Abstract
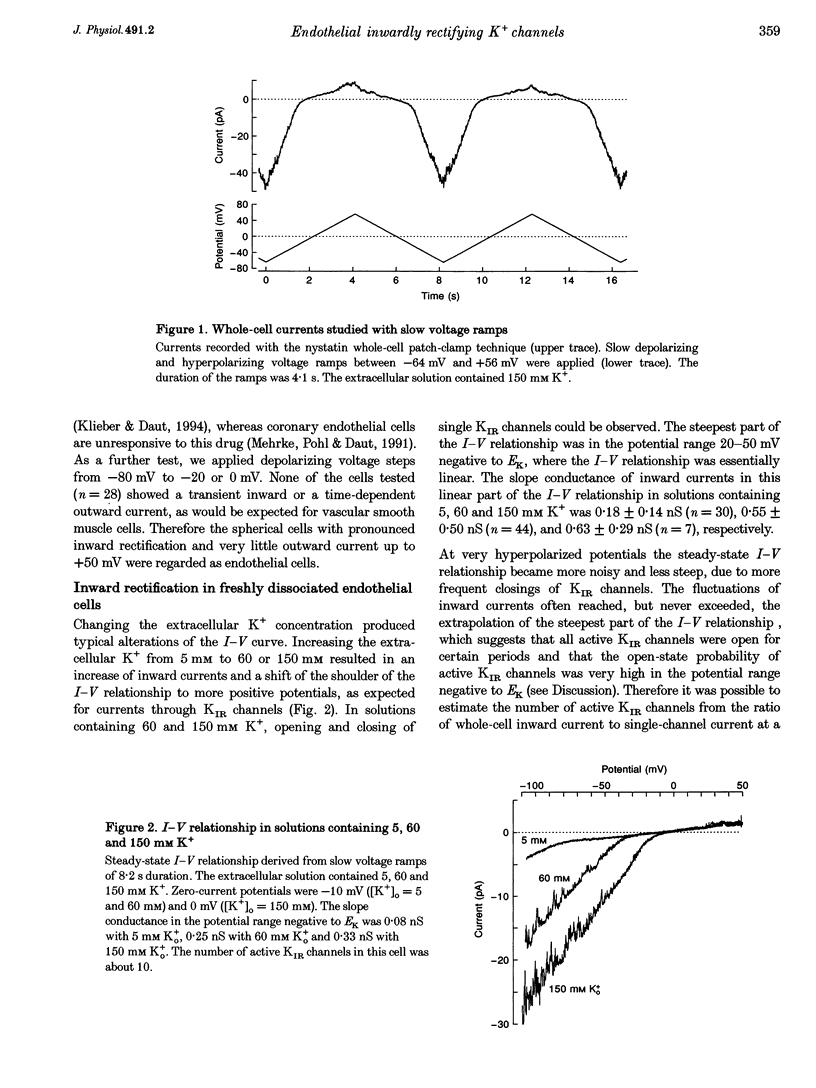
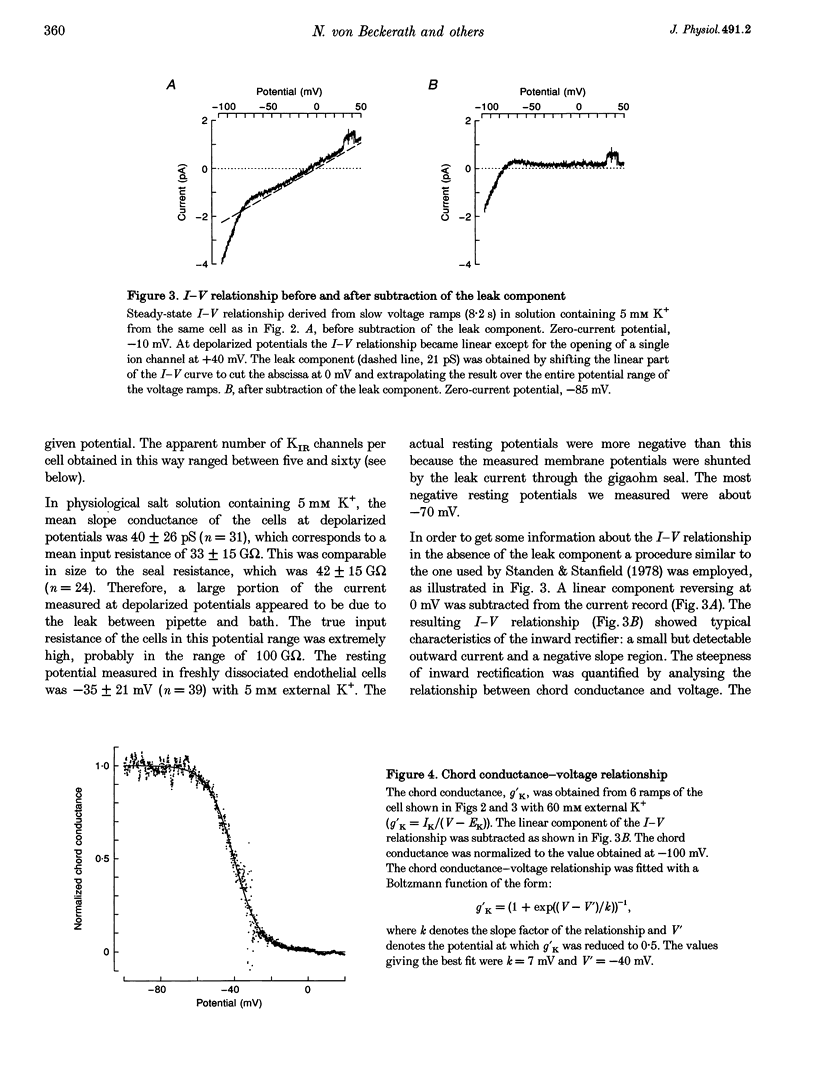
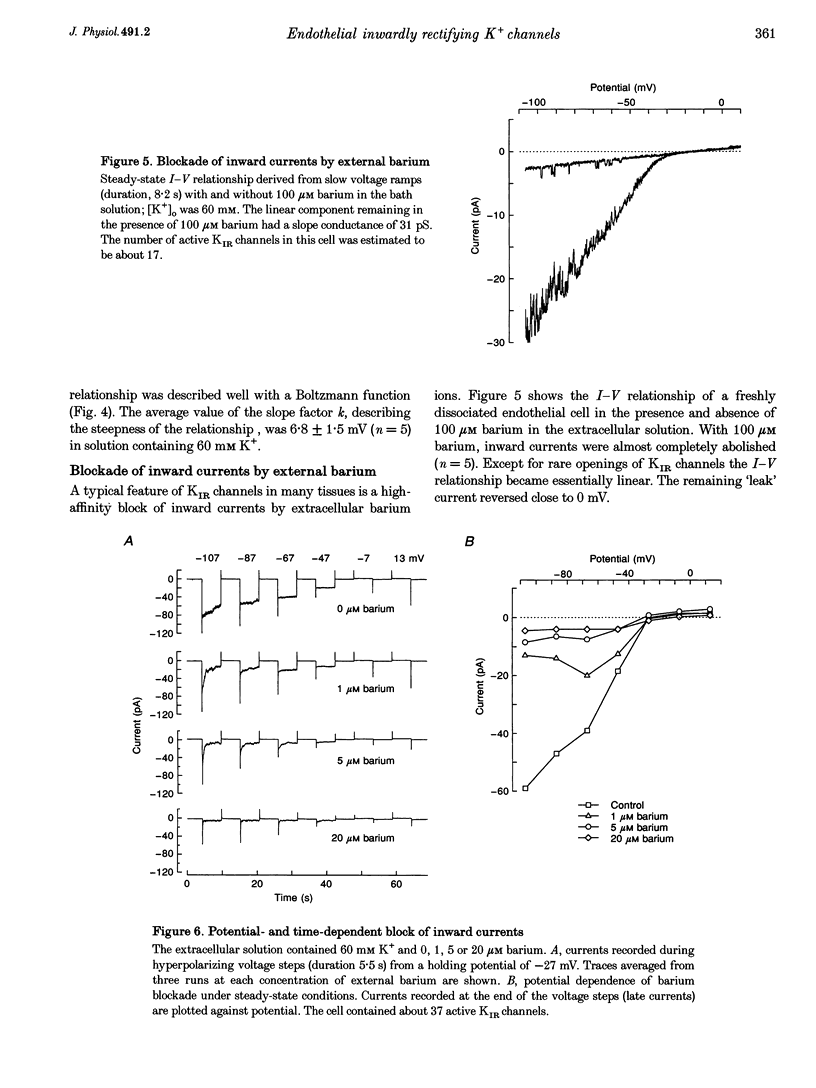
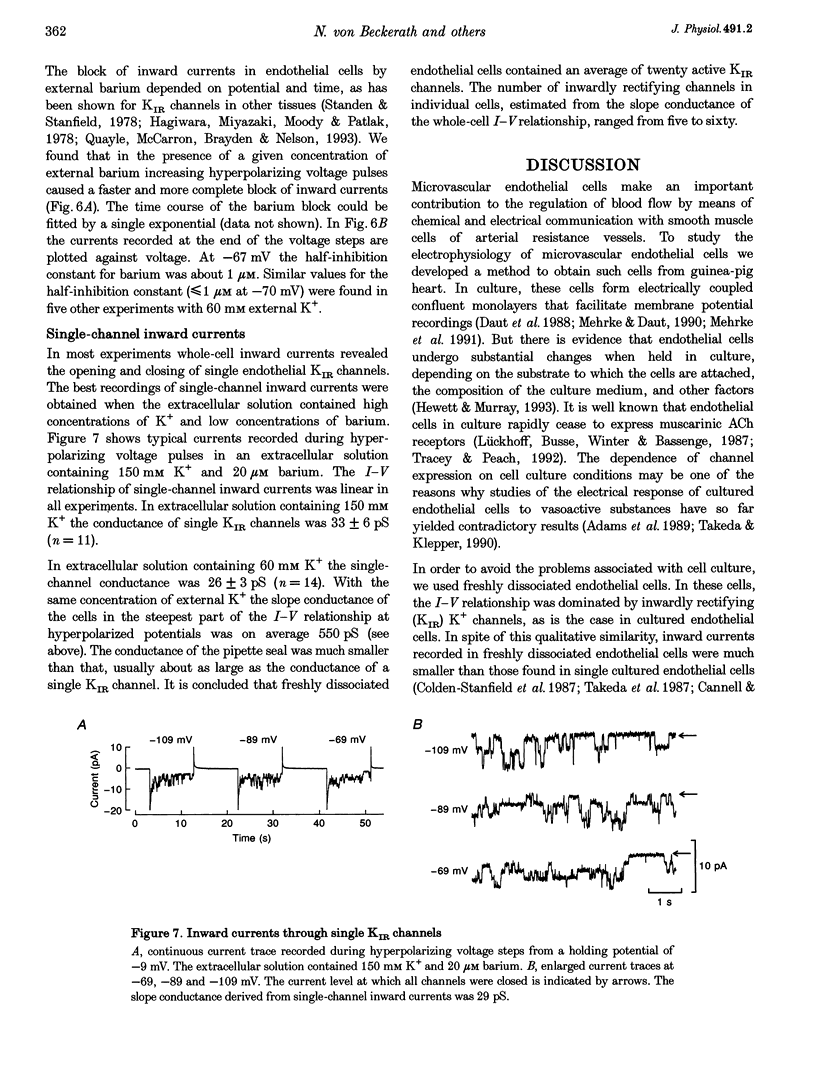
1. Inwardly rectifying K+ (IK(IR)) currents of freshly dissociated coronary endothelial cells from guinea-pig heart were investigated with the perforated-patch technique. 2. The whole-cell current-voltage relationship of endothelial cells showed strong inward rectification. Increasing the extracellular K+ resulted in an increase of inward currents. The slope conductance of the cells in the potential range negative to the calculated potassium equilibrium potential (EK) with 5, 60 and 150 mM external potassium was 0.18 +/- 0.14, 0.55 +/- 0.50 and 0.63 +/- 0.29 nS (mean +/- S.D.), respectively. 3. To quantify the steepness of inward rectification, the voltage dependence of the chord conductance of the cells was fitted with a Boltzmann function. The slope factor k describing the steepness of the relationship was 6.8 +/- 1.5 mV. 4. Extracellular barium induced a potential- and time-dependent block of inward currents through endothelial KIR channels. Half-maximum inhibition of IK(IR) currents was achieved with < or = 1 microM barium at a membrane potential of -70 mV in a solution containing 60 mM K+. 5. Whole-cell inward currents revealed the opening and closing of single KIR channels. The single-channel conductance was 26 +/- 3 pS with 60 mM external K+ and 33 +/- 6 pS with 150 mM external K+. 