Abstract
Tianeptine (1) is an unusual antidepressant in that its mechanism of action appears to be independent from any activity at serotonin receptors or monoamine transporters. In fact, tianeptine has been shown to be a moderately potent agonist for the mu opioid receptor (MOR) and to a lesser extent the delta opioid receptor (DOR). Additionally, tianeptine’s efficacy may be related to its action on glutamate-mediated pathways of neuroplasticity. Regardless of which neurotransmitter system is primarily responsible for the observed efficacy, the MOR agonist activity is problematic with respect to abuse liability. Increasing numbers of case reports have demonstrated that tianeptine is indeed being used recreationally at doses far beyond what are considered therapeutically relevant or safe, and scheduling reclassifications or outright bans on tianeptine products are ongoing around the world. It is the aim of this review to discuss the medicinal chemistry and pharmacology of tianeptine and to summarize this intriguing discrepancy between tianeptine’s historical use as a safe and effective antidepressant and its emerging potential for abuse.
Keywords: tianeptine, antidepressant, opioid, serotonin, drug abuse
Introduction
Depression is one of the most common mental disorders globally. According to the World Health Organization (WHO), approximately 3.8% of the population experiences depression during their lifetimes.1 Due to the long-lasting symptoms of depression, which include decreased concentration, fatigue, anxiety, and suppression of mood, depression is a major contributor to suicide (which is the fourth leading cause of death in young adults). In addition, depression is a common comorbidity in a broad group of other disorders including Parkinson’s disease (PD) and asthma.2,3
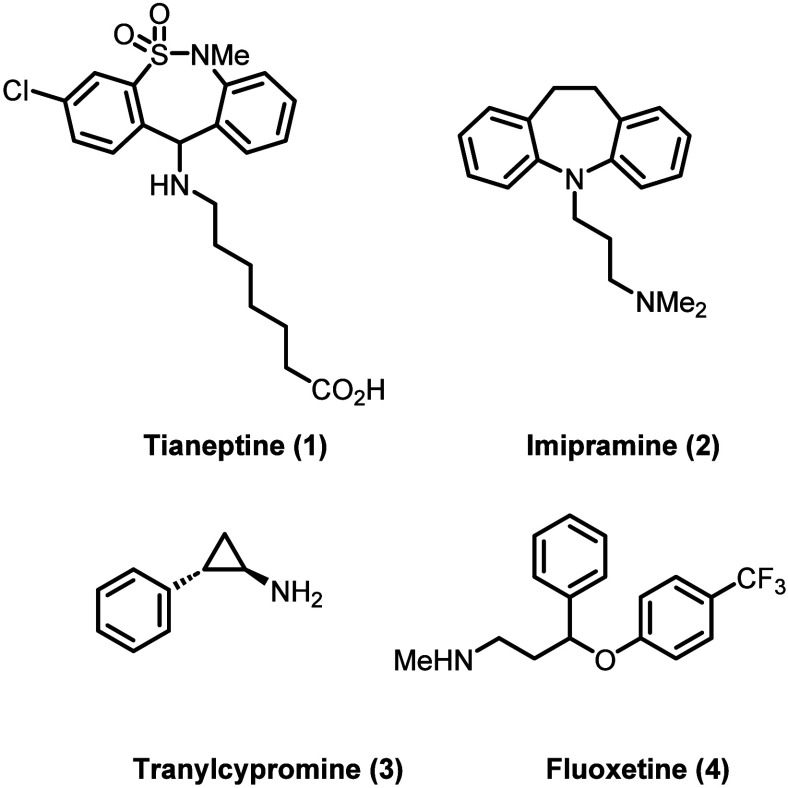
The global prominence of depression and the lack of a cure have spurred a vast number of drug discovery efforts. Monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), and selective serotonin reuptake inhibitors (SSRIs) have all been extensively studied from the mid to late 1900s. The serotonin (5-hydroxytryptamine, 5-HT) hypothesis of depression put forth by Alec Coppen and others became an early guiding principle for medicinal chemists working in this space,4−6 and the echoes of this principle are apparent in the structural design of many marketed antidepressants including imipramine (2, TCA), tranylcypromine (3, MAOI), and fluoxetine (4, SSRI; see Figure 1 for chemical structures).7−9
Figure 1.
Chemical structures of tianeptine and selected FDA-approved antidepressants.
Tianeptine (1, Figure 1), first synthesized in 1971 by the Science Union Et Cie, Societe Francaise de Recherche Medicale as an analog of the recently discovered TCAs, is something of a mechanistic outlier compared to the other major classes of antidepressants.10 Unlike SSRIs and structurally related TCAs, tianeptine was initially shown to enhance uptake of 5-HT in vivo without apparent activity at any serotonin receptors or monoamine transporters, although its mechanism of action may well be unrelated to serotonergic neurotransmission (see the pharmacology sections for a more detailed discussion).11
Tianeptine (brand names include Coaxil, Stablon, and Tatinol) is now used as an antidepressant in over 60 countries around the world (predominantly in Europe, Asia, and South America) and is not currently approved for use in the United States (among other countries including Canada and Australia).12,13 Despite this lack of FDA approval, tianeptine is easily obtained in gas stations and convenience stores and through online retailers. The compound is commonly sold through these channels under names including, but not limited to, Zaza, Tia, Tianna, Zaza Red, TD Red, and Pegasus; tianeptine is also colloquially known as “gas station heroin”.14 Recreational misuse of tianeptine can lead to opioid-like symptoms, such as an increase in dopamine levels, addiction, and respiratory depression.15,16 Cases of accidental overdose and intentional use in suicide have also been documented,17 and an overall increase in cases of tianeptine use has been reported worldwide, particularly in the United States and Europe. In the United States, the National Poison Data System (NPDS) reported 11 tianeptine exposure calls in the years between 2000 and 2017 and 207 calls between 2014 and 2017, and the FDA reported 151 cases in 2020.18−20 Due to this increase, some states have banned products containing tianeptine and have designated it a Schedule I or II controlled substance.21
Chemical Properties and Synthesis
Chemical Properties
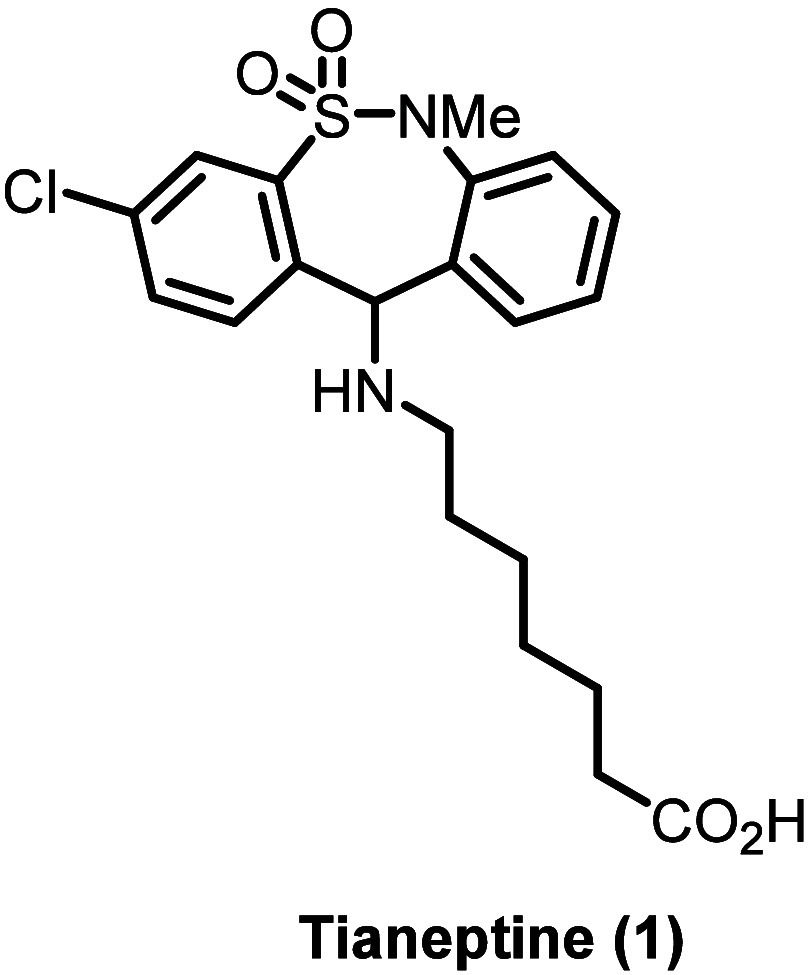
Tianeptine (CAS no. 72797-41-2; IUPAC: 7-[(3-chloro-6-methyl-5,5-dioxido-6,11-dihydrodibenzo[c,f][1,2]thiazepine-11-yl)amino]heptanoic acid, C21H25ClN2O4S) has an interesting tricyclic structure with a resemblance to that of other TCAs. It differs from the conventional TCAs, however, by the seven-membered sultam ring system and carboxylic acid tail (Scheme 1). Tianeptine has two hydrogen bond donors and six hydrogen bond acceptors and has a molecular weight of 436.953 g/mol. Tianeptine is amphoteric (pKa = 4.4 (acidic) and pKa = 6.86 (basic)) with a logP of 1.06 at pH 7.4, making the molecule compatible with Lipinski’s rule of five.22,23 Tianeptine (as the free carboxylic acid) has a melting point of 144–147 °C and is a white powder, while the sodium salt has a melting point of 148 °C and is generally encountered as a white to pale yellow powder.24,25 Tianeptine as well as the sodium salt (CAS no. 30123-17-2) are commercially available from a variety of chemical vendors.
