Abstract
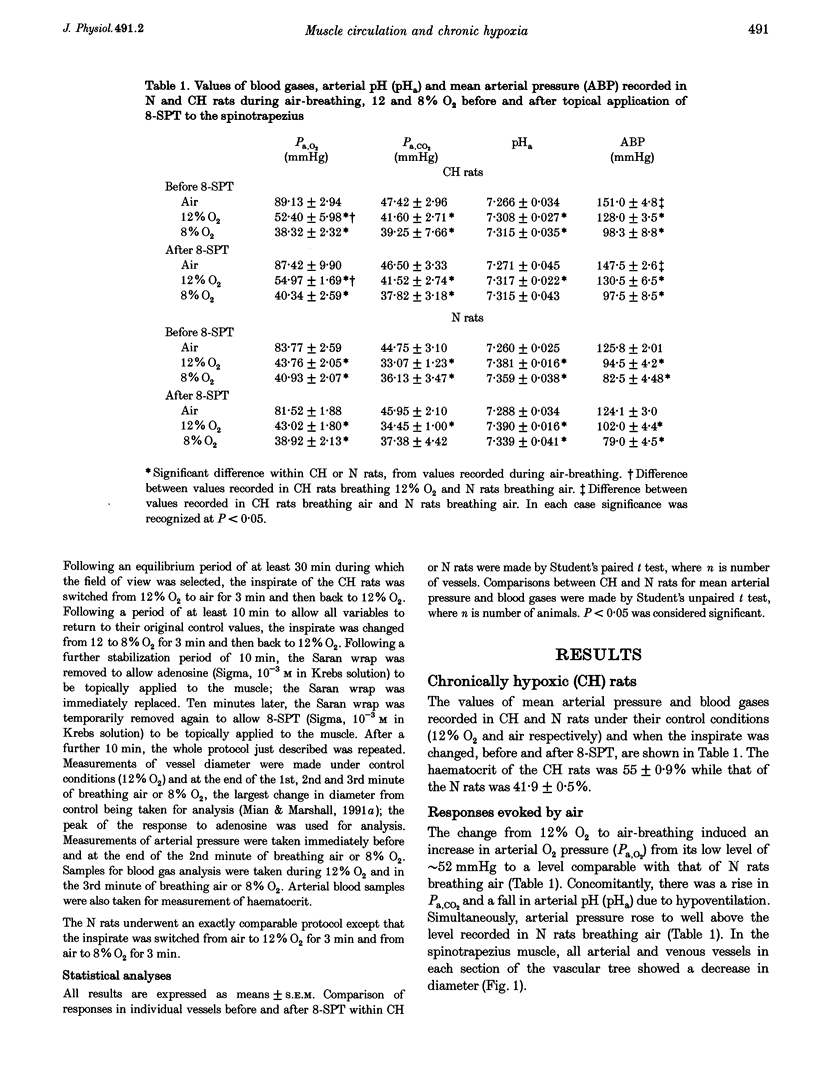
1. In rats housed in a hypoxic chamber at 12% O2 for 3-5 weeks (CH) and in normal rats housed in air (N), we directly observed responses of arterial and venous vessels of the spinotrapezius muscle to changes in O2 concentration in the inspirate. Both CH and N rats were anaesthetized with Saffan. They had haematocrits of 55.0 +/- 0.9% (mean +/- S.E.M.) and 41.9 +/- 0.5%, respectively. 2. In CH rats breathing 12% O2 and N rats breathing air, arterial and venous vessels from comparable anatomical positions in the vascular tree were of similar internal diameter. They also showed similar maximum dilator responses to topical adenosine (10(-3) M); 14.1 +/- 1.1 and 16.3 +/- 1.7% in all arterioles, 15.5 +/- 1.2 and 11.5 +/- 0.6% in all venules in CH and N rats, respectively. 3. In CH rats, the change from 12% O2 to air for 3 min induced constriction in all arterioles and venules (-12.9 +/- 1.0 and -14.3 +/- 1.7%, respectively), whereas in N rats, the change from air to 12% O2 for 3 min induced net dilatation (3.9 +/- 1.8% in arterioles and 4.7 +/- 0.8% in venules). Topical application of the adenosine receptor antagonist 8-sulphophenyltheophylline (8-SPT, 10(-3) M) had no effect on control diameters in CH or N rats, nor on constrictor responses to air in CH, but reversed or reduced dilator responses to 12% O2 in N rats (to -2.4 +/- 1.3% in arterioles and 2.0 +/- 0.9% in venules). 4. In CH rats, the change from 12 to 8% O2 produced net dilatation as great as that induced in N rats by the larger change from air to 8% O2: 8.5 +/- 2.6 and 5.0 +/- 3.7% in arterioles and 10.3 +/- 1.8 and 6.4 +/- 1.9% in venules, respectively. These responses were similarly reduced by 8-SPT to -4.3 +/- 1.9 and -5.2 +/- 2.7% in arterioles and to -6.9 +/- 2.0 and -1.5 +/- 2.0% in venules, respectively. 5. These results indicate that CH rats were acclimated to 12% O2 such that the resting tone of arterial and venous vessels of muscle was comparable to that of N rats breathing air. They also suggest that adenosine had little tonic dilator influence in CH rats breathing 12% O2 despite its contribution to the dilatation induced in N rats by acute exposure to 12% O2. This may reflect the greater haematocrit in CH rats which normalized the O2 supply to muscle. However, CH rats were more sensitive than N rats to the dilator influence of acute systemic hypoxia and this was largely mediated by adenosine.
