Abstract
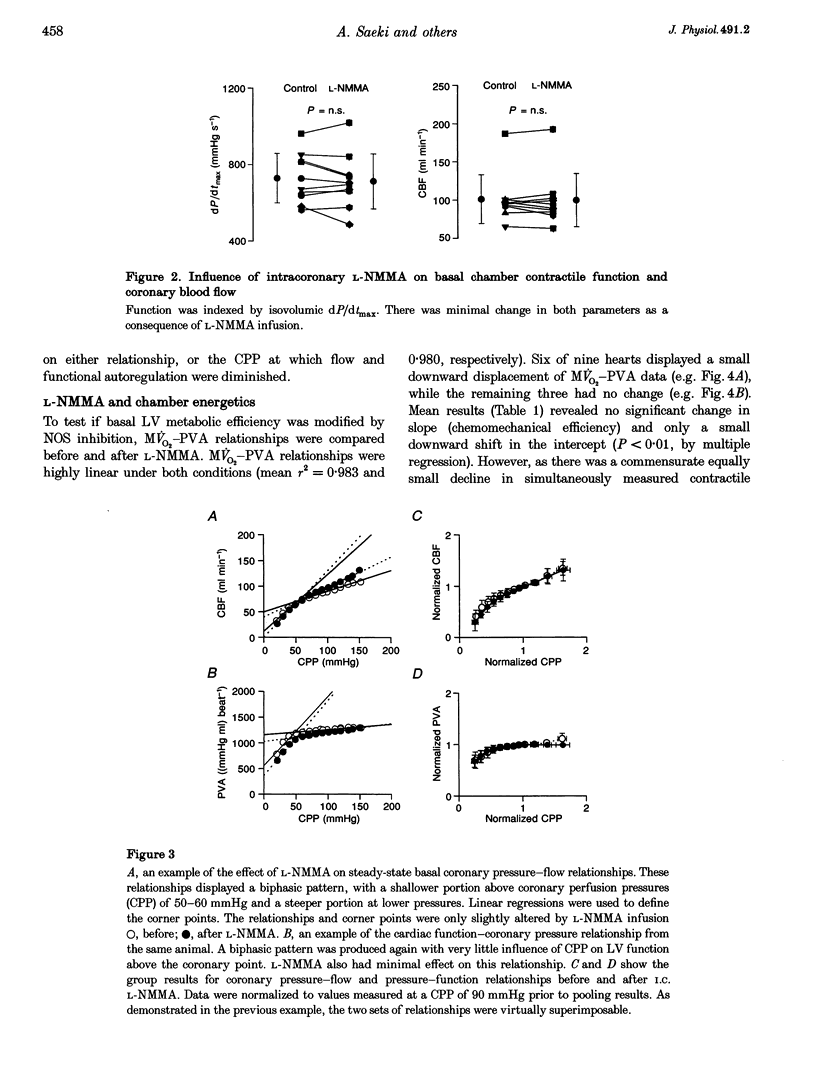
1. The role of nitric oxide (NO) in the regulation of basal coronary perfusion and ventricular chamber energetics was studied in isovolumetrically contracting isolated blood-perfused canine hearts. Hearts were cross-perfused by a donor animal prior to isolation, and chamber volume controlled by a servo-pump. Coronary sinus flow and arterial-coronary sinus oxygen difference were measured to determine energetic efficiency. 2. NO synthase (NOS) was competitively inhibited by NG-monomethyl-L-arginine (L-NMMA; 0.5 mg kg-1, intracoronary), resulting in a reduction of acetylcholine (50 micrograms min-1)-induced flow augmentation from 143 to 62% (P < 0.001). 3. NOS inhibition had no significant effect on basal coronary flow. Coronary pressure-flow relationships were determined at a constant cardiac workload by varying mean perfusion pressure between 20 and 150 mmHg. Neither the shape of the relationship, nor the low-pressure value at which flow regulation was substantially diminished were altered by NOS inhibition. 4. Myocardial efficiency was assessed by the relationship between myocardial oxygen consumption and total pressure-volume area (PVA), with cavity volume altered to generate varying PVAs. This relative load-independent measure of energetic efficiency was minimally altered by NOS inhibition. 5. These results contrast with isolated crystalloid-perfused heart experiments and suggest that in hearts with highly controlled ventricular loading and whole-blood perfusion, effects of basal NO production on coronary perfusion and left ventricular energetics are minimal.