Scheme 1. Initial Synthesis of Tianeptine (Top), and Modification of the Synthesis for Improved Yield (Bottom).
An X-ray structure of the hydrochloride salt revealed the seven-membered sultam ring adopts a boat-type conformation, and a dihedral angle between the mean planes of the benzene rings of 44.44(7)° was observed. Interestingly, an intramolecular hydrogen bond between a sultam oxygen and the amino nitrogen was also observed (Figure 2).26
Figure 2.
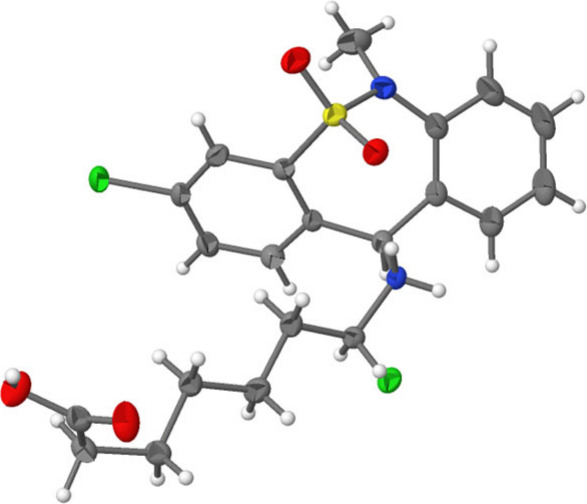
X-ray crystal structure of tianeptine hydrochloride. Reprinted with permission from ref (26). Copyright 2012 IUCr Journals.
Synthesis
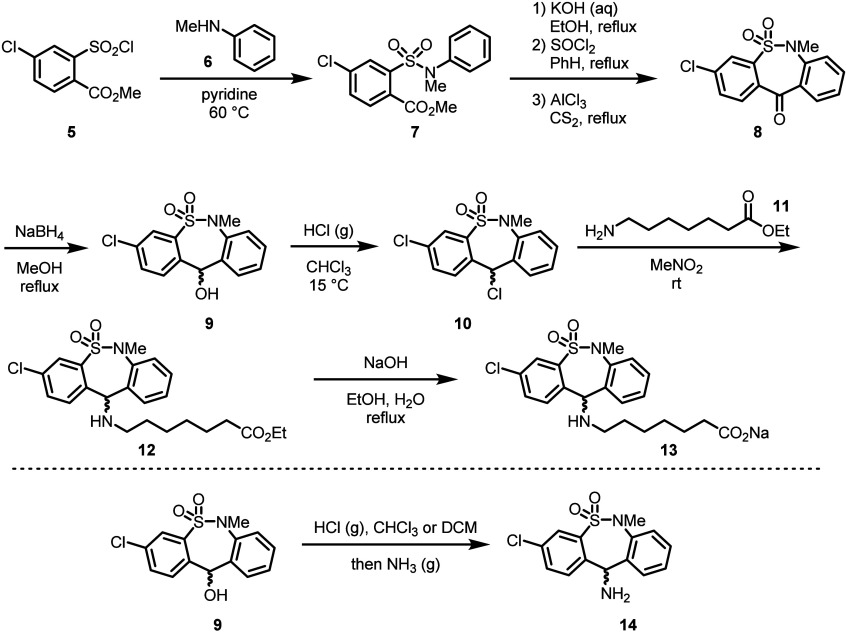
The initial synthesis of the tianeptine core was reported in 1970 by the Science Union Et Cie, Societe Francaise de Recherche Medicale. A separate synthesis of the tail component was disclosed in an additional patent in 1971.10,27,28 In the first step of the core synthesis, nucleophilic substitution with aniline 6 onto the aryl sulfonyl chloride 5 formed the corresponding aryl sulfonamide.29,30 Then, hydrolysis of the ester, chlorination to give the intermediate acyl chloride, and finally Friedel–Crafts acylation resulted in tricyclic intermediate 8. Reduction of the ketone and chlorination gave the tianeptine core 10 as the secondary alkyl chloride. Finally, nucleophilic substitution using ethyl 7-aminoheptanoate (11) and ester hydrolysis afforded tianeptine as the sodium salt (13).10
Various incremental improvements have been made to the synthesis of tianeptine to increase the yield and purity; next-generation efforts have primarily focused on the ethyl amino heptanoic acid tailpiece due to the instability of ethyl 7-aminoheptanoate in the reaction medium. Direct addition of the amine moiety to the tianeptine core to give intermediate 14 provided much higher yield and purity for large-scale synthesis.31,32 In addition, various functional group interconversions (FGIs) have been established to install the heptanoic acid tail in an efficient manner.33
Medicinal Chemistry and SAR
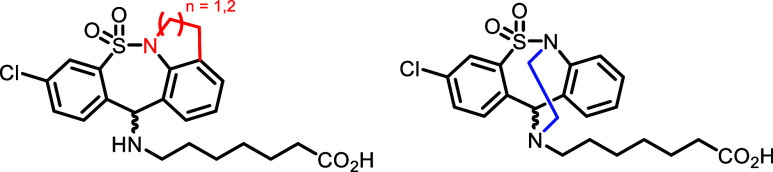
In recent literature, analogues of tianeptine have been synthesized from the Directorate of Drug Substance Development, Egis Pharmaceuticals Plc. with focus on modifications to the N-methyl moiety.34,35 The aim was to modify the N-methyl moiety to block N-demethylation, a known metabolic pathway for tianeptine (see the Drug Metabolism and Pharmacokinetics section for further details). Thus, tetracyclic analogues linking the sultam nitrogen to the aromatic ring (red) and amine tail (blue) were synthesized in turn (Figure 3).
