Abstract
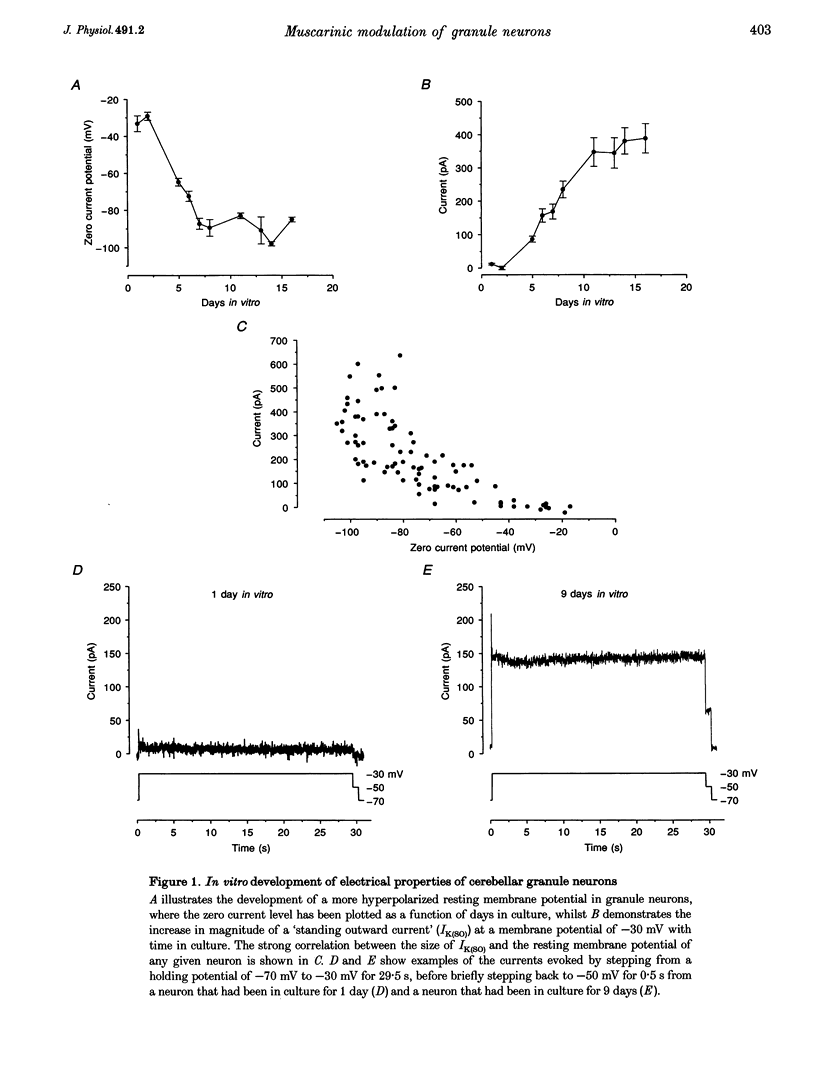
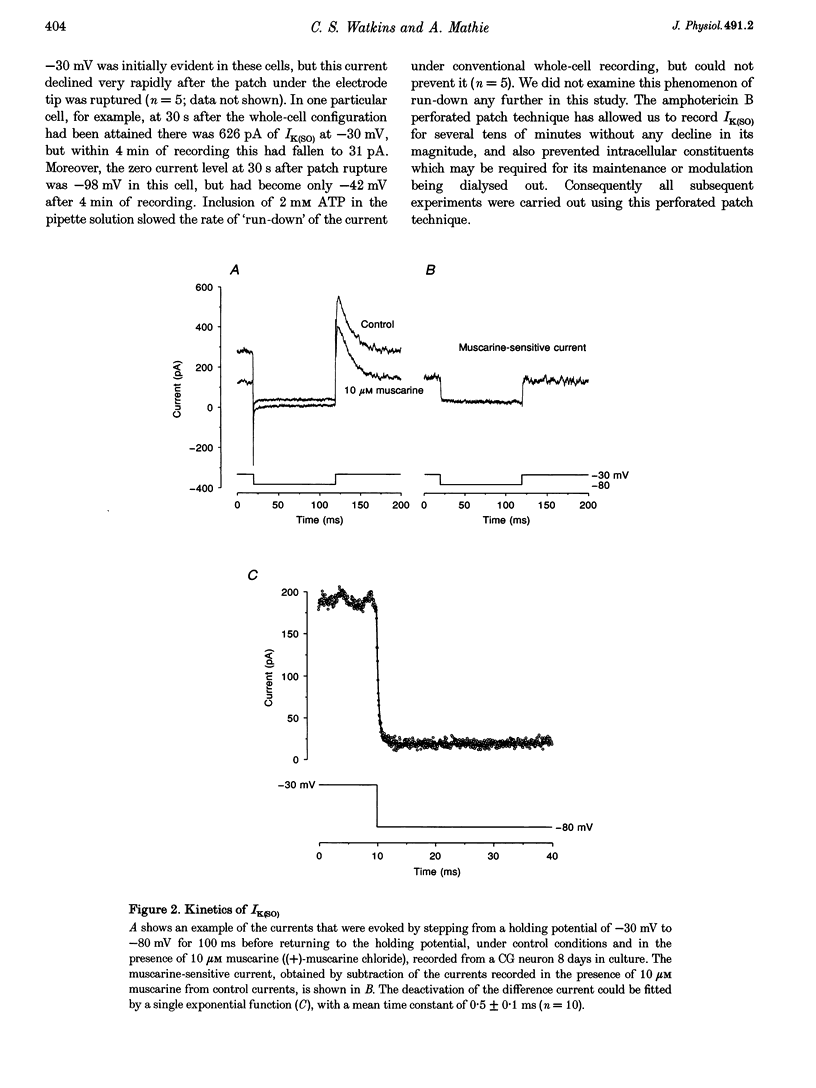
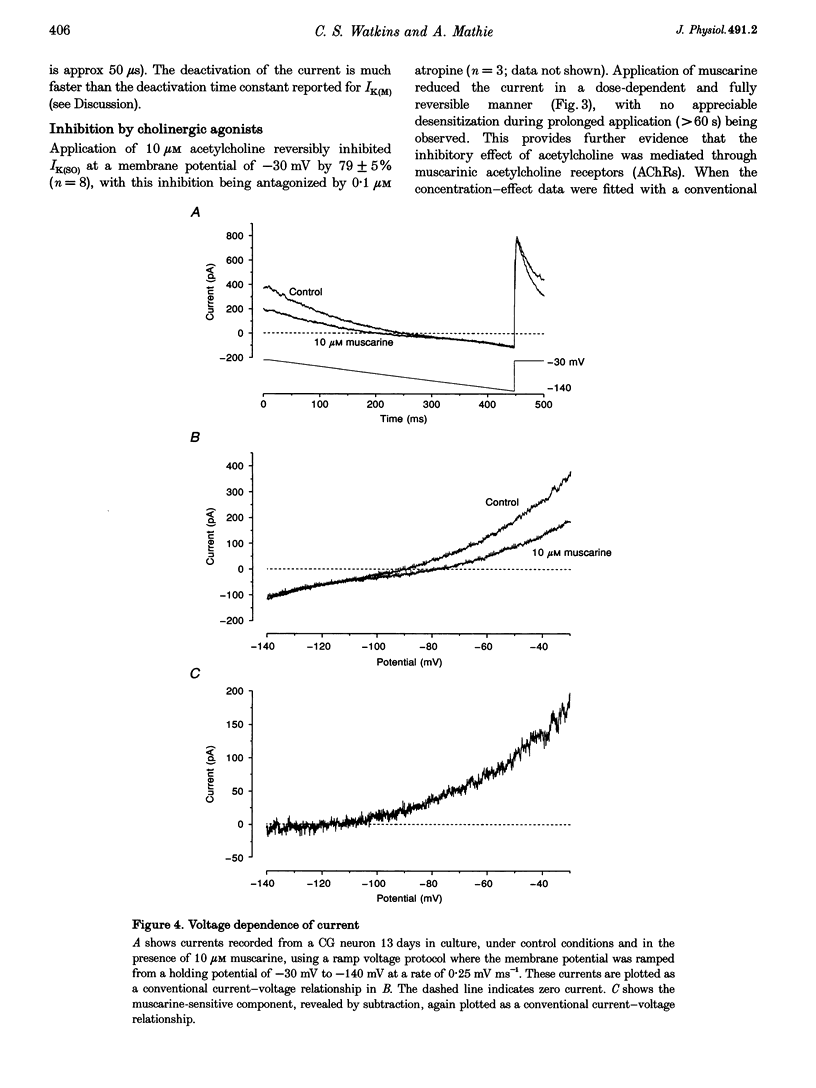
1. Whole-cell recordings were made from cultured cerebellar granule neurons using perforated patch clamp techniques. The primary cultures were prepared using 6- to 9-day-old Sprague-Dawley rats. 2. Neurons in culture for less than 48 h possessed resting membrane potentials of -29 mV. However, neurons in culture for 7 days had much more hyperpolarized resting membrane potentials (-89 mV). Over the same period, these neurons developed an additional component of outward current. 3. This non-inactivating current was activated by depolarization, exhibited outward rectification and reversed close to the potassium equilibrium potential. The kinetics of activation and deactivation were very rapid. 4. Muscarine ((+)-muscarine chloride) reversibly inhibited the current with an EC50 of 0.17 microM. The inhibition by muscarine was unaffected by pre-incubation for 17-20 h with 120 micrograms ml-1 pertussis toxin. 5. The current and its inhibition by muscarine were unaffected by 100 microM Cd2+. In Ca(2+)-free conditions, the current was significantly larger than in 0.5 mM Ca2+, but inhibition by 10 microM muscarine was significantly reduced. 6. The standing outward current was not obviously affected by 50 microM 5-HT, 50 microM noradrenaline, 50 microM 2-chloroadenosine or 5 mM tetraethylammonium. It was reduced by 10 microM La3+, 10 microM Zn2+ and 1 mM Ba2+. 