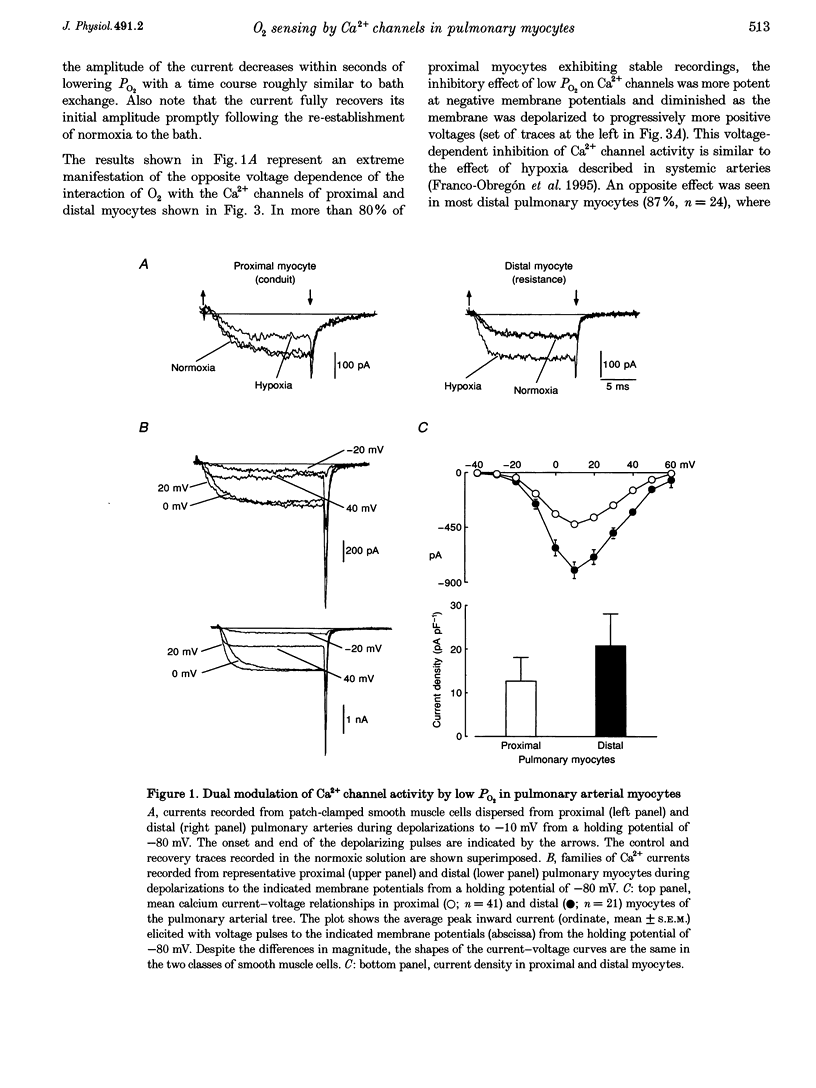
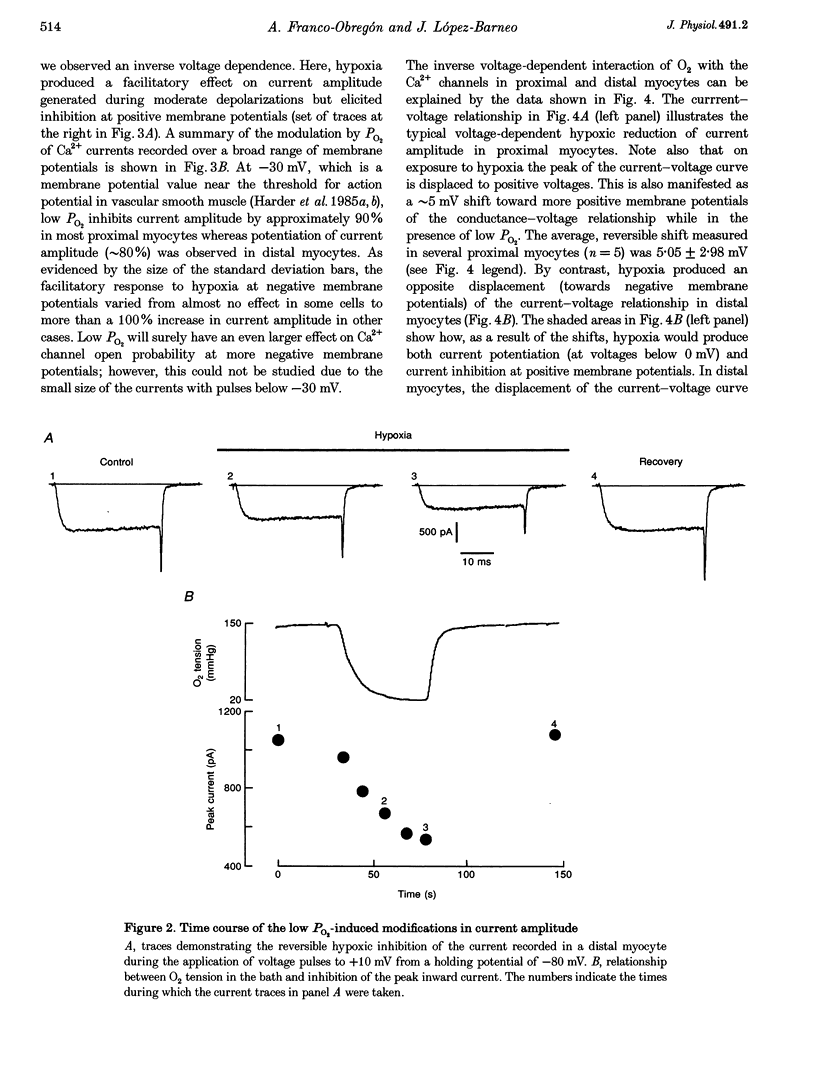
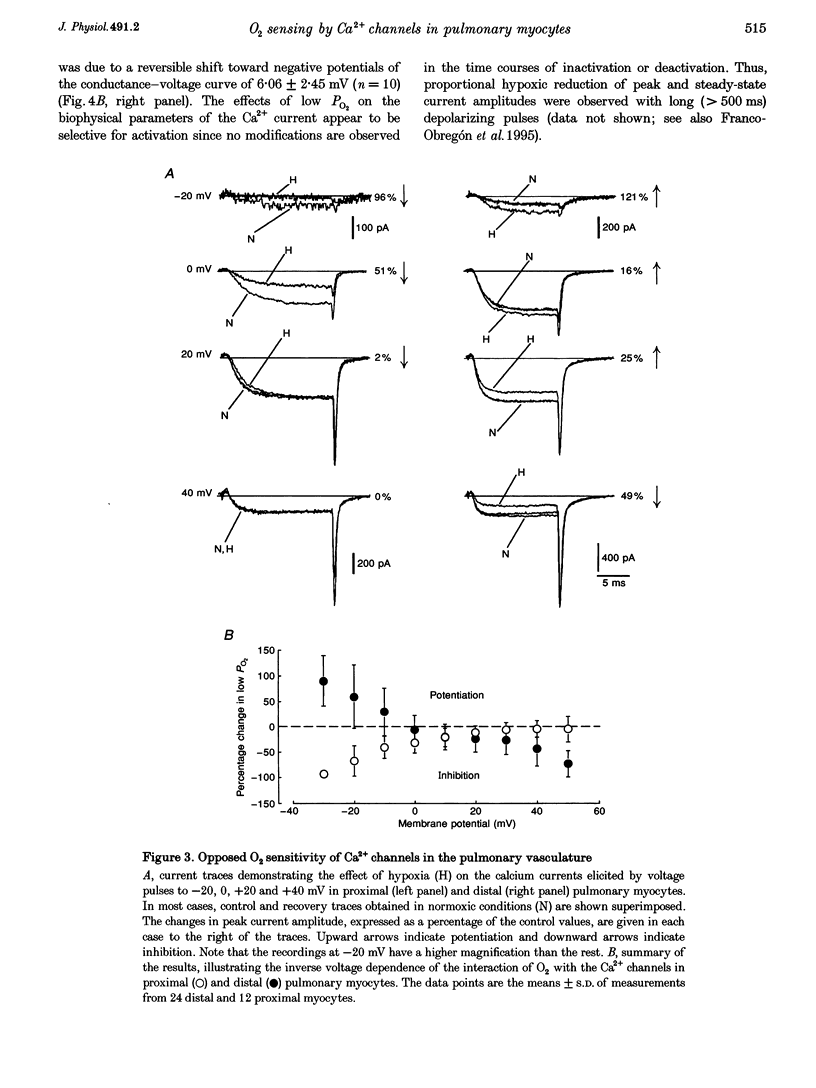
Abstract
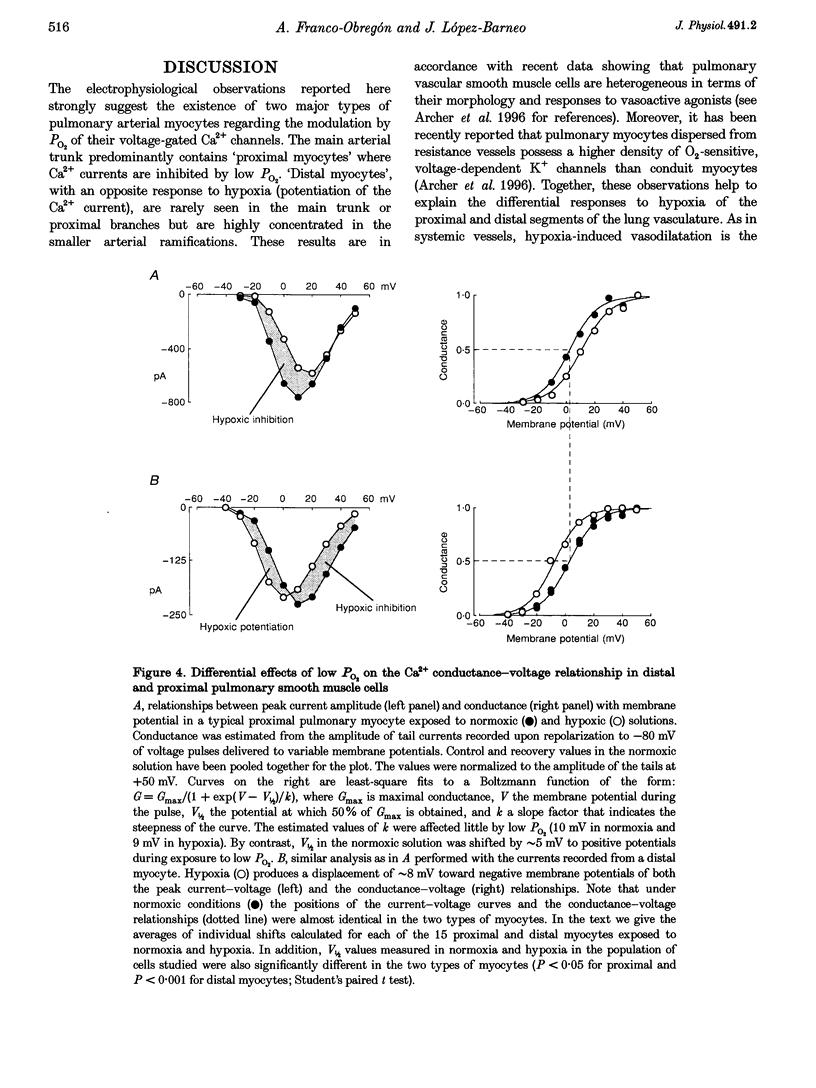
1. Calcium currents were recorded from smooth muscle cells dispersed from conduit and resistance rabbit pulmonary arteries. We tested the hypothesis that Ca2+ channel activity was regulated by environmental O2 tension. 2. Conduit (proximal) and resistance (distal) myocytes differ in their Ca2+ channel density and responses to low PO2. Ca2+ current density in distal myocytes (20.7 +/- 7.4 pA pF-1, n = 10) is almost twice the value in proximal myocytes (12.6 +/- 5.5 pA pF-1, n = 39). In proximal myocytes, the predominant response to reductions in PO2 is inhibition of the calcium current (n = 12) at membrane potentials below 0 mV, whereas potentiation of current amplitude is observed in distal myocytes (n = 24). 3. Hypoxia also produces opposite shifts in the conductance-voltage relationships along the voltage axis. The average displacements induced by low PO2 are +5.05 +/- 2.98 mV (n = 5) in proximal myocytes and -6.06 +/- 2.45 (n = 10) in distal myocytes. 