Figure 3.
Selected recent examples of known modifications to the tianeptine scaffold.
Due to the structural similarities between tianeptine and the TCAs, many of the SAR investigations for tianeptine have been reported as comparison studies with the latter, despite likely differences in therapeutic mechanisms. Because of the large numbers of reported TCA core scaffolds, there has historically been some difficulty in defining a precise set of SAR generalizations with respect to target potency. While unique SARs for specific TCAs have been studied, grouping multiple TCAs into one SAR model can be misleading due to the variety of biochemical mechanisms/pathways of these clinical TCAs.
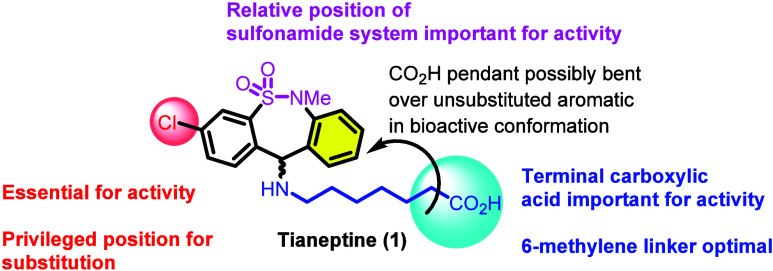
Regardless, an initial study of tianeptine SAR was conducted with an eye toward historical TCAs, specifically an examination of the reversal of reserpine-induced ptosis potency in mice.36 The study focused primarily on four structural aspects of tianeptine: (1) the carbon count of the heptanoic acid side chain, (2) the terminal carboxylic acid functional group, (3) the chlorine substituent on the aromatic ring, and (4) the nature of the tricyclic system. The results of each of these separate scaffold modifications are summarized as follows.
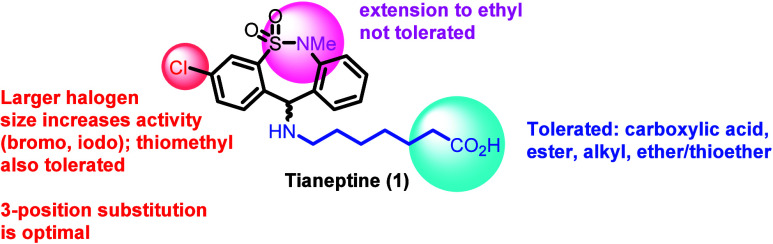
(1) The optimal length of the heptanoic acid side chain was found to be 6 methylene linkers (as in the structure of tianeptine itself; deviation of this count by either adding or removing carbon spacers was found to be detrimental). Additionally, the installation of methyl groups to the side chain did not appreciably change activity. (2) The carboxylic acid functional group was shown to be crucial for tianeptine’s activity in this context. Alkyl chains and imipramine-like tertiary amine tails were not tolerated. (3) The position of aromatic ring substitutions was also found to be important for activity in this context; aromatic substitution at C3 was found to be essential for maintenance of activity. Various substitutions for chlorine were also examined at the C3 position, but chlorine itself was ultimately found to have the optimal activity. Additionally, because of the loss of activity observed by adding substituents to C9 (eastern aromatic ring, as drawn), it was hypothesized that the side chain is bent toward the unsubstituted aromatic ring in the relevant bioactive conformation, a proposal that has been corroborated by variable-temperature NMR studies.37 (4) An electron-donating heteroatom at C5 (the sulfonamide S atom) is required for activity (see Figure 4 for summarized findings).
Figure 4.
Tianeptine SAR from reserpine-induced ptosis.
After the finding that tianeptine has agonist activity at both the mu and the delta opioid receptors (MOR and DOR, see the pharmacology sections for further details), an additional study on the SAR of tianeptine was performed by screening for activity at the three human opioid receptor subtypes (MOR, DOR, and the kappa opioid receptor (KOR)) using a G protein BRET assay.38 Based on recent X-ray crystal structures and 3D modeling, the sulfonamide functional group was theorized to be important in this context as well.26,39 As in the previous SAR study, tianeptine analogs were generated with a primary focus on structural modifications to the side chain and substitutions on the aromatic core.38
In contrast to the antireserpine-based SAR, a variety of modifications to the side chain were tolerated with respect to MOR activity (tianeptine itself is only very weakly DOR active, and the majority of analogs are either similarly weak or inactive). It was found that ester analogs of both tianeptine and one of its primary metabolites, MC5 (see the Drug Metabolism and Pharmacokinetics section for additional structural details), showed only a slight reduction in MOR potency compared to the parent analogs. Additionally, although both tianeptine and metabolite MC5 were found to be active at MOR, MC5 was found to be inactive at DOR. If MC5 plays any role in the antidepressant effects of tianeptine, it is therefore unlikely that these contributions are DOR-mediated. A variety of alkyl and ether/thioether functional groups displayed similar or only slightly diminished MOR potencies compared to tianeptine; however, the MOR potencies of the alcohol and aromatic derivatives were robustly diminished. Additionally, and in contrast to the reserpine study, larger halogens at the 3 position (bromo, iodo) were found to improve activity relative to the chloro substitution in the context of multiple side chains. Substitutions at other positions on the aromatic ring decreased activity; as in the previous reserpine-induced ptosis SAR study, the 3 position was shown to be a privileged position for functionalization on the aromatic rings. Extensions of the sulfonamide N-substitution (N-ethyl) were counterproductive (see Figure 5 for summarized findings).38
Figure 5.
Summary of SAR findings for tianeptine and analogs at the MOR.
