickens infected with A/Chicken/HK/G9/97 (H9N2) influenza virus showed no cross-reactivity with H5N1 influenza virus. Interestingly, A/Quail/HK/G1/97 (H9N2), unlike other H9N2 isolates, did not react well to sera from chickens infected with A/Chicken/HK/G9/97 (H9N2). This finding suggests that these two viruses may have different origins. In a mouse model, antibody to the extracellular domain of the M2 protein could protect mice from challenge with heterologous influenza A virus in a cross-reactive manner (24). The possible role of antibody to M2 protein of A/Chicken/HK/G9 (H9N2) influenza virus in protecting chickens from an H5N1 influenza virus appears to be minimal: we did not detect any cross-reactive neutralization antibody. However, we cannot rule out the possibility that differences in immune responses were due to species differences.
The number of H9N2 immunized chickens that died after being challenged with A/Chicken/HK/728/97 (H5N1) influenza virus increased as time elapsed. The increased number of deaths may be due to either a decrease in cross-reactive cellular immunity in chickens immunized with an H9N2 influenza virus or a delay in recruitment of memory CTLs from the lymphoid organs to respiratory sites. In humans after infection with influenza virus, memory CTL responses sharply decline (21): their half-life is 2 to 3 years. In a mouse model of influenza virus infection (40), splenic memory CTL precursors gradually lose the L-selectin-low phenotype, which is characteristic of recently generated CTL precursors. In mice, memory CTLs usually require 4 to 5 days to localize to the infected respiratory tract (9). Murine memory CTLs persist as long as 2 years after influenza virus infection (8). How long memory CTL responses last in chickens and how long memory CTLs take to localize to the respiratory tract in chickens remain unknown.
Although cell-mediated immunity was an important contributing factor in protecting chickens against lethal H5N1 influenza virus infection in the markets, our results showed that the cross-protective immunity was effective 15 days after immunization, but its effectiveness had diminished by day 30. One possibility is that genetic factors of chickens contributed to protection against lethal H5N1 infection, because some chickens died 10, 30, or 70 days after H9N2 immunization. Genetic factors influence the outcome of chickens infected with Rous sarcoma virus (RSV) and with Marek's disease virus (1, 37, 41). For example, RSV-infected chickens carrying the BF2 or BF21 chromosomal segment encoding the major histocompatibility complex (B) have a strong antitumor response, whereas BF24 confers a weaker response (1). A second possibility is that the chickens were reinfected with H9N2 and that their cell-mediated responses were reinstated, as was demonstrated in Table 2 with reinfection at 60 days. A third possibility is due to differences of CTL epitope hierarchy among outbred chickens. Some chickens have an immunodominant epitope that is shared between A/Chicken/HK/G9/97 (H9N2) and A/Chicken/HK/728/97 (H5N1), but some may respond more prominently to a nonshared epitope.
The adoptive transfer of a T-cell population depleted of CD4+ cells protected chickens against lethal H5N1 influenza virus infection; this result showed that cell-mediated immunity was responsible for the protection. In chickens, adoptive transfer of αβ+ CD8+ T cells protect chicks from infection by infectious bronchitis virus (31). For the mouse model of influenza infection, there are several examples in which adoptive transfer of immune T cells protects mice from lethal challenge of influenza virus (43, 36, 32). The transfer of primary or secondary influenza-immune spleen cells to naive mice results in significant clearance of virus from the lungs and protection from death (44). Influenza virus-specific CTL clones transferred to syngeneic mice protect them from death and mediate recovery from primary viral pneumonia (19).
It is interesting that H5N1 influenza virus was not isolated in chickens receiving CD4+ T cells on day 1 after the challenge, but the chickens had died by day 3. We propose two possible explanations for this result. One possibility is that virus may have been present in the lower respiratory tract, including the lungs, and would not have been detected by tracheal swabbing. The other possibility is that gamma interferon produced by CD4+ T cells suppressed the replication of the H5N1 influenza virus on day 1, but the quantity of gamma interferon was too low to further suppress replication on days 2 and 3. Influenza viruses are sensitive to the antiviral property of interferon and are efficient inducers of interferon expression (14, 23, 27). Whether H5N1 influenza viruses are also sensitive to interferon-induced antiviral action is not known. Our observation indicates that CD4+ T cells contribute to protection against an H5N1 influenza virus, although they are not a central element in the elimination of H5N1 influenza virus in chickens. The previous study supported our observation. In a study of B-cell-deficient μMT mice infected with the HKx31 influenza virus, CD4+ T-cell responses were inefficient for clearing virus in the absence of B cells and CD8+ T cells (38).