4. These findings demonstrate longitudinal differences in Ca2+ channel density and O2 sensitivity in myocytes along the pulmonary arterial tree. These results may help to understand the differential reactivity to hypoxia of the pulmonary vasculature: vasodilatation in conduit arteries and vasoconstriction in resistance vessels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson C. A. Role of pulmonary vasomotion in physiology of the lung. Physiol Rev. 1984 Apr;64(2):544–616. doi: 10.1152/physrev.1984.64.2.544. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Murray R. K., Kotlikoff M. I. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11914–11918. doi: 10.1073/pnas.91.25.11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregón A., Ureña J., López-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Madden J. A., Dawson C. A membrane electrical mechanism for hypoxic vasoconstriction of small pulmonary arteries from cat. Chest. 1985 Oct;88(4 Suppl):233S–235S. doi: 10.1378/chest.88.4_supplement.233s. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Madden J. A., Dawson C. Hypoxic induction of Ca2+-dependent action potentials in small pulmonary arteries of the cat. J Appl Physiol (1985) 1985 Nov;59(5):1389–1393. doi: 10.1152/jappl.1985.59.5.1389. [DOI] [PubMed] [Google Scholar]
- Marriott J. F., Marshall J. M. Effects of hypoxia upon contractions evoked in isolated rabbit pulmonary artery by potassium and noradrenaline. J Physiol. 1990 Mar;422:15–28. doi: 10.1113/jphysiol.1990.sp017969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry I. F., Davidson A. B., Reeves J. T., Grover R. F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res. 1976 Feb;38(2):99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Salvaterra C. G., Goldman W. F. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol. 1993 Mar;264(3 Pt 1):L323–L328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- Vadula M. S., Kleinman J. G., Madden J. A. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol. 1993 Dec;265(6 Pt 1):L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- Wadsworth R. M. Vasoconstrictor and vasodilator effects of hypoxia. Trends Pharmacol Sci. 1994 Feb;15(2):47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Yuan X. J., Tod M. L., Rubin L. J., Blaustein M. P. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol. 1990 Aug;259(2 Pt 2):H281–H289. doi: 10.1152/ajpheart.1990.259.2.H281. [DOI] [PubMed] [Google Scholar]