6. Our results suggest that the electrical properties of freshly dissociated endothelial cells are to a large extent determined by five to sixty active strong inwardly rectifying K+ (KIR) channels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Bond C. T., Pessia M., Xia X. M., Lagrutta A., Kavanaugh M. P., Adelman J. P. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2(3):183–191. [PubMed] [Google Scholar]
- Bény J. L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am J Physiol. 1990 Mar;258(3 Pt 2):H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994 Apr;266(4 Pt 2):H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Standen N. B., Nelson M. T. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994 Feb;5(2):154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Bond C., Glowatzki E., König C., Adelman J. P., Zenner H. P., Ruppersberg J. P. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 1994 Dec 19;356(2-3):199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett P. W., Murray J. C. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell Dev Biol Anim. 1993 Nov;29A(11):823–830. doi: 10.1007/BF02631356. [DOI] [PubMed] [Google Scholar]
- Hoyer J., Popp R., Meyer J., Galla H. J., Gögelein H. Angiotensin II, vasopressin and GTP[gamma-S] inhibit inward-rectifying K+ channels in porcine cerebral capillary endothelial cells. J Membr Biol. 1991 Jul;123(1):55–62. doi: 10.1007/BF01993963. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Mitsuiye T., Noma A., Takano M. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol. 1989 Dec;419:297–320. doi: 10.1113/jphysiol.1989.sp017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieber H. G., Daut J. A glibenclamide sensitive potassium conductance in terminal arterioles isolated from guinea pig heart. Cardiovasc Res. 1994 Jun;28(6):823–830. doi: 10.1093/cvr/28.6.823. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Little T. L., Xia J., Duling B. R. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995 Mar;76(3):498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R., Winter I., Bassenge E. Characterization of vascular relaxant factor released from cultured endothelial cells. Hypertension. 1987 Mar;9(3):295–303. doi: 10.1161/01.hyp.9.3.295. [DOI] [PubMed] [Google Scholar]
- Makhina E. N., Kelly A. J., Lopatin A. N., Mercer R. W., Nichols C. G. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994 Aug 12;269(32):20468–20474. [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Schwarz G., Droogmans G. Modulation by histamine of an inwardly rectifying potassium channel in human endothelial cells. J Physiol. 1993 Dec;472:359–371. doi: 10.1113/jphysiol.1993.sp019951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Bundgaard M. ATP-dependent closure and reactivation of inward rectifier K+ channels in endothelial cells. Circ Res. 1993 Sep;73(3):492–495. doi: 10.1161/01.res.73.3.492. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., McCarron J. G., Brayden J. E., Nelson M. T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993 Nov;265(5 Pt 1):C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Rakusan K., Moravec J., Hatt P. Y. Regional capillary supply in the normal and hypertrophied rat heart. Microvasc Res. 1980 Nov;20(3):319–326. doi: 10.1016/0026-2862(80)90032-1. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. R., DeCoursey T. E. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+. J Gen Physiol. 1990 Jul;96(1):109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Klepper M. Voltage-dependent and agonist-activated ionic currents in vascular endothelial cells: a review. Blood Vessels. 1990;27(2-5):169–183. doi: 10.1159/000158808. [DOI] [PubMed] [Google Scholar]
- Takeda K., Schini V., Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflugers Arch. 1987 Nov;410(4-5):385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Peach M. J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ Res. 1992 Feb;70(2):234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J Physiol. 1993 Nov;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]