It is unclear which internal proteins of the H9N2 influenza virus were involved in inducing an immune response that protected chickens from the lethal H5N1 influenza virus. PB1 and PB2 are the most likely candidates to have elicited a cross-protective immune response, because the PB1 and PB2 genes are highly (98 and 97%, respectively) homologous to those of the H5N1 virus. The second most likely candidates are the M and NS proteins, which are encoded by genes that are 96 and 94%, respectively, homologous to those of the H5N1 influenza virus. The least likely candidate is the NP protein; the NP gene is only 90% homologous to that of the H5N1 influenza virus.
In the mouse model of influenza infection, CTL responses to the polymerases (PA, PB1, and PB2) of influenza virus are, in some cases, equal to or better than CTL responses to NS1 or NP, which are synthesized in far greater quantities (4). O'Neill et al. (25) showed that mice infected with A/Quail/HK/G1/97 (H9N2) are protected from infection with an H5N1 influenza virus. They also showed that internal proteins other than NP might play a role in protecting mice from lethal infection with an H5N1 influenza virus.
A/Chicken/HK/G9/97 (H9N2), but not A/Quail/HK/G1/97 (H9N2), appears to be the main influenza virus that elicited cross-reactive cellular immunity in chickens of the Hong Kong poultry markets in 1997, because only one isolate of A/Quail/HK/G1/97-like virus was obtained from a quail and no isolates of this genotype were obtained from chickens (29). The dose of A/Quail/HK/G1/97 (H9N2) required to infect chickens is greater than that of A/Chicken/HK/G9/97. The fact that A/Quail/HK/G1/97 does not infect chickens as well as A/Chicken/HK/G9/97 may be the result of differences in host susceptibility. It seems that chickens can be a barrier to the crossing over of avian influenza viruses from one host species to another. The absence of A/Quail/HK/G1/97 and A/Chicken/HK/G9/97 reassortants supports this idea.
In summary, we found cross-reactive cellular immune responses induced by an H9N2 influenza virus protects chickens from the lethal H5N1 virus and that CD8+ T cells are the main effector cells of protective immunity. Thus, H9N2 influenza virus-primed CD8+ T cells probably protected chickens from lethal H5N1 virus infection in the Hong Kong markets in 1997.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI29680 and AI95357 and Cancer Center Support (CORE) grant CA-21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank Scott Krauss and Lijuan Zhang for excellent technical support, Nadine Finley and Alice Herren for manuscript preparation, Julia Cay Jones for editorial assistance, and Janice Riberdy for critical review of the manuscript. We also thank Kennedy Shortridge and Malik Peiris, University of Hong Kong, for providing the influenza virus isolates used in this study.
REFERENCES
- 1.Aeed P A, Briles W E, Zsigray R M, Collins W M. Influence of different B-complex recombinants on the outcome of Rous sarcomas in chickens. Anim Genet. 1993;24:177–181. doi: 10.1111/j.1365-2052.1993.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennink J R, Yewdell J W, Gerhard W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature. 1982;296:75–76. doi: 10.1038/296075a0. [DOI] [PubMed] [Google Scholar]
- 3.Bennink J R, Yewdell J W, Smith G L, Moss B. Recognition of cloned influenza virus hemagglutinin gene products by cytotoxic T lymphocytes. J Virol. 1986;57:786–791. doi: 10.1128/jvi.57.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennink J R, Yewdell J W, Smith G L, Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987;61:1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennink J R, Yewdell J W. Murine cytotoxic T lymphocyte recognition of individual influenza virus proteins. High frequency of nonresponder MHC class I alleles. J Exp Med. 1988;168:1935–1939. doi: 10.1084/jem.168.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braciale T J, Braciale V L, Henkel T J, Sambrook J, Gething M J. Cytotoxic T lymphocyte recognition of the influenza hemagglutinin gene product expressed by DNA-mediated gene transfer. J Exp Med. 1984;159:341–354. doi: 10.1084/jem.159.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauthen A N, Swayne D E, Schultz-Cherry S, Perdue M L, Suarez D L. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J Virol. 2000;74:6592–6599. doi: 10.1128/jvi.74.14.6592-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 9.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 10.Gianfrani C, Oseroff C, Sidney J, Chesnut R W, Alessandro S. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum Immunol. 2000;61:438–452. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 11.Gotch F, McMichael A, Smith G, Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987;165:408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Shortridge K F, Krauss S, Chin P S, Dyrting K C, Ellis T M, Webster R G, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill D A, Baron S, Perkins J C, Worthington M, Van Kirk J E, Mills J, Kapikian A Z, Chanock R M. Evaluation of an interferon inducer in viral respiratory disease. JAMA. 1972;219:1179–1184. [PubMed] [Google Scholar]