Tianeptine is typically encountered and prescribed as a racemic mixture of enantiomers at the side chain linkage position, and at present, it is unclear how each enantiomer differentiates with respect to opioid receptor binding (preliminary docking experiments suggest only marginal differences in MOR binding).38 However, in the context of classical 5-HT-induced behaviors in rodents, the (−)-enantiomer appears to be responsible for the increased neuronal uptake of 5-HT in vivo.40,41 The (+)-enantiomer, in contrast, was found to have marginal activity but did not appear to significantly inhibit the activity of the (−)-enantiomer. Interestingly, the “less active” (+)-enantiomer has been described as a potential treatment for memory and cognitive disorders due to its high bioavailability relative to the racemate and its lack of effect on serotonergic transmission, although further studies are certainly needed to shed light on the medicinal chemistry and pharmacology of each enantiomer. Additionally, the absolute stereochemistry of each enantiomer has yet to be defined.31,38,42
Drug Metabolism and Pharmacokinetics
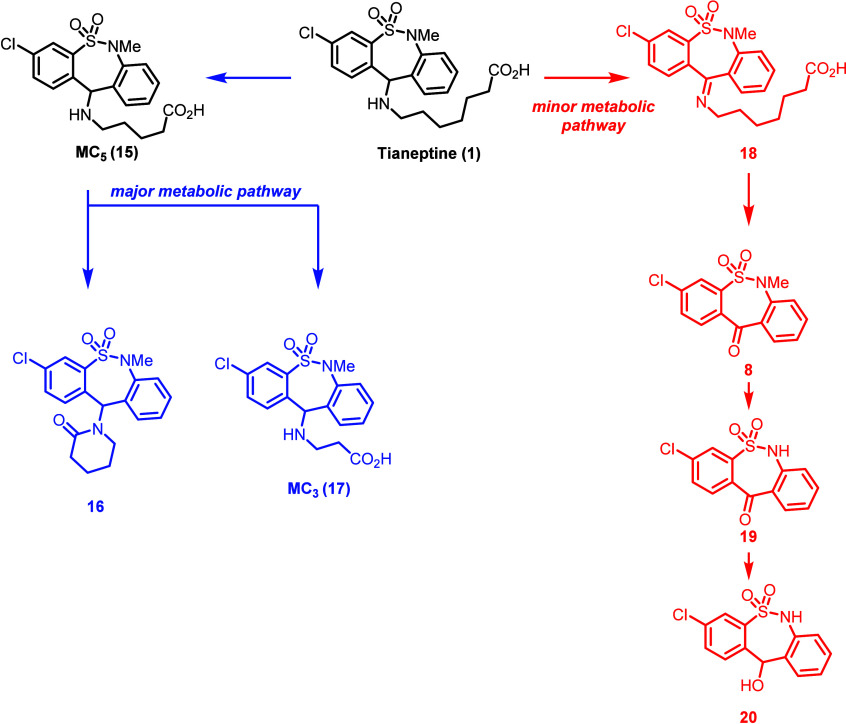
Prescription tianeptine is administered orally. Unlike conventional TCA metabolism, tianeptine is not primarily metabolized by cytochrome P450s (CYPs), although CYP-mediated generation of reactive covalent metabolites is known.43−50 In one study using [14C]-labeled tianeptine administered to six healthy male volunteers, two primary routes of metabolism were observed (Scheme 2).51 The major metabolic pathway observed for tianeptine was β-oxidation of the heptanoic acid tail to a pentanoic acid tail (MC5, 15). Metabolite MC5 underwent further β-oxidation to give a propionic tail (MC3, 17). Lactonization of the MC5 pentanoic tail was also observed, forming the corresponding δ-lactam compound 16. A minor metabolic pathway for tianeptine was found to be oxidation of the amine tail to give imine 18 followed by imine hydrolysis to ketone 8 (an intermediate in tianeptine synthesis), N-dealkylation of the methylsulfonamide to give 19, and reduction of the ketone to give alcohol 20. Tianeptine has been found to be extensively metabolized via the major β-oxidation pathway; 24 h after administration, the parent molecule accounted for less than 3% of the administered dose in urine with the 3 primary metabolites all products of the β-oxidation pathway. In feces and plasma samples, the metabolite profile was found to be comparable. Similar metabolic pathways have been observed in other species.52
Scheme 2. Metabolic Pathways of Tianeptine.
After oral ingestion of a single dose of tianeptine sodium salt (12.5 mg) to 12 healthy volunteers (6 men and 6 women), the average peak plasma level (Cmax) was found to be 334 ± 79 ng/mL with a Tmax of 0.94 ± 0.47 h. The absorption half-life was 0.19 ± 0.14 h, and the terminal half-life was 2.5 ± 1.1 h. Compared to tianeptine, the average peak plasma level for major metabolite MC5 was lower (63 ± 14 ng/mL) with a Tmax of 2.23 ± 0.42 h and a plasma elimination half-life of 7.2 ± 5.7 h. The average systemic bioavailability of tianeptine after oral administration was high in fasting young healthy subjects (99%, with no single measured value lower than 66%).53 In contrast to other TCAs, tianeptine is rapidly absorbed after administration without an extensive first-pass effect.53−57 The same trend for relative Cmax, Tmax, and terminal half-life values for both tianeptine and MC5 was observed in rats after chronic (i.p.) administration.43,58 The influence of food on tianeptine absorption has also been studied; when tianeptine was given at the end of a meal, absorption was slightly delayed (with lower Cmax and higher Tmax values), although this influence was found to be marginal from a clinical standpoint.59 Additionally, alcohol consumption resulted in a modest reduction in absorption rate for the parent compound (30% reduction in peak plasma concentration, with a ∼20 min delay in achieving Tmax) but did not significantly affect plasma levels of MC5.53
Pharmacodynamics
Early experiments suggested that tianeptine’s mechanism of action was serotonergic, although the observed enhancement of 5-HT uptake was directly opposed to the uptake inhibition associated with the SSRIs. Both acute and repeated doses of tianeptine were observed to increase 5-HT uptake without affecting the release, binding, or uptake of an array of neurotransmitters (acetylcholine, dopamine, epinephrine, GABA, glutamate, histamine, norepinephrine) along with an apparent lack of amine-oxidase activity.60−63 The finding that 5-HT uptake inhibitors (classical TCAs) and putative serotonin uptake enhancers (tianeptine) both show antidepressant activity continues to challenge the monoamine hypothesis of depression. Indeed, follow-up studies have suggested that an enhancement of serotonin uptake may not be the main driver of tianeptine’s antidepressant effects. Specifically, imipramine and tianeptine were found to have different actions on 5-HT2 and 5-HT1A receptors, but both decreased [3H]-paroxetine binding to 5-HT transporter sites, suggesting a different mechanism for the two drugs independent of their effects on 5-HT reuptake.64 In addition, an in vivo microdialysis study showed that tianeptine did not change extracellular concentrations of 5-HT ([5-HT]ext), in contrast to the previous studies in which tianeptine inhibited the K+-induced increase of [5-HT]ext in the ventral hippocampus and frontal cortex.65−67 This apparent discrepancy could imply limited resources with which to detect 5-HT in the initial studies, and a growing body of evidence suggests that an involvement of 5-HT in the efficacy of tianeptine is unlikely.68
Hypotheses on tianeptine’s mechanism of action have gradually shifted from serotonergic to glutamatergic. Specifically, tianeptine may be acting by influencing the expression of synaptic plasticity via the modulation of glutamatergic transmission and is known to modulate the phosphorylation state of glutamate receptors.69,70 In one study, long-term tianeptine administration was examined at hippocampal CA3 commissural associational (c/a) glutamate receptor ion channels (NMDA and AMPA) and was found to normalize the amplitude ratio of NMDA to AMPA/kainate receptor-mediated currents and prevent the stress-induced attenuation of NMDA-excitatory postsynaptic currents. This effect was attenuated by administration of a kinase inhibitor, suggesting that tianeptine acts via a postsynaptic phosphorylation cascade at the CA3 c/a synapse.71 Follow-up studies have corroborated both AMPA involvement and a postsynaptic site of action, and specific inhibitors for protein kinase A (PKA) and calmodulin-dependent protein kinase II (CaMKII) indicated that both kinases are important players in tianeptine’s effect on AMPA responses.72
Tianeptine has also been proposed to act as an agonist of the adenosine A1 receptor (A1R),73 although no direct A1R activity was observed in calcium functional assays.74 A screen of tianeptine across a broad panel of CNS receptors (>50 targets, Psychoactive Drug Screening Program, University of North Carolina) revealed the mu opioid receptor (MOR) as the only hit at a 10 μM screening concentration. In follow-up concentration–response experiments, tianeptine was found to be an agonist for both human and mouse MOR with weaker activity at the delta opioid receptor (DOR) and no appreciable activity for the kappa opioid receptor (KOR) (see Table 1).74 The compound is best characterized as a moderately potent but highly efficacious and selective MOR agonist (Emax > 100% relative to DAMGO).74,75 In mice, using a combination of knockout (KO) studies and pharmacological inhibition (naloxone), both the analgesic and the antidepressant effects of tianeptine were found to be MOR dependent. These results validate the involvement of MOR as an important CNS target for tianeptine and suggest that the receptor is at least partially involved in its antidepressant efficacy. Metabolite MC5, which is an approximately 3-fold weaker agonist at both human and mouse MOR in functional assays, mimicked the behavioral effects of tianeptine in a MOR-dependent manner. The human MOR potency for metabolite MC3 is very weak (EC50 = 16 μM) and is therefore unlikely to play a role in the observed behavioral effects. In the same study, tianeptine was not found to display the tolerance or withdrawal behaviors associated with classical opioids in mice, raising the intriguing possibility that a MOR-preferring antidepressant may be relatively safe and well tolerated.76 Another study suggested that tianeptine may have a lower potential to cause tolerance and dependence in rodents compared to other classical opioids of abuse while still noting MOR agonist-like acute adverse effects (constipation, respiratory depression, etc.).77 Case studies involving robust withdrawal symptoms after recreational use, however, cast doubt on the translatability of these findings to human subjects.