7. Muscarinic agonists increased the input resistance of neurons and shifted the zero current level in the depolarized direction when voltage clamped. This enhanced excitability was evident under current clamp, where 10 microM muscarine depolarized granule neurons such that action potentials became evident.
Full text
PDF

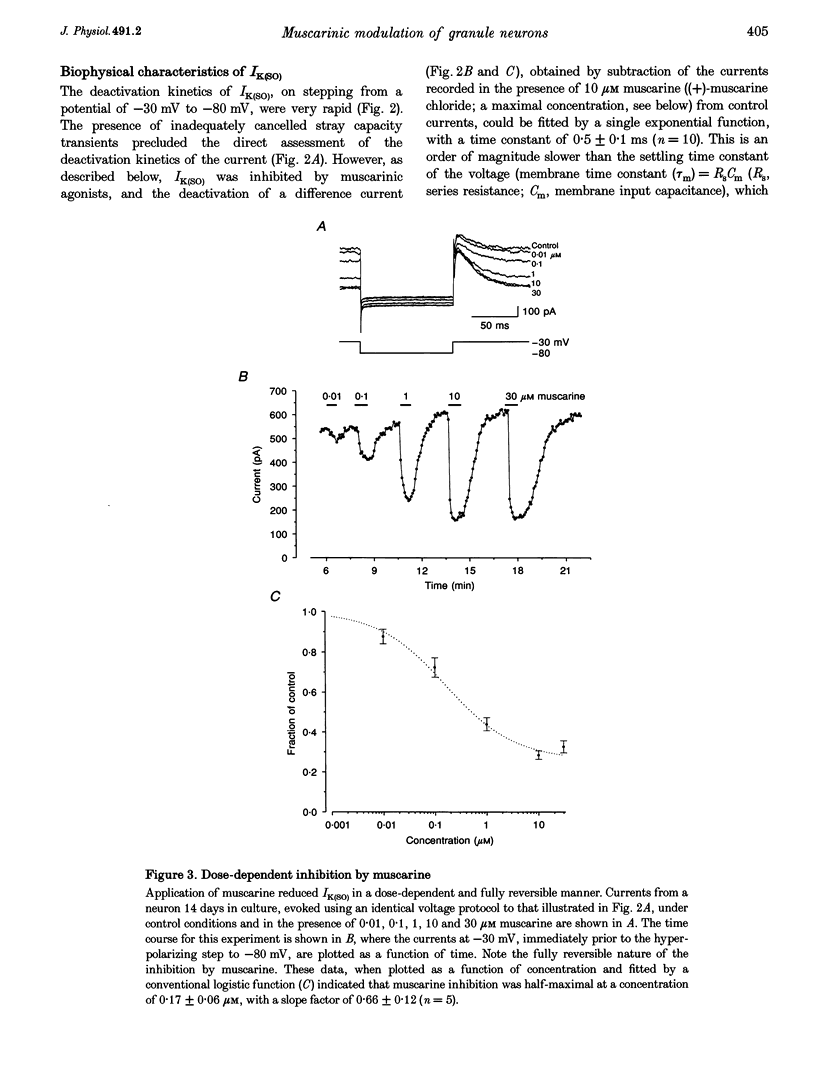
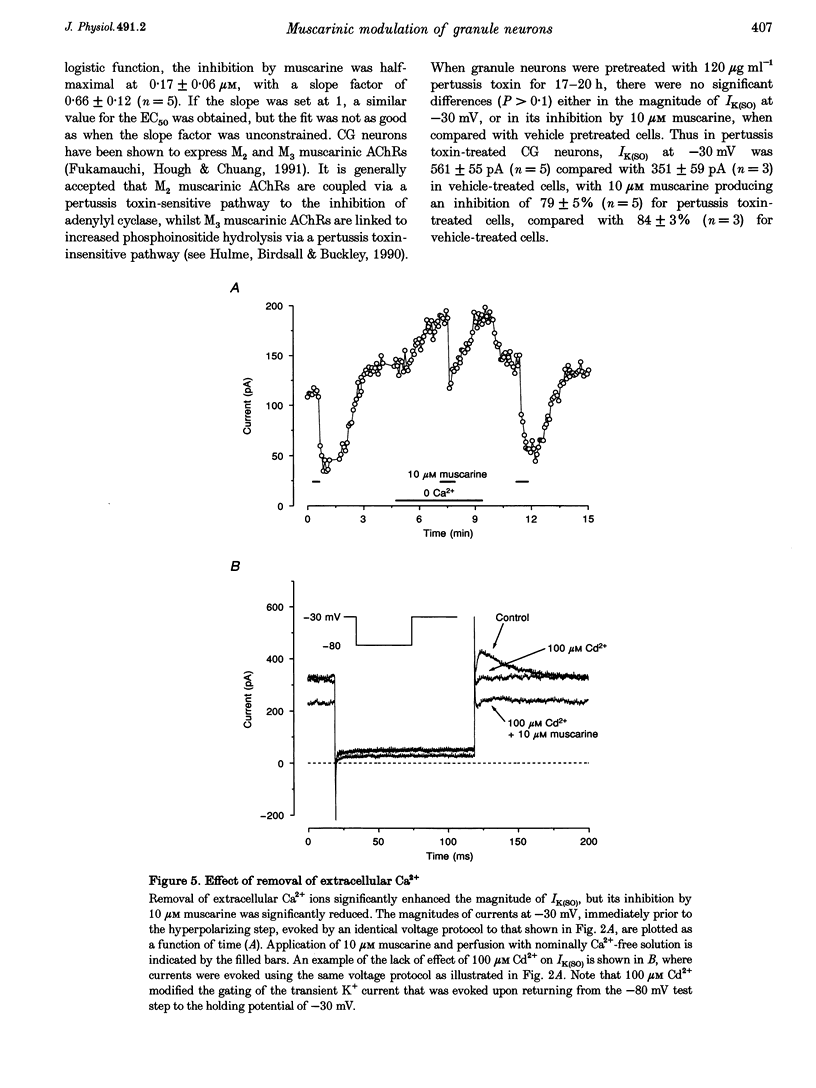
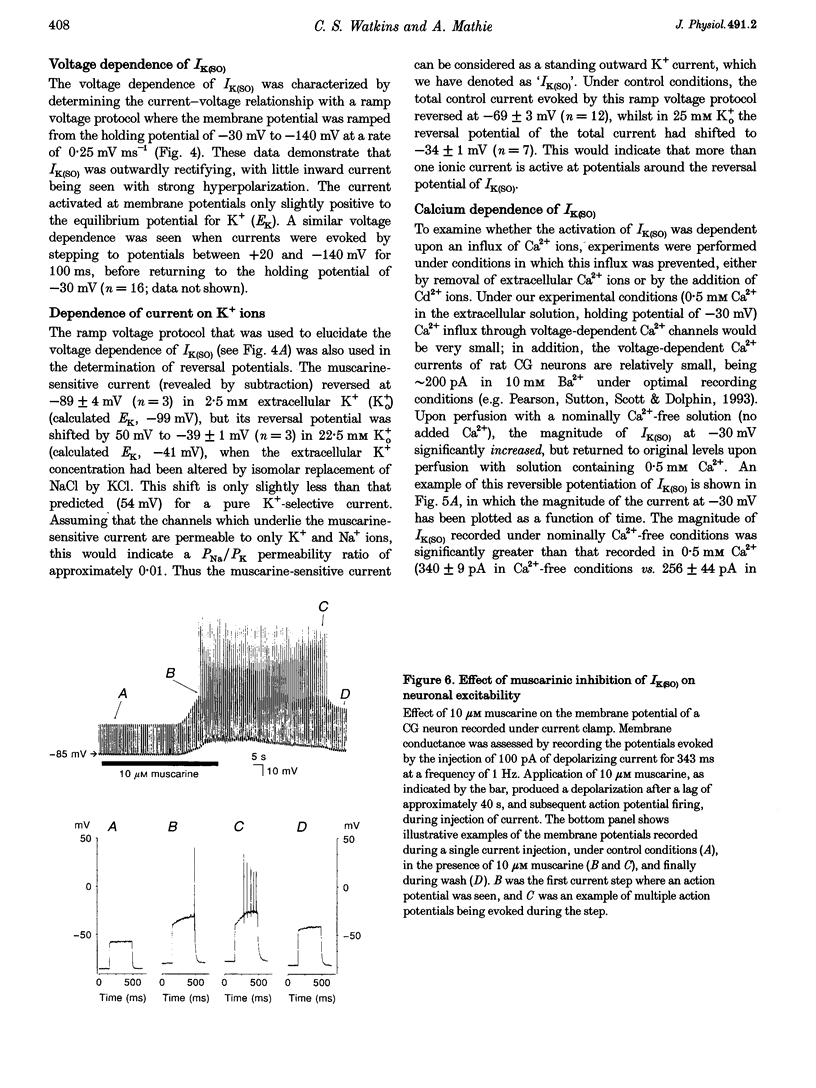




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico C., Marchetti C., Nobile M., Usai C. Pharmacological types of calcium channels and their modulation by baclofen in cerebellar granules. J Neurosci. 1995 Apr;15(4):2839–2848. doi: 10.1523/JNEUROSCI.15-04-02839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R., Belluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol. 1993 Jun;69(6):2222–2231. doi: 10.1152/jn.1993.69.6.2222. [DOI] [PubMed] [Google Scholar]
- Barmack N. H., Baughman R. W., Eckenstein F. P. Cholinergic innervation of the cerebellum of rat, rabbit, cat, and monkey as revealed by choline acetyltransferase activity and immunohistochemistry. J Comp Neurol. 1992 Mar 15;317(3):233–249. doi: 10.1002/cne.903170303. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. M., Blitzer R. D., Landau E. M. