Abstract
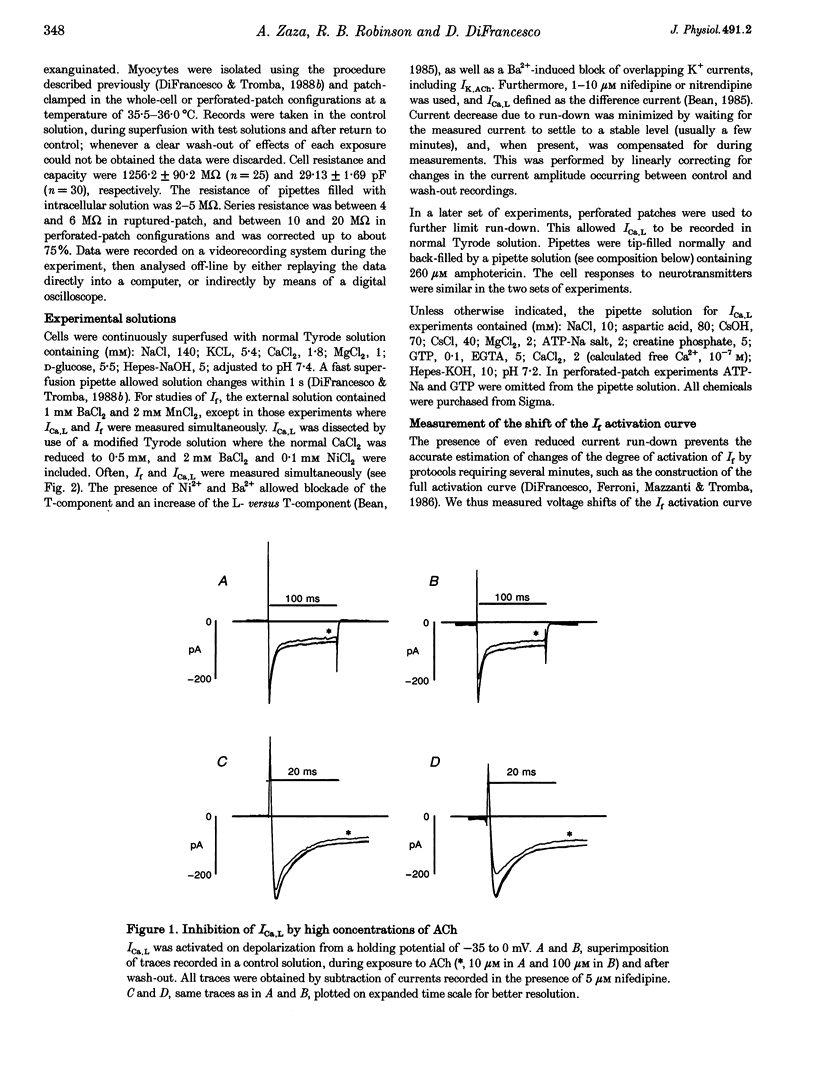
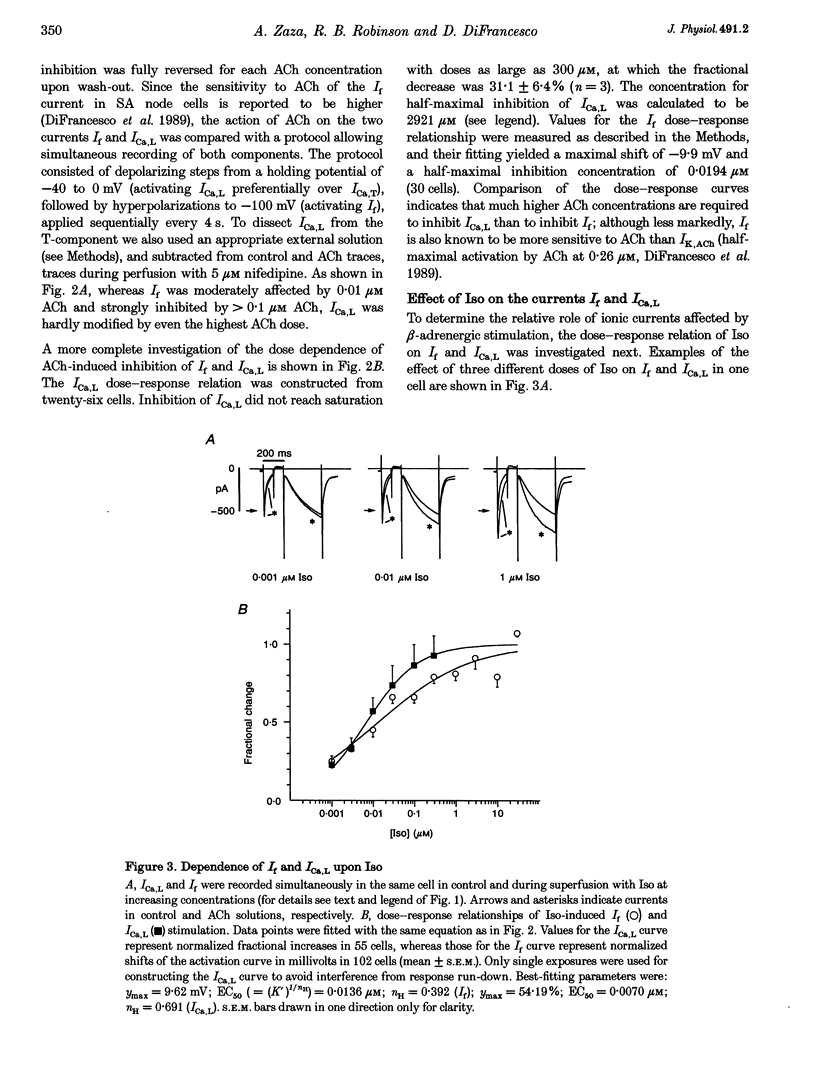
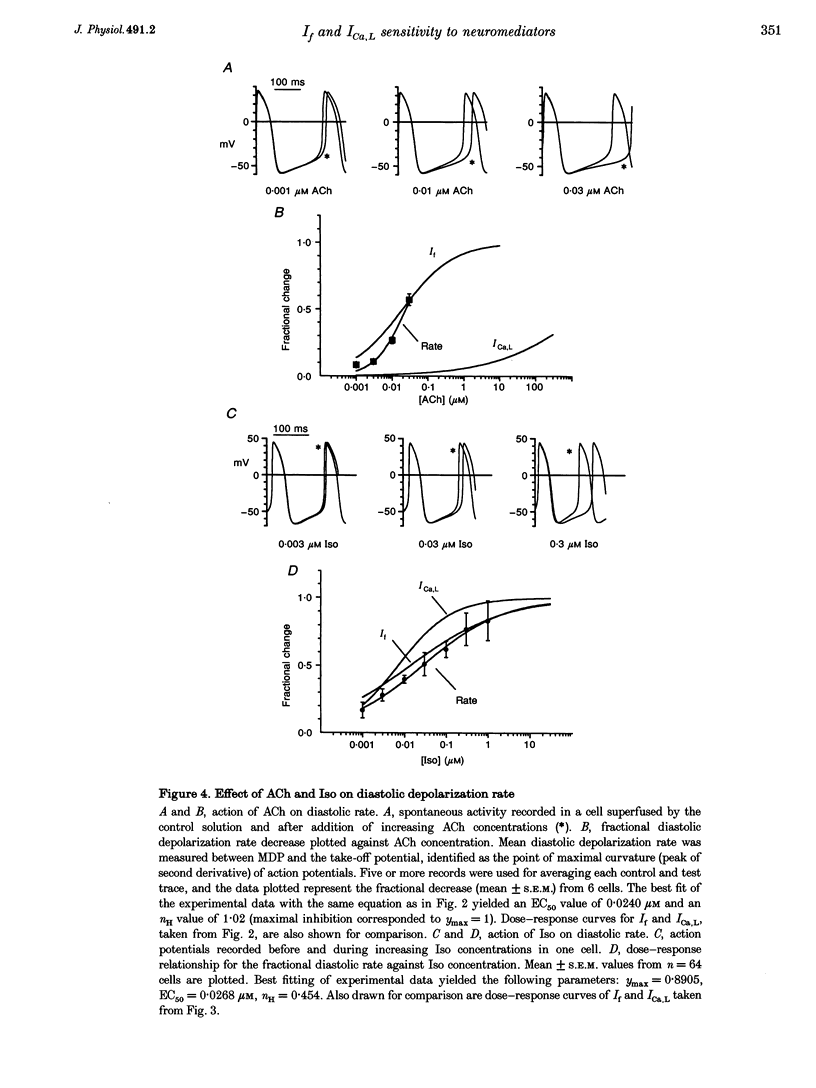
1. The dose dependence of the cholinergic agonist acetylcholine (ACh) and the beta-adrenergic agonist isoprenaline (Iso) were determined for the hyperpolarization-activated current (If) and the L-type Ca2+ current (ICa,L) in single cells isolated from the rabbit sino-atrial (SA) node. 2. ACh inhibited If by a negative shift of its activation curve with a maximal effect of -9.9 mV; half-maximal effect was produced by 0.019 microM ACh. High ACh concentrations were required to inhibit ICa,L only partially (31% inhibition at 300 microM). 3. In contrast, If and ICa,L responded to Iso over a similar dose range, with concentrations for half-maximal enhancement of 0.0136 and 0.0070 microM, respectively. 4. The effects on spontaneous activity of ACh (range 0.001-0.03 microM) and Iso (range 0.001-1 microM) were investigated. ACh decreased the slope of diastolic depolarization at concentrations similar to those inhibiting If (> 50% at 0.03 microM). Iso enhanced diastolic depolarization at concentrations similar to those affecting both If and ICa,L (half-maximal effect at 0.027 microM). 5. In a ramp-clamp protocol simulating diastolic depolarization, the threshold for activation of inward nifedipine-sensitive current was -41.22 +/- 0.68 mV. Although enhancing ICa,L, Iso did not affect this threshold. 