Table 1. Opioid Receptor Binding and Efficacy Data for Tianeptinea.
Adverse Effects and Dosage
The typical dosage of tianeptine is 12.5 mg/day three times a day for adults and 25 mg/day for the elderly.78 In clinical studies, a relative lack of adverse effects relative to classical TCAs has been observed.79 While reductions in attention and memory are common adverse effects associated with the use of TCAs, clinical studies with tianeptine have shown that these cognitive functions are largely unaffected.80−82 Adverse effects on sleep quality are known but largely context dependent; one study using a single dose of tianeptine (12.5 mg) reported more subjects with restless sleep versus the placebo, but another study using higher doses (37.5 mg) found sleep was more effective with tianeptine.79,81 In elderly populations, tianeptine had an advantage over mianserin, a tetracyclic antidepressant (TeCA), in terms of decreasing risk of falling and impaired vigilance.83 Additionally, another study comparing tianeptine with mianserin showed that average tianeptine doses did not have a measurable effect on driving skills.84
Adverse effects have also been observed through unregulated use of tianeptine and include nausea, vomiting, and abdominal pain.85 The case reports on tianeptine abuse vary greatly from the clinical studies and are difficult to compare due to the extremely high doses typically ingested (from 87.5 mg to 10 g).86,18 At least two cases of reported suicide associated with tianeptine use are known and further underscore the danger of the drug when taken at abnormally high doses.87,88 Further issues related to tianeptine overdose will be discussed in the Current Issues and Concerns section.
History and Importance in Neuroscience
As a microcosm for the transition from serotonergic, to glutamatergic, to other hypotheses of depression (opioid and beyond), the tianeptine story is a fascinating case study. In the early history of antidepressants, it was thought that the TCA mechanism of action involved the uptake inhibition of either or both noradrenaline and serotonin.89 That tianeptine appeared to directly contradict this hypothesis while still providing relief from depression symptoms sparked renewed interest in untangling the root causes of depression and related mood disorders.
Tianeptine (Stablon) has been marketed by Laboratoires Servier in France since 1989 (approximately 20 years after the disclosure of its synthesis) and is now available in 15 countries in the European Union (France, Luxembourg, Portugal, Bulgaria, Romania, Slovakia, Poland, Malta, Hungary, Lithuania, Slovenia, Czech Republic, Austria, Latvia, and Estonia).90 In the decades following tianeptine’s initial launch, several countries outside the EU have approved the drug for marketing (e.g., Singapore in 1999 through license no. SIN11182P),91 and tianeptine is now available in at least 66 countries around the world.90 Tianeptine has never been marketed in the United States for reasons that predate research into its potential for abuse; the projected profits for the drug were not expected to outweigh the cost for running a clinical trial.92 As of 2024, however, a clinicaltrials.gov search for “tianeptine” yields 9 total studies examining the utility of tianeptine (alone or as a combination therapy) across a diverse range of indications including treatment-resistant depression, bipolar depression, postmastectomy pain after breast cancer surgery, and brain fog symptoms related to COVID-19.93 Additional reports have also suggested that the utility of tianeptine may extend beyond depression treatment. Tianeptine has demonstrated significant efficacy as an anxiolytic with one study noting that both tianeptine and paroxetine, an SSRI, were similarly efficacious in the 35% CO2 panic challenge (administration of a harmless mixture of 35% CO2/65% O2 gas which stimulates panic through chemoreceptor-mediated CO2 sensitivity).94 Tianeptine has also demonstrated efficacy as a treatment for asthma, irritable bowel syndrome (IBS), convulsions, fibromyalgia, and attention-deficit hyperactivity disorder (ADHD).95−99
In 2014, researchers at Columbia University demonstrated that tianeptine is an agonist for the MOR74,76 and accordingly produces significant antinociception in rodent pain models (corroborating similar findings conducted prior to a definitive understanding that the antinociceptive effects were MOR mediated).100 It is well established that MOR activation can lead to drug tolerance, dependence and abuse,101 and these issues were observed with tianeptine use prior to this mechanistic finding. In 2012, the Haute Autorité de Santé (French National Authority for Health) published a document aiming to reassess the benefit/risk ratio of tianeptine (Stablon) considering increasing reports of abuse and drug dependence in France. Specifically, in 2005, Stablon underwent an addiction vigilance survey which led to the addition of a warning about the risk of abuse and dependence in patient leaflets. In 2011, the National Narcotics and Psychotropics Committee found “persisting cases of tianeptine abuse and drug dependence and requested that the benefit/risk ratio of the substance be re-assessed.”90 As another metric to assess the abuse potential of a given substance, “doctor shopping” (the simultaneous consultation of multiple physicians) has also been associated with tianeptine in France.102 Ultimately, the benefit/risk ratio for tianeptine was found to be positive, although the criteria for prescribing and dispensing the drug in France have been tightened.90
Although tianeptine is not approved by the FDA, similar assessments are actively ongoing in the United States, where tianeptine is widely available through retailers as an unregulated supplement. Currently, outright bans on tianeptine have been enacted in at least 9 states, where the drug is officially regulated as either a schedule I or a schedule II substance.103 In Tennessee, bill HB2043 (2022) added tianeptine and “any salt, sulfate, free acid, or other preparation of tianeptine, and any salt, sulfate, free acid, compound, derivative, precursor, or preparation thereof that is substantially chemically equivalent or identical with tianeptine, as a schedule II controlled substance”.104 Reassessments of the risk associated with tianeptine extend beyond the United States and France. In Turkey, where tianeptine can be obtained without a prescription, citizens of Georgia buy the drug frequently in large quantities, and tianeptine is often referred to as “the Georgian drug” in the region (where intravenous administration is also common).105
Current Issues and Concerns
Prior to 2014, few cases of tianeptine abuse were known, but reports have been increasing steadily. The FDA Center for Food Safety and Applied Nutrition (CFSAN) Adverse Event Reporting System (CAERS) received more such reports in 2022 than in the previous 3 years combined.106 These incidents of abuse are typically encountered either as acute effects from drug withdrawal or as death from an overdose (whether intentional or unintentional). The opioid antagonists naloxone and/or buprenorphine are often effective for overdose prevention, and several uses of this strategy have been documented.107−109 Recently, Tonix Pharmaceuticals patented a tianeptine oxalate/naloxone combination for the treatment of depression with a minimization of abuse potential.110
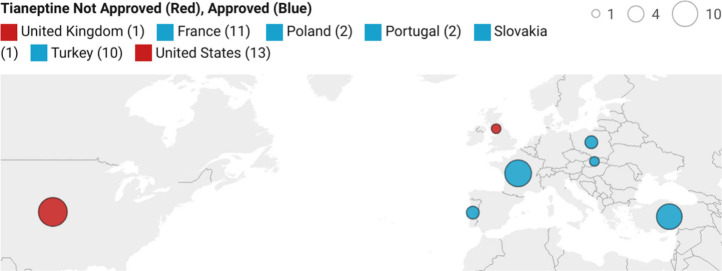
In the United States, tianeptine is part of a billion dollar nootropic supplement industry and can be easily obtained in many states despite explicit warnings from the FDA against its sale and distribution (see Figure 6).111,112 Although tianeptine is well recognized to be an opioid by recreational users, the drug is often perceived on social media as being less dangerous than other opioids or substances of abuse and is taken in doses far beyond a typical prescription.113 Literature case reports paint a similar picture. In 2018, Lauhan and colleagues disclosed a thorough meta-analysis of tianeptine abuse case studies, collated through PubMed searches for “tianeptine abuse” and “tianeptine dependence”. These searches yielded 25 articles describing 65 total patients (of which more than 80% were outside the United States) taking an average dose of ∼2000 mg daily (nearly 100× times the upper dosages of typical prescriptions). Excluding the 24 patients from unreported locations in eastern Europe, the breakdown of abuse cases by specific country is summarized in Figure 7. A range of withdrawal symptoms and complications is described in these studies, and in many instances, tianeptine was taken concurrently with other substances.114
Figure 6.