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol. 1988 Oct;404:479–496. doi: 10.1113/jphysiol.1988.sp017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cambray-Deakin M. A. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res. 1988 Jan-Mar;472(1):77–101. doi: 10.1016/0165-0173(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chavis P., Nooney J. M., Bockaert J., Fagni L., Feltz A., Bossu J. L. Facilitatory coupling between a glutamate metabotropic receptor and dihydropyridine-sensitive calcium channels in cultured cerebellar granule cells. J Neurosci. 1995 Jan;15(1 Pt 1):135–143. doi: 10.1523/JNEUROSCI.15-01-00135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., De Filippi G., Rossi P., Taglietti V. Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J Physiol. 1995 Apr 15;484(Pt 2):397–413. doi: 10.1113/jphysiol.1995.sp020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C. The G.L. Brown Prize Lecture. Voltage-dependent calcium channels and their modulation by neurotransmitters and G proteins. Exp Physiol. 1995 Jan;80(1):1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- Fagni L., Bossu J. L., Bockaert J. Activation of a Large-conductance Ca2+-Dependent K+ Channel by Stimulation of Glutamate Phosphoinositide-coupled Receptors in Cultured Cerebellar Granule Cells. Eur J Neurosci. 1991;3(8):778–789. doi: 10.1111/j.1460-9568.1991.