6. Half-maximal ACh concentrations for inhibition of automaticity and If are similar and are lower than the threshold concentrations for modulation of ICa,L; this argues against a role of ICa,L in direct muscarinic modulation of pacemaking. In contrast, modulation of If, ICa,L and automaticity occur at similar Iso concentrations. The difference between maximum diastolic potential (-61.95 +/- 0.93 mV) and the threshold for Iso-stimulated ICa,L (-39.54 +/- 1.03 mV) suggests that this current plays a role only at later stages of diastolic depolarization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H., Roberts A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J Physiol. 1988 Oct;404:613–635. doi: 10.1113/jphysiol.1988.sp017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ducouret P., Robinson R. B. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989 Feb 3;243(4891):669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Mangoni M. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J Physiol. 1994 Feb 1;474(3):473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Ono K., Noma A. A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. J Physiol. 1995 Feb 15;483(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi Y., Lu L. L., Morikawa J., Yoshimura M. Angiotensin II inhibition of L-type Ca2+ current in sinoatrial node cells of rabbits. Am J Physiol. 1995 Mar;268(3 Pt 2):H1053–H1060. doi: 10.1152/ajpheart.1995.268.3.H1053. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Ito H., Ono K., Noma A. Background conductance attributable to spontaneous opening of muscarinic K+ channels in rabbit sino-atrial node cells. J Physiol. 1994 Apr 1;476(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- Noble D., Denyer J. C., Brown H. F., DiFrancesco D. Reciprocal role of the inward currents ib, Na and i(f) in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc Biol Sci. 1992 Dec 22;250(1329):199–207. doi: 10.1098/rspb.1992.0150. [DOI] [PubMed] [Google Scholar]
- Petit-Jacques J., Bois P., Bescond J., Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993 Apr;423(1-2):21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- Verheijck E. E., van Ginneken A. C., Bourier J., Bouman L. N. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res. 1995 Apr;76(4):607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Regulation of cardiac pacemaker current If in excised membranes from sinoatrial node cells. Am J Physiol. 1990 Jun;258(6 Pt 2):H1947–H1951. doi: 10.1152/ajpheart.1990.258.6.H1947. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Chang F., Cohen I. S. Phosphatase inhibition by calyculin A increases i(f) in canine Purkinje fibers and myocytes. Pflugers Arch. 1993 Mar;422(6):614–616. doi: 10.1007/BF00374010. [DOI] [PubMed] [Google Scholar]


