Abstract
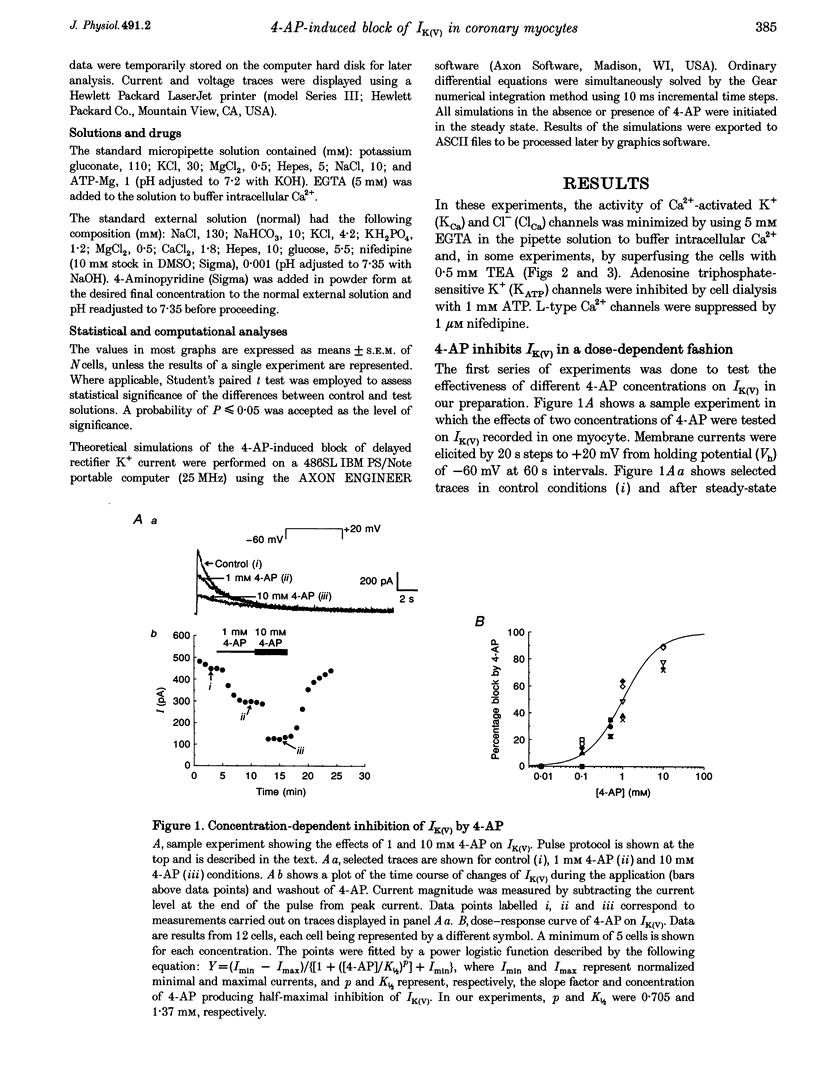
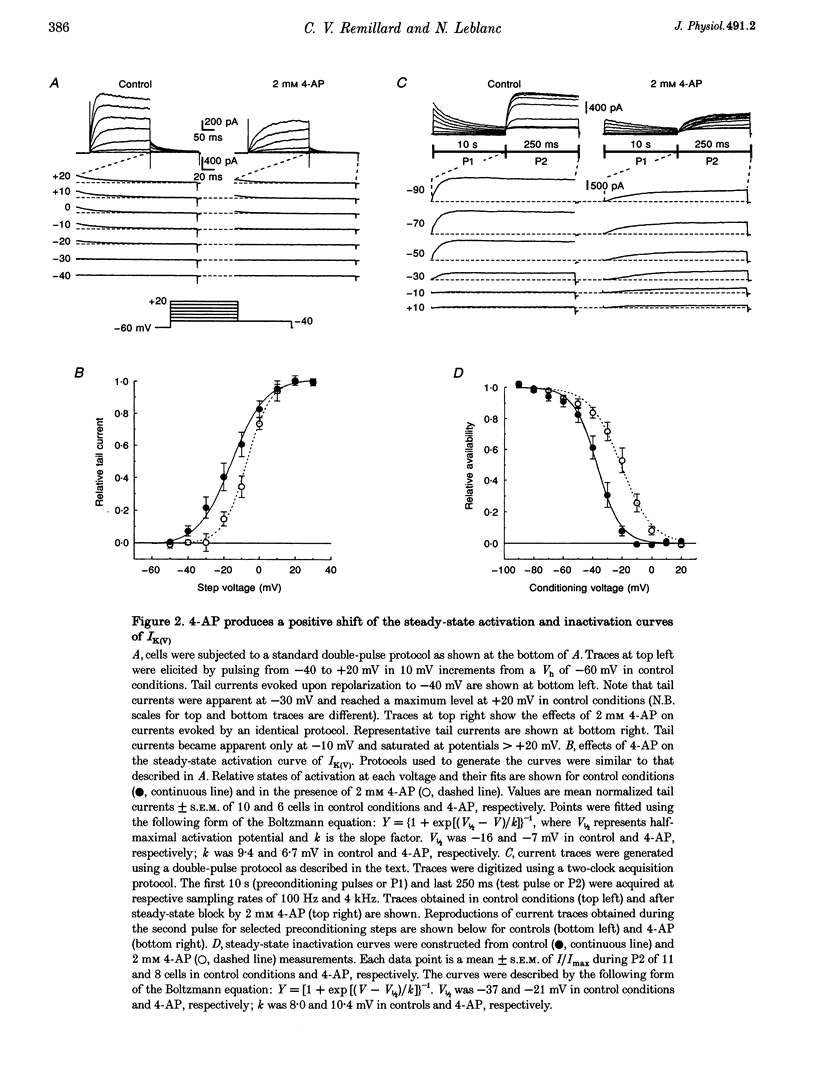
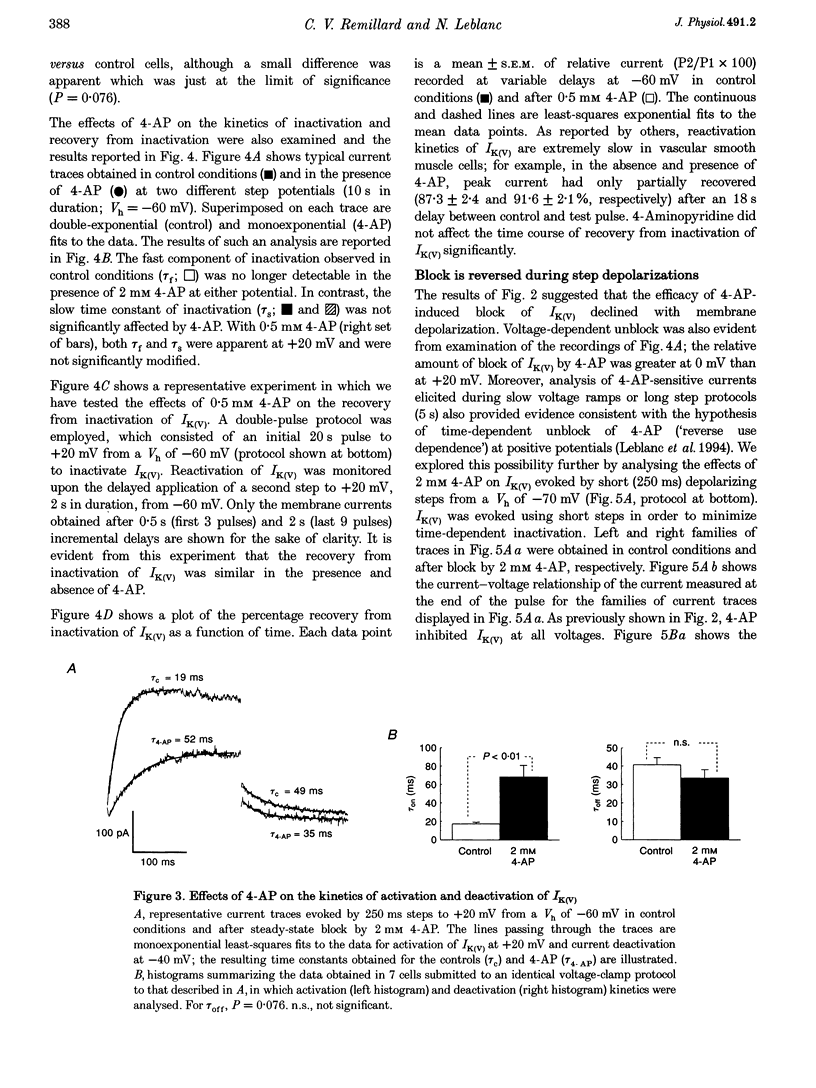
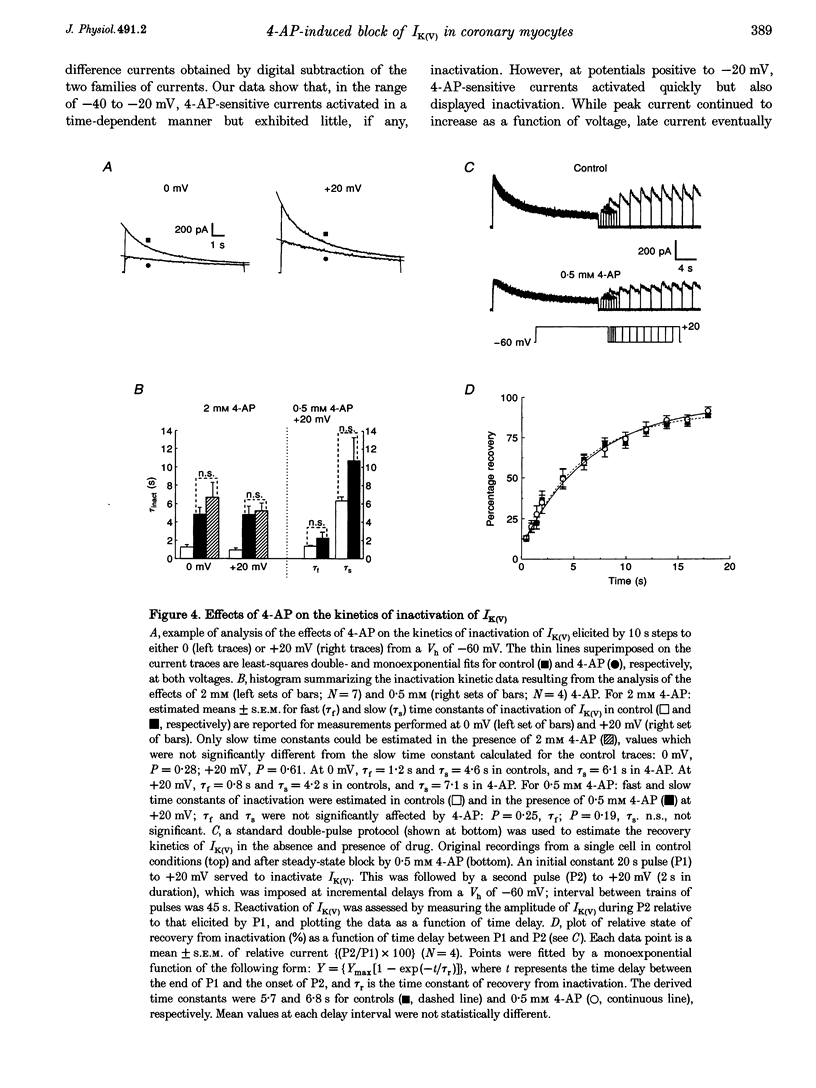
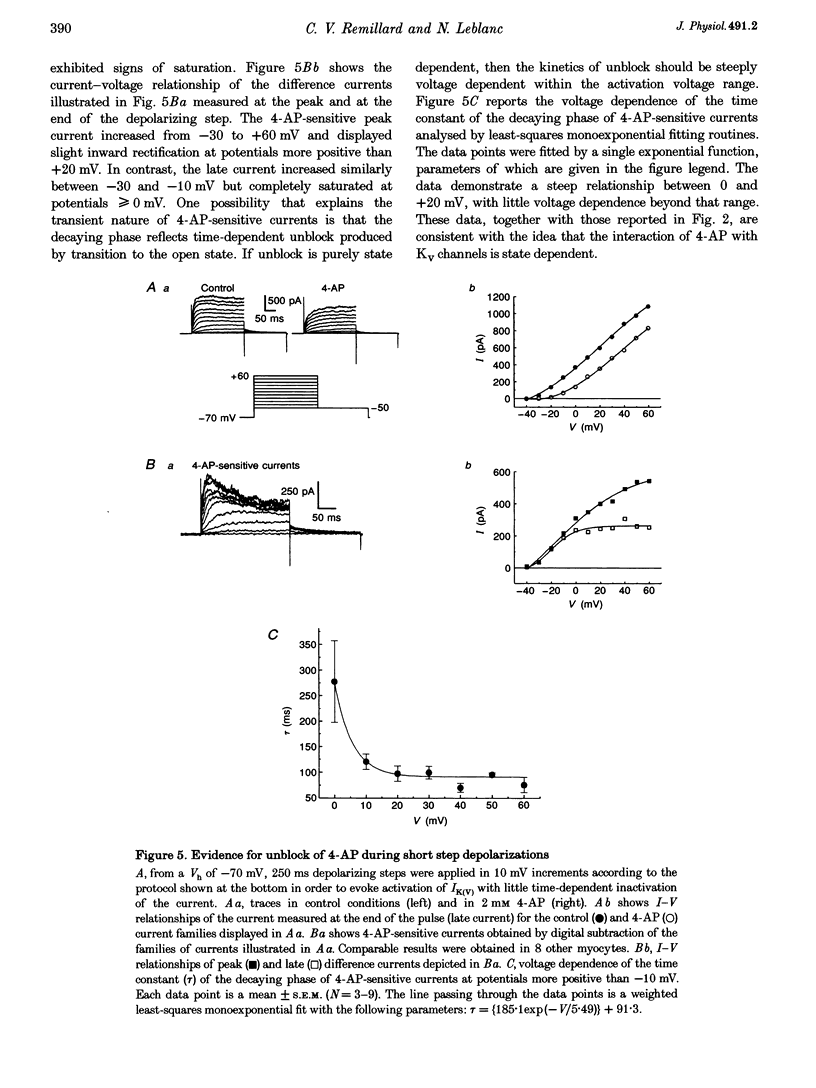
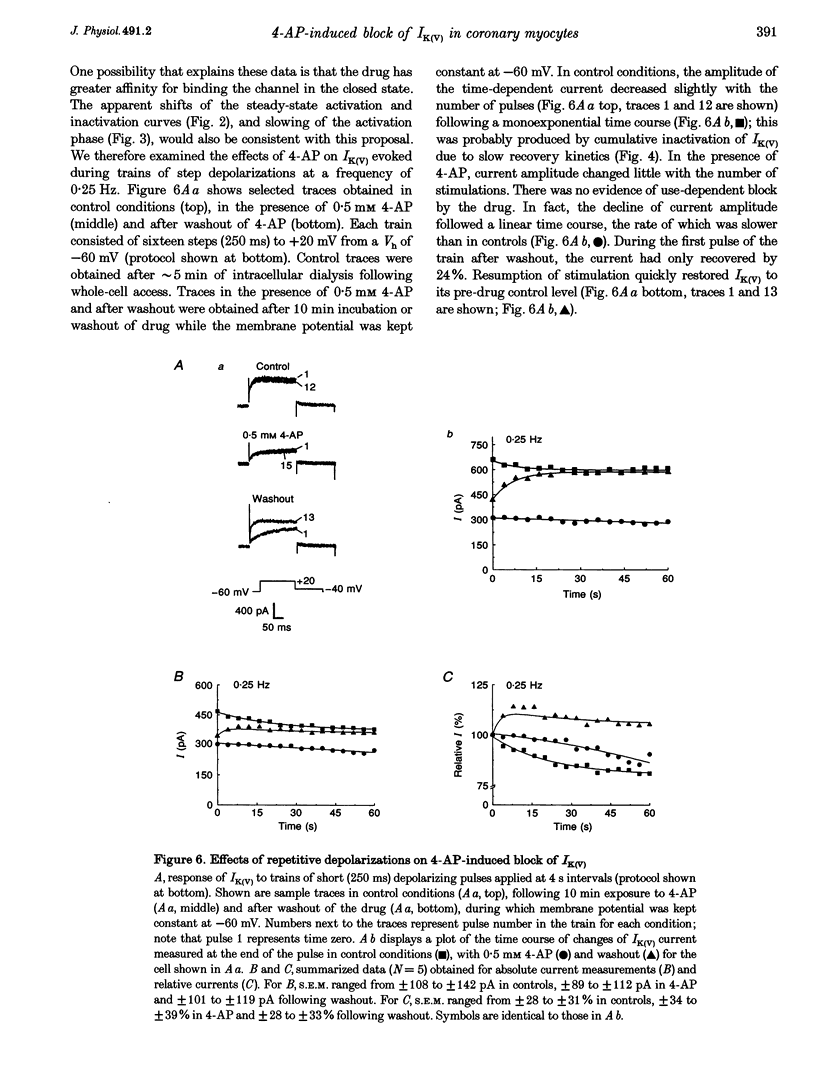
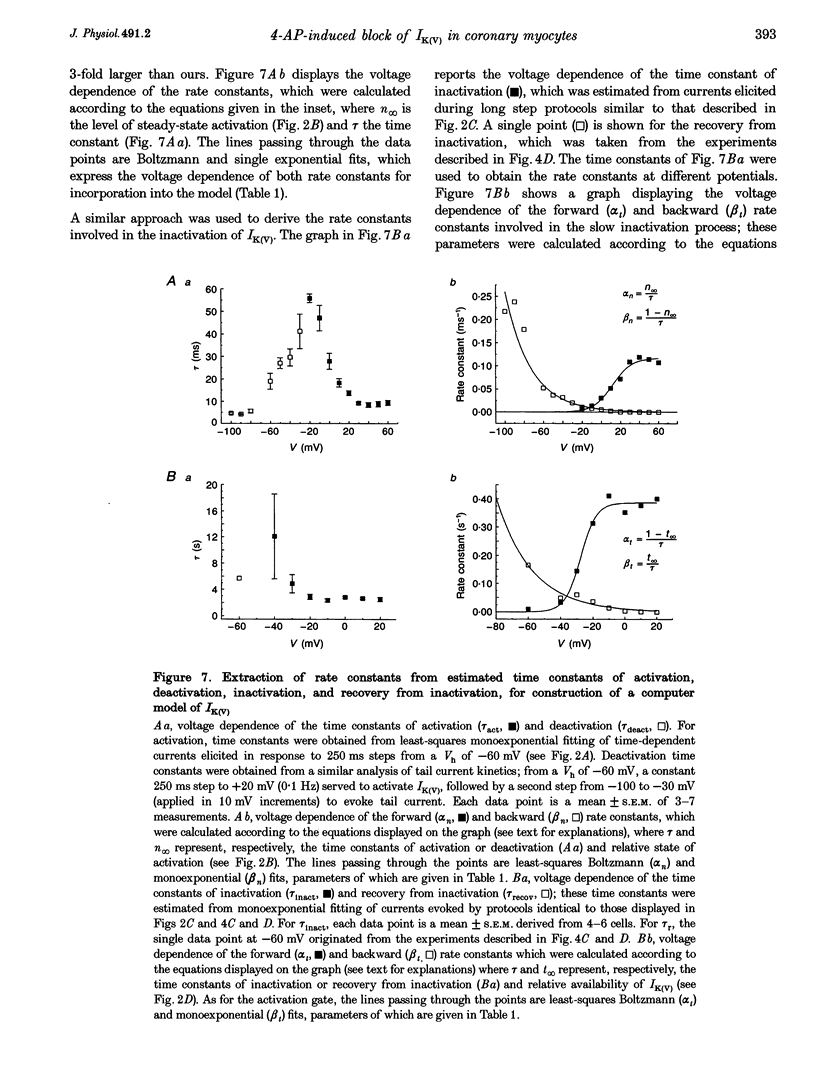
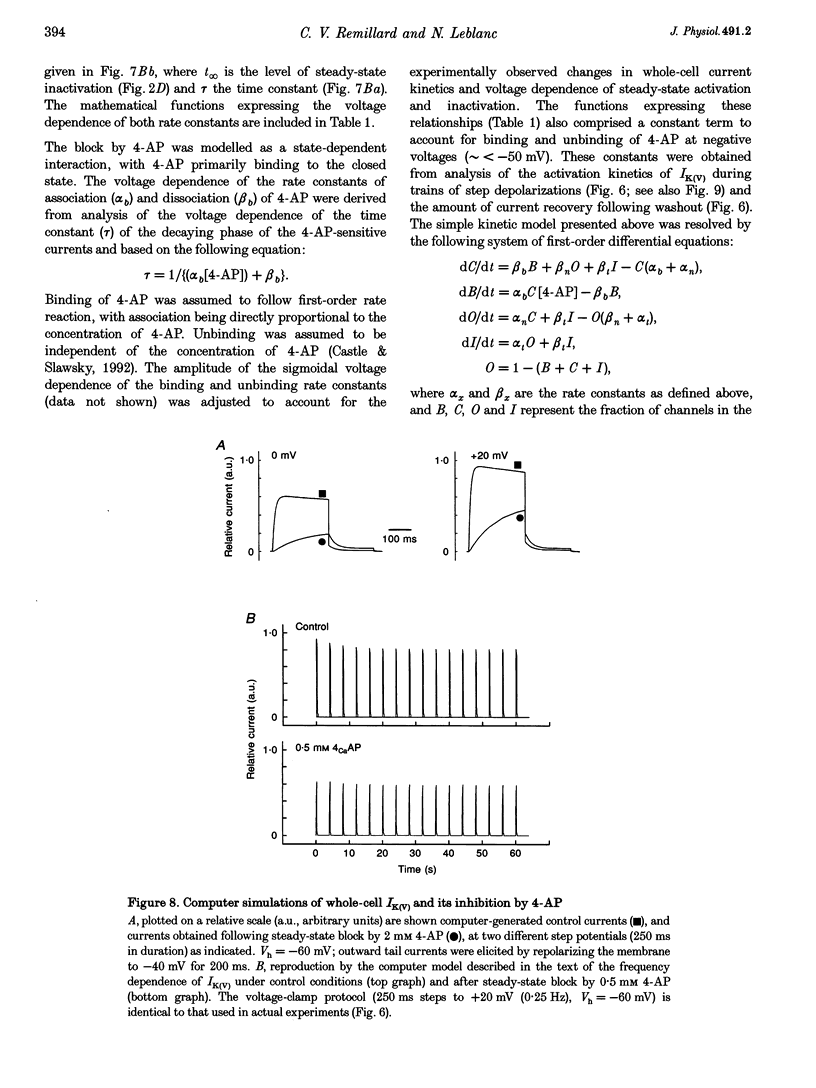
1. The mechanisms involved in the 4-aminopyridine (4-AP)-induced block of delayed rectifier K+ current (IK(V)) in vascular smooth muscle cells were studied in cells enzymatically isolated from the rabbit coronary artery. 2. 4-AP inhibited slowly inactivating IK(V) in a dose-dependent manner (concentration producing half-maximal inhibition, K1/2, = 1.37 mM), and shifted the steady-state activation and inactivation curves of IK(V) by +9 and +16 mV, respectively. 3. The time constant of activation was significantly increased by 4-AP at +20 mV; deactivation kinetics were unaffected upon repolarization to -40 mV. The fast (tau f approximately 1 s) and slow (tau s approximately 5 s) time constants of inactivation (0 and +20 mV), and the recovery kinetics (tau r approximately 6 s) at -60 mV were not significantly affected by 0.5 mM 4-AP. However, tau f disappeared in the presence of 2 mM 4-AP while tau s remained unaffected. 