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Fukamauchi F., Hough C., Chuang D. M. Expression and agonist-induced down-regulation of mRNAs of m2- and m3-muscarinic acetylcholine receptors in cultured cerebellar granule cells. J Neurochem. 1991 Feb;56(2):716–719. doi: 10.1111/j.1471-4159.1991.tb08210.x. [DOI] [PubMed] [Google Scholar]
- Galdzicki Z., Lin F., Moran O., Novelli A., Puia G., Sciancalepore M. Development of voltage-dependent ionic currents in rat cerebellar granule cells grown in primary culture. Int J Neurosci. 1991 Jan-Feb;56(1-4):193–200. doi: 10.3109/00207459108985416. [DOI] [PubMed] [Google Scholar]
- Guérineau N. C., Gähwiler B. H., Gerber U. Reduction of resting K+ current by metabotropic glutamate and muscarinic receptors in rat CA3 cells: mediation by G-proteins. J Physiol. 1994 Jan 1;474(1):27–33. doi: 10.1113/jphysiol.1994.sp019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Horne A. L. Membrane properties of the granule cells of the islands of Calleja of the rat studied in vitro. J Physiol. 1995 Sep 1;487(Pt 2):421–440. doi: 10.1113/jphysiol.1995.sp020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992 Aug;9(2):187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Immunocytochemical and electrophysiological differentiation of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1370–1383. doi: 10.1523/JNEUROSCI.07-05-01370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Huston E., Cullen G., Sweeney M. I., Pearson H., Fazeli M. S., Dolphin A. C. Pertussis toxin treatment increases glutamate release and dihydropyridine binding sites in cultured rat cerebellar granule neurons. Neuroscience. 