Examples of products containing tianeptine. Such products are often labeled in a misleading manner; see the “turmeric supplement” on the right-hand side. Photos courtesy of the FDA’s Office of Regulatory Affairs, Health Fraud Branch.
Figure 7.
Global case reports of tianeptine abuse.114
In one case study from the United States, a 28-year-old male was discovered unresponsive and lying on the floor of his residence. Despite a history of alcohol, tobacco and illicit drug use, he had reportedly been clean for “some time”, and indeed, a toxicology report indicated no significant evidence for substances other than tianeptine in his blood. Tianeptine itself was reported at a concentration of 2.0 mg/L, and while this number is somewhat lower than other reported fatal tianeptine concentrations, the autopsy findings of pulmonary edema and urinary retention are “suggestive of a medication toxicity with respiratory depression”. Significant amounts of metabolite MC5 were also identified in the TOF spectrum.115 These results suggest that tianeptine intoxication was the primary contributor to the cause of death.
It can be difficult to reconcile the reports of abuse and overdose with the encouraging clinical outcomes often observed for tianeptine monotherapy. In one such study, a 72-year-old female reported a long history of depression symptoms (>25 years) with >5 previous antidepressants all failing to give a positive result. This patient was given tianeptine at 12.5 mg/day for 2 years with no additional treatments, and her HDMS score improved from 28 (severe depression) to 6 (normal) over the course of the study with remission lasting out to at least 2 additional years.116 A subsequent report supports the idea that a MOR agonist may indeed be an effective treatment for major depressive disorder provided that the compound is administered alongside a MOR antagonist.117
The contrast between these case studies highlights two of the most fundamental yet often neglected tenets of medicine and toxicology: (1) that the importance of context cannot be overstated (the mindset, environment, and physiological disposition of the patient) and (2) that no compound is intrinsically toxic or nontoxic; toxicity is a function of dose.
Conclusion
The discrepancy between tianeptine as a well-tolerated depression treatment and as a recreationally abused opioid has never been more apparent. From a basic research standpoint, tianeptine remains a useful and underutilized tool with which to (1) understand how a mechanistically differentiating TCA can elicit a positive signal in classical models of depression and (2) to specifically understand the contributions of glutamate neurotransmission and/or the MOR in these models. Structurally, the tianeptine scaffold is conducive to creative medicinal chemistry, and the pharmacology of new synthetic analogs remains a fascinating prospect (recently, structural analogs of tianeptine have been reported as class I histone deacetylase (HDAC) inhibitors).118 Additionally, the finding that coadministration of a MOR antagonist (samidorphan) alongside a MOR agonist (buprenorphine) can be an effective treatment for depression in patient populations raises the intriguing possibility of other coadministration or mixed-efficacy approaches.117 For example, MOR agonists with concomitant DOR antagonism as part of their polypharmacology have been explored as a viable strategy to attenuate the negative side effects associated with pure MOR agonists,119−121 although it remains to be seen how such an approach might fare in the context of depression.
Conversely, a disturbing increase in reports of recreational use, particularly in Eastern Europe and the United States, has challenged the case for the drug’s widespread availability and less restrictive scheduling. That the same molecule can be encountered as a legal antidepressant in one country and as “gas station heroin” in another speaks to the pressing need for the dissemination of accurate drug information (regardless of any stances on regulatory approval). It is our hope that this need continues to be met for tianeptine as well as for any compound with a complicated neuroscientific history.
Acknowledgments
The authors thank the William K. Warren Family and Foundation for funding the William K. Warren, Jr. Chair in Medicine and support of our programs.
Author Contributions
Y.N. and A.M.B. researched and wrote the manuscript with additional input and editing from C.W.L.
The authors declare no competing financial interest.
References
- World Health Organization. Depressive disorder (depression); https://www.who.int/news-room/fact-sheets/detail/depression (accessed 2024–03–11).
- Aarsland D.; Påhlhagen S.; Ballard C. G.; Ehrt U.; Svenningsson P. Depression in Parkinson Disease—Epidemiology, Mechanisms and Management. Nat. Rev. Neurol. 2012, 8, 35–47. 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- Ciprandi G.; Schiavetti I.; Rindone E.; Ricciardolo F. L. M. The Impact of Anxiety and Depression on Outpatients with Asthma. Ann. of Allergy Asthma Immunol. 2015, 115, 408–414. 10.1016/j.anai.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Coppen A. The Biochemistry of Affective Disorders. Br. J. Psychiatry. 1967, 113, 1237–1264. 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- Schildkraut J. J. Neuropsychopharmacology and the Affective Disorders. N. Engl. J. Med. 1969, 281, 248–255. 10.1056/NEJM196907312810506. [DOI] [PubMed] [Google Scholar]
- Lingjærde O. The Biochemistry of Depression: A Survey of Monoaminergic, Neuroendocrinological, and Bio-rhythmic Disturbances in Endogenous Depression. Acta. Psychiatr. Scand. 1983, 67, 36–51. 10.1111/j.1600-0447.1983.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Kocsis J. H. Imipramine Treatment for Chronic Depression. Arch. Gen. Psychiatry 1988, 45, 253. 10.1001/archpsyc.1988.01800270071008. [DOI] [PubMed] [Google Scholar]
- Frieling H.; Bleich S. Tranylcypromine: New Perspectives on an “Old” Drug. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 268–273. 10.1007/s00406-006-0660-8. [DOI] [PubMed] [Google Scholar]
- Gilaberte I.; Montejo A. L.; De La Gandara J.; Perez-Sola V.; Bernardo M.; Massana J.; Martin-Santos R.; Santiso A.; Noguera R.; Casais L.; Perez-Camo V.; Arias M.; Judge R. Fluoxetine in the Prevention of Depressive Recurrences: A Double-Blind Study. J. Clin. Psychopharmacol. 2001, 21, 417–424. 10.1097/00004714-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Malen C.; Danree B.; Poignant J. C.. New Tricyclic Derivatives and Process for Their Manufacture. FR2104728A1, 1971.
- Fattaccini C. M.; Bolaños-Jimenez F.; Gozlan H.; Hamon M. Tianeptine Stimulates Uptake of 5-Hydroxytryptamine in Vivo in the Rat Brain. Neuropharmacology 1990, 29, 1–8. 10.1016/0028-3908(90)90076-4. [DOI] [PubMed] [Google Scholar]
- Galust H.; Seltzer J. A.; Hardin J. R.; Friedman N. A.; Minns A. Tianeptine Abuse via Novel, Extended-Release, Star-Shaped, Drug Delivery Device. Toxicol. Rep. 2023, 11, 162–164. 10.1016/j.toxrep.2023.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration Memorandum . Regulatory status and review of available information pertaining to Tianpetine: lack of general recognition of safety for its use in conventional foods; U.S. Food & Drug Administration, 2018; https://www.fda.gov/media/175867/download (accessed 2024–08–07).