4. Use-dependent unblock of IK(V) was revealed at potentials > or = -10 mV from analyses of the voltage dependence of 4-AP-sensitive currents and the frequency-dependent changes ('reverse use dependence') of IK(V) during the application of repetitive steps (-60 to +20 mV for 250 ms at a rate of 0.25 Hz) in control conditions, in the presence of 0.5 mM 4-AP, and after washout of the drug. These results suggested that 4-AP preferentially binds to the channel in the closed state, and unbinding is promoted by transitions to the open state. 5. The channel was modelled as a simple three-state mathematical loop model incorporating single closed, open and inactivated states. The block by 4-AP was modelled as a state-dependent interaction with 4-AP primarily binding to the closed state. Computer simulations support the hypothesis that 4-AP-induced block of the delayed rectifier K+ (KV) channel in the closed state is relieved during membrane depolarization. 6. Closed state binding of 4-AP to the KV channel depolarizes vascular smooth muscle cells by shifting the activation curve of these channels to more positive potentials.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. P., Tomasic M., Kotlikoff M. I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol. 1992 Feb;447:329–350. doi: 10.1113/jphysiol.1992.sp019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Campbell D. L., Qu Y., Rasmusson R. L., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. II. Closed state reverse use-dependent block by 4-aminopyridine. J Gen Physiol. 1993 Apr;101(4):603–626. doi: 10.1085/jgp.101.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A. Multiple components of delayed rectifier K+ current in canine colonic smooth muscle. J Physiol. 1995 Apr 15;484(Pt 2):339–353. doi: 10.1113/jphysiol.1995.sp020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Fadous S., Logothetis D. E., Wang G. K. Aminopyridine block of Kv1.1 potassium channels expressed in mammalian cells and Xenopus oocytes. Mol Pharmacol. 1994 Jun;45(6):1242–1252. [PubMed] [Google Scholar]