- 15.Jameson J, Cruz J, Ennis F A. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol. 1998;72:8682–8689. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodihalli S, Kobasa D L, Webster R G. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000;18:2592–2599. doi: 10.1016/s0264-410x(99)00485-5. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano K, Scott M, Young J F, Ennis F A. HA2 subunit of influenza A H1 and H2 subtype viruses induces a protective cross-reactive cytotoxic T lymphocyte response. J Immunol. 1988;140:1264–1268. [PubMed] [Google Scholar]
- 18.Kuwano K, Tamura M, Ennis F A. Cross-reactive protection against influenza A virus infections by an NS1-specific CTL clone. Virology. 1990;178:174–179. doi: 10.1016/0042-6822(90)90391-4. [DOI] [PubMed] [Google Scholar]
- 19.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y L, Askonas B A. Biological properties of an influenza A virus-specific killer T cell clone inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMichael A J, Gotch F M, Dongworth D W, Clark A, Potter C W. Declining T-cell immunity to influenza, 1977–82. Lancet. 1983;ii2:762–764. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- 22.McMichael A J, Gotch F M, Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986;164:1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy B R, Baron S, Chalhub E G, Uhlendorf C P, Chanock R M. Temperature-sensitive mutants of influenza virus. IV. Induction of interferon in the nasopharynx by wild-type and a temperature-sensitive recombinant virus. J Infect Dis. 1973;128:488–493. doi: 10.1093/infdis/128.4.488. [DOI] [PubMed] [Google Scholar]
- 24.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou W M, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill E, Krauss S L, Riberdy J M, Webster R G, Woodland D L. Heterologous protection against lethal A/HongKong/156/97 (H5N1) infection in C57BL/6 mice. J Gen Virol. 2000;81:2689–2696. doi: 10.1099/0022-1317-81-11-2689. [DOI] [PubMed] [Google Scholar]
- 26.Reay P A, Jones I M, Gotch F M, McMichael A J, Brownlee G G. Recognition of the PB1, neuraminidase, and matrix proteins of influenza virus A/NT/60/68 by cytotoxic T lymphocytes. Virology. 1989;170:477–485. doi: 10.1016/0042-6822(89)90439-x. [DOI] [PubMed] [Google Scholar]
- 27.Richman D D, Murphy B R, Baron S, Uhlendorf C. Three strains of influenza A virus (H3N2): interferon sensitivity in vitro and interferon production in volunteers. J Clin Microbiol. 1976;3:223–226. doi: 10.1128/jcm.3.3.223-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 29.Shortridge K F. Poultry and the influenza H5N1 outbreaks in Hong Kong, 1997: abridged chronology and virus isolation. Vaccine. 1999;17(Suppl. 1):S26–S29. doi: 10.1016/s0264-410x(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 30.Seo S H, Collisson E W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo S H, Pei J, Briles W E, Dzielawat J, Collisson E W. Adoptive transfer of infectious bronchitis virus primed αβ T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson P G, Hawke S, Bangham C R. Protection against influenza virus encephalitis by adoptive lymphocyte transfer. Virology. 1997;232:158–166. doi: 10.1006/viro.1997.8535. [DOI] [PubMed] [Google Scholar]
- 33.Suaraz D L, Perdue M L, Cox N, Rowe R, Bender C, Huang J, Swayne D E. Comparison of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 35.Taylor J, Weinberg R, Kawaoka Y, Webster R G, Paoletti E. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine. 1988;6:504–508. doi: 10.1016/0264-410x(88)90101-6. [DOI] [PubMed] [Google Scholar]
- 36.Taylor P M, Askonas B A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor R L, Jr, Clare R A, Ward P H, Briles R W, Briles W E. Anti-Rous sarcoma response of major histocompatibility (B) complex haplotypes B23, B24, and B30. Anim Genet. 1988;19:277–284. doi: 10.1111/j.1365-2052.1988.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 38.Topham D J, Doherty P C. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsend A R, McMichael A J, Carter N P, Huddleston J A, Brownlee G G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 40.Tripp R A, Hou S, Doherty P C. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 41.Vallejo R L, Pharr G T, Liu H C, Cheng H H, Witter R L, Bacon L D. Non-association between Rfp-Y major histocompatibility complex-like genes and susceptibility to Marek's disease virus-induced tumors in 6(3) × 7(2) F2 intercross chickens. Anim Genet. 1997;28:331–337. doi: 10.1111/j.1365-2052.1997.00178.x. [DOI] [PubMed] [Google Scholar]
- 42.Webster R G, Kawaoka Y, Taylor J, Weinberg R, Paoletti E. Efficacy of nucleoprotein and haemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine. 1991;9:303–308. doi: 10.1016/0264-410x(91)90055-b. [DOI] [PubMed] [Google Scholar]
- 43.Wells M A, Ennis F A, Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981;126:1042–1046. [PubMed] [Google Scholar]
- 44.Yap K L, Ada G L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immnol. 1978;7:389–397. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 45.Yewdell J W, Bennink J R, Smith G L, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinkernagel R M, Doherty P C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T cell restriction specificity, function and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]