- New Jersey Department of Health . Health Alert: Tianeptine or “Gas Station Heroin”; https://www.nj.gov/health/populationhealth/documents/healthalerttianeptine.pdf (accessed 2024–03–11).
- Sacchetti G.; Bonini I.; Waeterloos G. C.; Samanin R. Tianeptine Raises Dopamine and Blocks Stress-Induced Noradrenaline Release in the Rat Frontal Cortex. Eur. J. Pharmacol. 1993, 236, 171–175. 10.1016/0014-2999(93)90586-7. [DOI] [PubMed] [Google Scholar]
- Invernizzi R.; Pozzi L.; Garattini S.; Samanin R. Tianeptine Increases the Extracellular Concentrations of Dopamine in the Nucleus Accumbens by a Serotonin-Independent Mechanism. Neuropharmacology 1992, 31, 221–227. 10.1016/0028-3908(92)90171-K. [DOI] [PubMed] [Google Scholar]
- Edinoff A. N.; Sall S.; Beckman S. P.; Koepnick A. D.; Gold L. C.; Jackson E. D.; Wenger D. M.; Cornett E. M.; Murnane K. S.; Kaye A. M.; Kaye A. D. Tianeptine, an Antidepressant with Opioid Agonist Effects: Pharmacology and Abuse Potential, a Narrative Review. Pain. Ther. 2023, 12, 1121–1134. 10.1007/s40122-023-00539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffa J. M.; Stork C. M.; Hoffman R. S.; Su M. K. Poison Control Center Experience with Tianeptine: An Unregulated Pharmaceutical Product with Potential for Abuse. Clin. Toxicol. 2018, 56, 1155–1158. 10.1080/15563650.2018.1476694. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. Tianeptine; 2024; https://www.deadiversion.usdoj.gov/drug_chem_info/tianeptine.pdf (accessed 2024–03–12).
- U.S. Food & Drug Administration . Tianeptine Products Linked to Serious Harm, Overdose, Death; https://www.fda.gov/consumers/consumer-updates/tianeptine-products-linked-serious-harm-overdoses-death (accessed 2024–03–12).
- Michigan Legislature. Senate Bill 801 of 2018 (Public Act 107 of 2018), 2018; https://www.legislature.mi.gov/Bills/Bill?ObjectName=2018-SB-0801 (accessed 2024–03–12).
- Zini R.; Morin D.; Salvadori C.; Tillement J. Tianeptine Binding to Human Plasma Proteins and Plasma from Patients with Hepatic Cirrhosis or Renal Failure. Br. J. Clin. Pharmacol. 1990, 29, 9–18. 10.1111/j.1365-2125.1990.tb03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings 1PII of Original Article: S0169–409X(96)00423–1. (The Article Was Originally Published in Adv. Drug Delivery Rev.1997, 23, 3–25.). Adv. Drug Delivery Rev. 2001, 46 (1–3), 3–26. 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Santa Cruz Biotechnology. Tianeptine Certificate of Analysis; https://datasheets.scbt.com/coa/sample/sc-213044_SAMPLE.pdf (accessed 2024–03–15).
- Santa Cruz Biotechnology. Safety Data Sheet Tianeptine Sodium Salt SC-204345; https://datasheets.scbt.com/sds/aghs/en/sc-204345.pdf (accessed 2024–03–15).
- Mishnev A.; Zvirgzdins A.; Actins A.; Delina M. 7-[(3-Chloro-6-methyl-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl)amino]-heptanoic acid S,S-Dioxide Hydrochloride. Acta Crystallogr. Sect. E. Struct. Rep. Online 2012, 68 (11), o3136. 10.1107/S1600536812042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malen C.; Laubie M.. New 5-Chloro-10-Dioxo-11-Alkyl-Dibenzo (c,f) Thiazepine (1,2) Derivatives and process for preparing them. GB1179109, 1970.
- Malen C.; Danree B.; Poignant J. C.. Tricyclic Compounds. US3758528A, 1973.
- Meerwein H.; Dittmar G.; Göllner R.; Hafner K.; Mensch F.; Steinfort O. Untersuchungen Über Aromatische Diazoverbindungen, II. Verfahren Zur Herstellung Aromatischer Sulfonsäurechloride, Eine Neue Modifikation Der Sandmeyerschen Reaktion. Chem. Ber. 1957, 90, 841–852. 10.1002/cber.19570900602. [DOI] [Google Scholar]
- Sean O. F.Method of Preparing Ortho Sulfonyl Chloride Benzoic Acid Esters. US2667503A, 1954.
- Kamoun A.; Delalleau B.; Deslandes A.. Use of a Tricyclic Derivative for Producing a Medicament for the Treatment of Cognitive and Memory Disorders. EP0671173A1, 1995.
- Blanchard J.; Turbe H.; Brigot D.. Process For the Preparation of 11-Amino-3-Chloro-6, 11- Dihydro-5,5-Dioxo-6-Methyl-Dibenzo[c,f][1,2]Thiazepine and Application to the Synthesis of Tianeptine. US6441165B2, 2002.
- Rangisetty J. B.; Pullagurla M. R.; Bhudeti R.. Novel Process for the Preparation of 7-((3-Chloro-6-methyl-5,5-dioxo-6,11-dihydrodibenzo(c,f)(1,2)thiazepine-11-yl)amino)heptanoate. WO2010/070667A2, 2010.
- Berecz G.; Dancsó A.; Lauritz M. T.; Simig G.; Volk B. Synthesis of Bridged Analogues of the Antidepressant Drug Tianeptine. Representatives of a New Ring System. Tetrahedron 2023, 134, 133300 10.1016/j.tet.2023.133300. [DOI] [Google Scholar]
- Berecz G.; Dancsó A.; Németh D. R.; Kiss L.; Simig G.; Volk B. Towards Tianeptine Analogues: Synthesis of New Ring Systems Containing a Dibenzo[c,f][1,2]Thiazepine S,S-Dioxide Core. Synthesis 2022, 54, 3874–3882. 10.1055/s-0040-1719885. [DOI] [Google Scholar]
- Labrid C.; Moleyre J.; Poignant J. C.; Malen C.; Mocaër E.; Kamoun A. Structure-Activity Relationships of Tricyclic Antidepressants, with Special Reference to Tianeptine. Clin. Neuropharmacol. 1988, 11, S21–S31. [PubMed] [Google Scholar]
- Platzer N.; Bouchet J. P.; Malen C.; Labrid C.; Mocaer E. 1 H and 13 C NMR Studies on the Antidepressant Drug Tianeptine. MRC 1992, 30, 1212–1219. 10.1002/mrc.1260301210. [DOI] [Google Scholar]
- Kruegel A. C.Chemical and Biological Explorations of Novel Opioid Receptor Modulators. Ph.D. Dissertation, Columbia University, NY, 2015; https://academiccommons.columbia.edu/doi/10.7916/D8V1242F (accessed 2024–03–26).