Full text
PDF

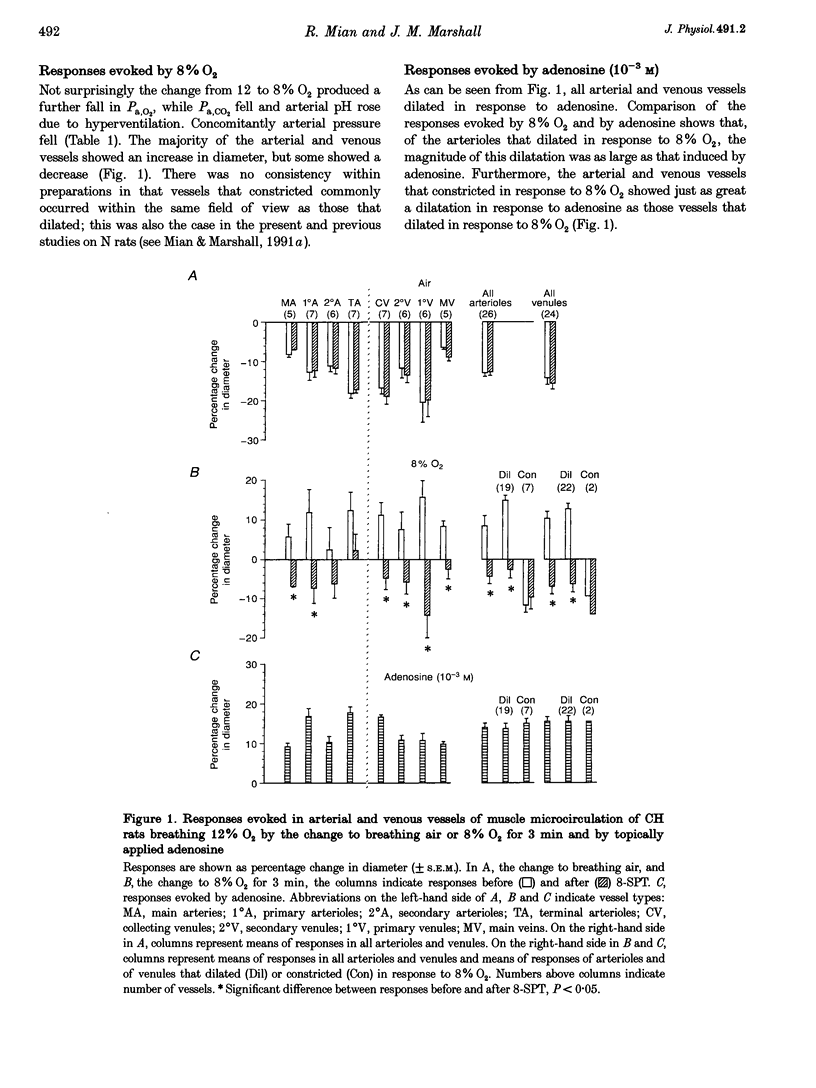
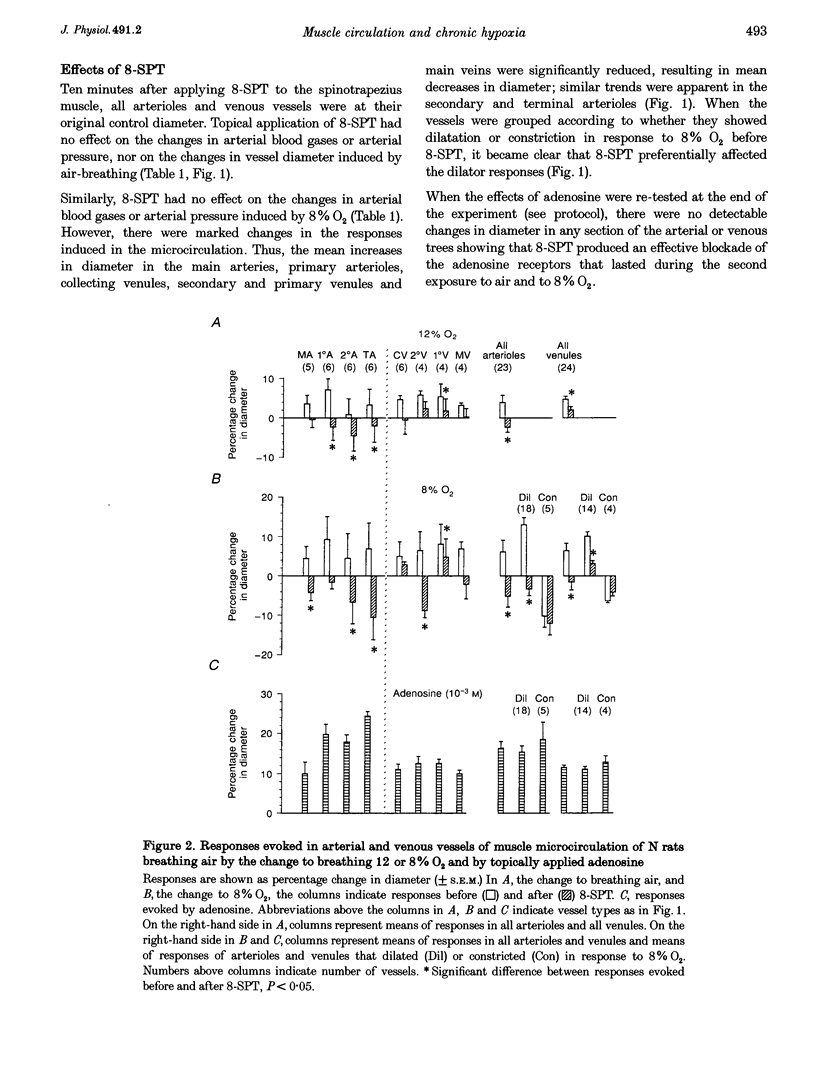





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockman E. L., McKenzie J. E. Tissue adenosine content in active soleus and gracilis muscles of cats. Am J Physiol. 1983 Apr;244(4):H552–H559. doi: 10.1152/ajpheart.1983.244.4.H552. [DOI] [PubMed] [Google Scholar]
- Deussen A., Möser G., Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986 Jun;406(6):608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Walker B. R. Attentuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol. 1991 Jun;260(6 Pt 2):R1114–R1122. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. J., Duling B. R. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978 Nov;235(5):H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- Harrison D. K., Kessler M., Knauf S. K. Regulation of capillary blood flow and oxygen supply in skeletal muscle in dogs during hypoxaemia. J Physiol. 1990 Jan;420:431–446. doi: 10.1113/jphysiol.1990.sp017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert M. T., Marshall J. M. Direct observations of the effects of baroreceptor stimulation on skeletal muscle circulation of the rat. J Physiol. 1988 Jun;400:45–59. doi: 10.1113/jphysiol.1988.sp017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A., Itoh K., Oishi Y., Itoh M., Hirofuji C., Hayashi H. Effects of hypobaric hypoxia on histochemical fibre-type composition and myosin heavy chain isoform component in the rat soleus muscle. Pflugers Arch. 1995 Mar;429(5):601–606. doi: 10.1007/BF00373980. [DOI] [PubMed] [Google Scholar]
- Jackson W. F. Arteriolar oxygen reactivity is inhibited by leukotriene antagonists. Am J Physiol. 1989 Nov;257(5 Pt 2):H1565–H1572. doi: 10.1152/ajpheart.1989.257.5.H1565. [DOI] [PubMed] [Google Scholar]
- Lang D. J., Johnson P. C. Elevated ambient oxygen does not affect autoregulation in cat mesentery. Am J Physiol. 1988 Jul;255(1 Pt 2):H131–H137. doi: 10.1152/ajpheart.1988.255.1.H131. [DOI] [PubMed] [Google Scholar]