Full text
PDF


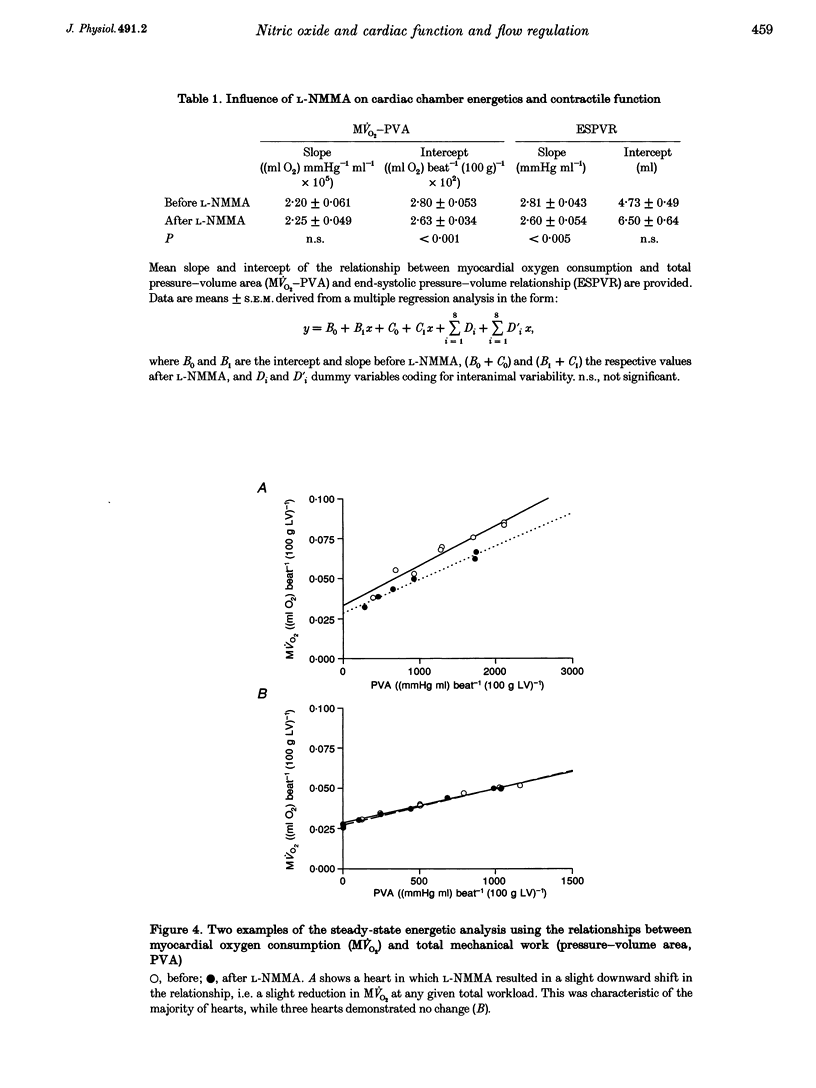




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrani M., O'Shea J., Allen N. J., Harding S. E., Jayakumar J., Pepper J. R., Moncada S., Yacoub M. H. Role of basal release of nitric oxide on coronary flow and mechanical performance of the isolated rat heart. J Physiol. 1992 Oct;456:681–687. doi: 10.1113/jphysiol.1992.sp019361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma P., Ferdinandy P., Sipkema P., Allaart C. P., Westerhof N. Nitric oxide is an important determinant of coronary flow in the isolated blood perfused rat heart. Basic Res Cardiol. 1992 Nov-Dec;87(6):570–584. doi: 10.1007/BF00788667. [DOI] [PubMed] [Google Scholar]
- Brown I. P., Thompson C. I., Belloni F. L. Role of nitric oxide in hypoxic coronary vasodilatation in isolated perfused guinea pig heart. Am J Physiol. 1993 Mar;264(3 Pt 2):H821–H829. doi: 10.1152/ajpheart.1993.264.3.H821. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Canty J. M., Jr Coronary pressure-function and steady-state pressure-flow relations during autoregulation in the unanesthetized dog. Circ Res. 1988 Oct;63(4):821–836. doi: 10.1161/01.res.63.4.821. [DOI] [PubMed] [Google Scholar]
- Chu A., Chambers D. E., Lin C. C., Kuehl W. D., Palmer R. M., Moncada S., Cobb F. R. Effects of inhibition of nitric oxide formation on basal vasomotion and endothelium-dependent responses of the coronary arteries in awake dogs. J Clin Invest. 1991 Jun;87(6):1964–1968. doi: 10.1172/JCI115223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole W. P. Autoregulation of the coronary circulation. Prog Cardiovasc Dis. 1987 Jan-Feb;29(4):293–323. doi: 10.1016/s0033-0620(87)80005-1. [DOI] [PubMed] [Google Scholar]
- Duncker D. J., Bache R. J. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res. 1994 Apr;74(4):629–640. doi: 10.1161/01.res.74.4.629. [DOI] [PubMed] [Google Scholar]
- Feigl E. O., Neat G. W., Huang A. H. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol. 1990 Apr;22(4):375–390. doi: 10.1016/0022-2828(90)91474-l. [DOI] [PubMed] [Google Scholar]
- Harasawa Y., de Tombe P. P., Sheriff D. D., Hunter W. C. Basal metabolism adds a significant offset to unloaded myocardial oxygen consumption per minute. Circ Res. 1992 Aug;71(2):414–422. doi: 10.1161/01.res.71.2.414. [DOI] [PubMed] [Google Scholar]
- Hare J. M., Keaney J. F., Jr, Balligand J. L., Loscalzo J., Smith T. W., Colucci W. S. Role of nitric oxide in parasympathetic modulation of beta-adrenergic myocardial contractility in normal dogs. J Clin Invest. 1995 Jan;95(1):360–366. doi: 10.1172/JCI117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkebøen K. A., Naess P. A., Offstad J., Ilebekk A. Effects of regional inhibition of nitric oxide synthesis in intact porcine hearts. Am J Physiol. 1994 Apr;266(4 Pt 2):H1516–H1527. doi: 10.1152/ajpheart.1994.266.4.H1516. [DOI] [PubMed] [Google Scholar]
- Lefroy D. C., Crake T., Uren N. G., Davies G. J., Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993 Jul;88(1):43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Tunin C., Kass D. A. Transient time course of cocaine-induced cardiac depression versus sustained peripheral vasoconstriction. J Am Coll Cardiol. 1993 Jan;21(1):260–268. doi: 10.1016/0735-1097(93)90746-n. [DOI] [PubMed] [Google Scholar]