- Choquet D., Korn H. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol. 1992 Feb;99(2):217–240. doi: 10.1085/jgp.99.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Washabau R. J., Kotlikoff M. I. Control of resting membrane potential by delayed rectifier potassium currents in ferret airway smooth muscle cells. J Physiol. 1993 Sep;469:625–638. doi: 10.1113/jphysiol.1993.sp019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband C. H., Hume J. R. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res. 1992 Oct;71(4):745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hart P. J., Overturf K. E., Russell S. N., Carl A., Hume J. R., Sanders K. M., Horowitz B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9659–9663. doi: 10.1073/pnas.90.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnel U., Klemm P., Nawrath H. Different mechanisms of the inhibition of the transient outward current in rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1994 Jan;349(1):87–94. doi: 10.1007/BF00178211. [DOI] [PubMed] [Google Scholar]
- Kehl S. J. 4-Aminopyridine causes a voltage-dependent block of the transient outward K+ current in rat melanotrophs. J Physiol. 1990 Dec;431:515–528. doi: 10.1113/jphysiol.1990.sp018344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J Gen Physiol. 1993 Nov;102(5):797–816. doi: 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Yeh J. Z., Oxford G. S. Modulation of aminopyridine block of potassium currents in squid axon. Biophys J. 1986 Oct;50(4):637–644. doi: 10.1016/S0006-3495(86)83503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Wan X., Leung P. M. Physiological role of Ca(2+)-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994 Jun;266(6 Pt 1):C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Morales E., Leblanc N. R., Cole W. C. Metabolic inhibition enhances Ca(2+)-activated K+ current in smooth muscle cells of rabbit portal vein. Am J Physiol. 1993 Dec;265(6 Pt 2):H2184–H2195. doi: 10.1152/ajpheart.1993.265.6.H2184. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Differential inhibition of a transient K+ current by chlorpromazine and 4-aminopyridine in neurones of the rat dorsal root ganglia. Br J Pharmacol. 