- Langdown A. J., Marshall J. M. Analysis of responses observed in mesenteric microcirculation of the rat during systemic hypoxia. J Physiol. 1995 Feb 1;482(Pt 3):669–677. doi: 10.1113/jphysiol.1995.sp020549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. Analysis of cardiovascular responses evoked following changes in peripheral chemoreceptor activity in the rat. J Physiol. 1987 Dec;394:393–414. doi: 10.1113/jphysiol.1987.sp016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Lloyd J., Mian R. The influence of vasopressin on the arterioles and venules of skeletal muscle of the rat during systemic hypoxia. J Physiol. 1993 Oct;470:473–484. doi: 10.1113/jphysiol.1993.sp019870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Influences on the cardiovascular response to graded levels of systemic hypoxia of the accompanying hypocapnia in the rat. J Physiol. 1989 Mar;410:381–394. doi: 10.1113/jphysiol.1989.sp017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982 Nov;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Thomas T., Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993 Dec;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. Responses observed in individual arterioles and venules of rat skeletal muscle during systemic hypoxia. J Physiol. 1991 May;436:485–497. doi: 10.1113/jphysiol.1991.sp018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon M., Marshall J. M. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Sullivan S. M., Johnson P. C. Effect of oxygen on arteriolar dimensions and blood flow in cat sartorius muscle. Am J Physiol. 1981 Oct;241(4):H547–H556. doi: 10.1152/ajpheart.1981.241.4.H547. [DOI] [PubMed] [Google Scholar]
- Thomas T., Elnazir B. K., Marshall J. M. Differentiation of the peripherally mediated from the centrally mediated influences of adenosine in the rat during systemic hypoxia. Exp Physiol. 1994 Sep;79(5):809–822. doi: 10.1113/expphysiol.1994.sp003809. [DOI] [PubMed] [Google Scholar]
- Thomas T., Marshall J. M. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995 Sep 1;487(Pt 2):513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]