- MOSHER P., ROSS J., Jr, MCFATE P. A., SHAW R. F. CONTROL OF CORONARY BLOOD FLOW BY AN AUTOREGULATORY MECHANISM. Circ Res. 1964 Mar;14:250–259. doi: 10.1161/01.res.14.3.250. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Myers P. R., Banitt P. F., Guerra R., Jr, Harrison D. G. Characteristics of canine coronary resistance arteries: importance of endothelium. Am J Physiol. 1989 Aug;257(2 Pt 2):H603–H610. doi: 10.1152/ajpheart.1989.257.2.H603. [DOI] [PubMed] [Google Scholar]
- Noe D. A., Weedn V., Bell W. R. Direct spectrophotometry of serum hemoglobin: an Allen correction compared with a three-wavelength polychromatic analysis. Clin Chem. 1984 May;30(5):627–630. [PubMed] [Google Scholar]
- Parent R., Paré R., Lavallée M. Contribution of nitric oxide to dilation of resistance coronary vessels in conscious dogs. Am J Physiol. 1992 Jan;262(1 Pt 2):H10–H16. doi: 10.1152/ajpheart.1992.262.1.H10. [DOI] [PubMed] [Google Scholar]
- Paulus W. J., Vantrimpont P. J., Shah A. M. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994 May;89(5):2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V., Berdeaux A., la Rochelle C. D., Giudicelli J. F. Regional coronary haemodynamic effects of two inhibitors of nitric oxide synthesis in anaesthetized, open-chest dogs. Br J Pharmacol. 1991 Sep;104(1):59–64. doi: 10.1111/j.1476-5381.1991.tb12385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. P., Jr, Canty J. M., Jr Modulation of coronary autoregulatory responses by nitric oxide. Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res. 1993 Aug;73(2):232–240. doi: 10.1161/01.res.73.2.232. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Münzel T., Bassenge E. Reversal of acetylcholine-induced coronary resistance vessel dilation by hemoglobin. Eur J Pharmacol. 1987 Apr 14;136(2):239–242. doi: 10.1016/0014-2999(87)90717-5. [DOI] [PubMed] [Google Scholar]
- Suga H., Hisano R., Goto Y., Yamada O., Igarashi Y. Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res. 1983 Sep;53(3):306–318. doi: 10.1161/01.res.53.3.306. [DOI] [PubMed] [Google Scholar]
- Suga H. Ventricular energetics. Physiol Rev. 1990 Apr;70(2):247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- Sunagawa K., Maughan W. L., Friesinger G., Guzman P., Chang M. S., Sagawa K. Effects of coronary arterial pressure on left ventricular end-systolic pressure-volume relation of isolated canine heart. Circ Res. 1982 May;50(5):727–734. doi: 10.1161/01.res.50.5.727. [DOI] [PubMed] [Google Scholar]
- Ueeda M., Silvia S. K., Olsson R. A. Nitric oxide modulates coronary autoregulation in the guinea pig. Circ Res. 1992 Jun;70(6):1296–1303. doi: 10.1161/01.res.70.6.1296. [DOI] [PubMed] [Google Scholar]
- Van Winkle D. M., Feigl E. O. Acetylcholine causes coronary vasodilation in dogs and baboons. Circ Res. 1989 Dec;65(6):1580–1593. doi: 10.1161/01.res.65.6.1580. [DOI] [PubMed] [Google Scholar]
- Wennmalm A., Benthin G., Edlund A., Jungersten L., Kieler-Jensen N., Lundin S., Westfelt U. N., Petersson A. S., Waagstein F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res. 1993 Dec;73(6):1121–1127. doi: 10.1161/01.res.73.6.1121. [DOI] [PubMed] [Google Scholar]
- Yamabe H., Okumura K., Ishizaka H., Tsuchiya T., Yasue H. Role of endothelium-derived nitric oxide in myocardial reactive hyperemia. Am J Physiol. 1992 Jul;263(1 Pt 2):H8–14. doi: 10.1152/ajpheart.1992.263.1.H8. [DOI] [PubMed] [Google Scholar]