1993 Feb;52(4):787–798. doi: 10.1016/0306-4522(93)90529-o. [DOI] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992 Oct;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalonen T., Johansson S., Holopainen I., Oja S. S., Arhem P. Single-channel and whole-cell currents in rat cerebellar granule cells. Brain Res. 1990 Dec 3;535(1):33–38. doi: 10.1016/0006-8993(90)91820-7. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Zucker R. S., Marsh S. J., Adams P. R. Modulation of M-current by intracellular Ca2+. Neuron. 1991 Apr;6(4):533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Ojima H., Kawajiri S., Yamasaki T. Cholinergic innervation of the rat cerebellum: qualitative and quantitative analyses of elements immunoreactive to a monoclonal antibody against choline acetyltransferase. J Comp Neurol. 1989 Dec 1;290(1):41–52. doi: 10.1002/cne.902900104. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Sutton K. G., Scott R. H., Dolphin A. C. Ca2+ currents in cerebellar granule neurones: role of internal Mg2+ in altering characteristics and antagonist effects. Neuropharmacology. 1993 Nov;32(11):1171–1183. doi: 10.1016/0028-3908(93)90011-q. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Activation of the pharmacologically defined M3 muscarinic receptor depolarizes hippocampal pyramidal cells. Brain Res. 1990 Nov 26;534(1-2):257–262. doi: 10.1016/0006-8993(90)90137-z. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Robbins J., Trouslard J., Marsh S. J., Brown D. A. Kinetic and pharmacological properties of the M-current in rodent neuroblastoma x glioma hybrid cells. J Physiol. 1992;451:159–185. doi: 10.1113/jphysiol.1992.sp019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Bredt D., Snyder S. H. Messenger molecules in the cerebellum. Trends Neurosci. 1990 Jun;13(6):216–222. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- Watkins C. S., Mathie A. Modulation of the gating of the transient outward potassium current of rat isolated cerebellar granule neurons by lanthanum. Pflugers Arch. 1994 Oct;428(3-4):209–216. doi: 10.1007/BF00724499. [DOI] [PubMed] [Google Scholar]
- Xu J., Chuang D. M. Serotonergic, adrenergic and histaminergic receptors coupled to phospholipase C in cultured cerebellar granule cells of rats. Biochem Pharmacol. 1987 Jul 15;36(14):2353–2358. doi: 10.1016/0006-2952(87)90603-4. [DOI] [PubMed] [Google Scholar]
- del Río E., Nicholls D. G., Downes C. P. Involvement of calcium influx in muscarinic cholinergic regulation of phospholipase C in cerebellar granule cells. J Neurochem. 1994 Aug;63(2):535–543. doi: 10.1046/j.1471-4159.1994.63020535.x. [DOI] [PubMed] [Google Scholar]