1993 Aug;109(4):1239–1246. doi: 10.1111/j.1476-5381.1993.tb13755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Overturf K. E., Russell S. N., Carl A., Vogalis F., Hart P. J., Hume J. R., Sanders K. M., Horowitz B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol. 1994 Nov;267(5 Pt 1):C1231–C1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Nelson M. T. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol. 1994 Dec;267(6 Pt 1):C1589–C1597. doi: 10.1152/ajpcell.1994.267.6.C1589. [DOI] [PubMed] [Google Scholar]
- Russell S. N., Overturf K. E., Horowitz B. Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. Am J Physiol. 1994 Dec;267(6 Pt 1):C1729–C1733. doi: 10.1152/ajpcell.1994.267.6.C1729. [DOI] [PubMed] [Google Scholar]
- Russell S. N., Publicover N. G., Hart P. J., Carl A., Hume J. R., Sanders K. M., Horowitz B. Block by 4-aminopyridine of a Kv1.2 delayed rectifier K+ current expressed in Xenopus oocytes. J Physiol. 1994 Dec 15;481(Pt 3):571–584. doi: 10.1113/jphysiol.1994.sp020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simurda J., Simurdová M., Christé G. Use-dependent effects of 4-aminopyridine on transient outward current in dog ventricular muscle. Pflugers Arch. 1989 Nov;415(2):244–246. doi: 10.1007/BF00370600. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Robertson T. P., Ward J. P., Aaronson P. I. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994 Jan;266(1 Pt 2):H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- Stephens G. J., Garratt J. C., Robertson B., Owen D. G. On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. J Physiol. 1994 Jun 1;477(Pt 2):187–196. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Lang R. J. Identification of single transiently opening ("A-type") K channels in guinea-pig colonic myocytes. Pflugers Arch. 1994 Dec;429(2):160–164. doi: 10.1007/BF00374307. [DOI] [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk K. A., Shibata E. F. Single delayed rectifier potassium channels from rabbit coronary artery myocytes. Am J Physiol. 1993 Apr;264(4 Pt 2):H1146–H1153. doi: 10.1152/ajpheart.1993.264.4.H1146. [DOI] [PubMed] [Google Scholar]
- Yao J. A., Tseng G. N. Modulation of 4-AP block of a mammalian A-type K channel clone by channel gating and membrane voltage. Biophys J. 1994 Jul;67(1):130–142. doi: 10.1016/S0006-3495(94)80462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