- O’Boyle N. M.; Banck M.; James C. A.; Morley C.; Vandermeersch T.; Hutchison G. R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix P.; Rocher N.; Deslandes A. Antidepressant Effects of Tianeptine, of Its Two Enantiomers and Its Predominant Metabolite in the Learned Helplessness Test in Rats. Eur. Neuropsychopharmacol. 1996, 6, S4–S70. 10.1016/0924-977X(96)83017-3. [DOI] [Google Scholar]
- Oluyomi A. O.; Datla K. P.; Curzon G. Effects of the (+) and (−) Enantiomers of the Antidepressant Drug Tianeptine on 5-HTP-Induced Behaviour. Neuropharmacology 1997, 36, 383–387. 10.1016/S0028-3908(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Morris R. G. M.; Kelly S.; Burney D.; Anthony T.; Boyer P. A.; Spedding M. Tianeptine and Its Enantiomers: Effects on Spatial Memory in Rats with Medial Septum Lesions. Neuropharmacology 2001, 41, 272–281. 10.1016/S0028-3908(01)00058-2. [DOI] [PubMed] [Google Scholar]
- Szafarz M.; Wencel A.; Pociecha K.; Fedak F. A.; Wlaź P.; Wyska E. Pharmacokinetic Study of Tianeptine and Its Active Metabolite MC5 in Rats Following Different Routes of Administration Using a Novel Liquid Chromatography Tandem Mass Spectrometry Analytical Method. Naunyn-Schmiedeberg’s Arch. Pharmacol 2018, 391, 185–196. 10.1007/s00210-017-1448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepan A. F.; Walker D. P.; Bauman J.; Price D. A.; Baillie T. A.; Kalgutkar A. S.; Aleo M. D. Structural Alert/Reactive Metabolite Concept as Applied in Medicinal Chemistry to Mitigate the Risk of Idiosyncratic Drug Toxicity: A Perspective Based on the Critical Examination of Trends in the Top 200 Drugs Marketed in the United States. Chem. Res. Toxicol. 2011, 24, 1345–1410. 10.1021/tx200168d. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S.; Dalvie D. Predicting Toxicities of Reactive Metabolite–Positive Drug Candidates. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 35–54. 10.1146/annurev-pharmtox-010814-124720. [DOI] [PubMed] [Google Scholar]
- Larrey D.; Tinel M.; Lettéron P.; Maurel P.; Loeper J.; Belghiti J.; Pessayre D. Metabolic Activation of the New Tricyclic Antidepressant Tianeptine by Human Liver Cytochrome P450. Biochem. Pharmacol. 1990, 40, 545–550. 10.1016/0006-2952(90)90554-X. [DOI] [PubMed] [Google Scholar]
- Letteron P.; Descatoire V.; Tinel M.; Maurel P.; Labbe G.; Loeper J.; Larrey D.; Freneaux E.; Pessayre D. Metabolic Activation of the Antidepressant Tianeptine. I. Cytochrome P-450-Mediated in Vitro Covalent Binding. Biochem. Pharmacol. 1989, 38, 3241–3246. 10.1016/0006-2952(89)90620-5. [DOI] [PubMed] [Google Scholar]
- Letteron P.; Labbe G.; Descatoire V.; Degott C.; Loeper J.; Tinel M.; Larrey D.; Pessayre D. Metabolic Activation of the Antidepressant Tianeptine. II. In Vivo Covalent Binding and Toxicological Studies at Sublethal Doses. Biochem. Pharmacol. 1989, 38, 3247–3251. 10.1016/0006-2952(89)90621-7. [DOI] [PubMed] [Google Scholar]
- Larrey D.; Tinel M.; Lettéron P.; Maurel P.; Loeper J.; Belghiti J.; Pesssayre D. Metabolic Activation of the New Tricyclic Antidepressant Tianeptine into a Reactive Metabolite in Human Liver. Role of Cytochrome P-450 IIIA3, an Isoenzyme Inducible by Glucocorticoids. Eur. J. Pharmacol. 1990, 183, 1644. 10.1016/0014-2999(90)91933-3. [DOI] [Google Scholar]
- Khalil S. M.; MacKenzie K. R.; Maletic-Savatic M.; Li F. Metabolic Bioactivation of Antidepressants: Advance and Underlying Hepatotoxicity. Drug Metabolism Reviews 2024, 56, 97–126. 10.1080/03602532.2024.2313967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grislain L.; Gele P.; Bertrand M.; Luijten W.; Bromet N.; Salvadori C.; Kamoun A. The Metabolic Pathways of Tianeptine, a New Antidepressant, in Healthy Volunteers. Drug. Metab. Dispos. 1990, 18, 804–808. [PubMed] [Google Scholar]
- Campbell D. B.; Taylor A. R.. A Species Comparison of the Pharmacokinetics and Metabolism of 14C-Tianeptine. In Biological Psychiatry: Proceedings of the 3rd World Congress of Biological Psychiatry, June 28–July 3, Stockholm, Sweden; Struwe G., Janssen B., Eds.; Elsevier/North-Holland, 1981; pp 585–588. [Google Scholar]
- Salvadori C.; Ward C.; Defrance R.; Hopkins R. The Pharmacokinetics of the Antidepressant Tianeptine and Its Main Metabolite in Healthy Humans – Influence of Alcohol Co-administration. Fundam. Clin. Pharmacol. 1990, 4, 115–125. 10.1111/j.1472-8206.1990.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Royer R. J.; Albin H.; Barrucand D.; Salvadori-Failler C.; Kamoun A. Pharmacokinetic and Metabolic Parameters of Tianeptine in Healthy Volunteers and in Populations with Risk Factors. Clin. Neuropharmacol. 1988, 11, S90–S96. [PubMed] [Google Scholar]
- Carlhant D.; Garrec J.; Guedes Y.; Salvadori C.; Mottier D.; Riche C. Pharmacokinetics and Bioavailability of Tianeptine in the Elderly. Drug Invest 1990, 2, 167–172. 10.1007/BF03259191. [DOI] [Google Scholar]
- Rudorfer M. V.; Potter W. Z. Metabolism of Tricyclic Antidepressants. Cell. Mol. Neurobiol. 1999, 19, 373–409. 10.1023/A:1006949816036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar G.; Gupta R. N. Plasma Levels and Tricyclic Antidepressant Therapy: Part 2. Pharmacokinetic, Clinical and Toxicologic Aspects. Biopharm. Drug Dispos. 1980, 1, 283–305. 10.1002/bdd.2510010602. [DOI] [PubMed] [Google Scholar]
- Couet W.; Girault J.; Latrille F.; Salvadori C.; Fourtillan J. B. Kinetic Profiles of Tianeptine and Its MC5Metabolite in Plasma, Blood and Brain after Single and Chronic Intraperitoneal Administration in the Rat. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 69–74. 10.1007/BF03190130. [DOI] [PubMed] [Google Scholar]
- Dresse A.; Rosen J. M.; Brems H.; Masset H.; Defrance R.; Salvadori C. Influence of Food on Tianeptine and Its Main Metabolite Kinetics. J. Clin. Pharmacol. 1988, 28, 1115–1119. 10.1002/j.1552-4604.1988.tb05726.x. [DOI] [PubMed] [Google Scholar]
- Kato G.; Weitsch A. F. Neurochemical Profile of Tianeptine, a New Antidepressant Drug. Clin. Neuropharmacol. 1988, 11, S43–S50. [PubMed] [Google Scholar]
- Mennini T.; Mocaer E.; Garattini S. Tianeptine, a Selective Enhancer of Serotonin Uptake in Rat Brain. Naunyn-Schmiedeberg’s Arch. Pharmacol 1987, 336, 478–482. 10.1007/BF00169302. [DOI] [PubMed] [Google Scholar]
- Fattaccini C. M.; Bolaños-Jimenez F.; Gozlan H.; Hamon M. Tianeptine Stimulates Uptake of 5-Hydroxytryptamine in Vivo in the Rat Brain. Neuropharmacology 1990, 29, 1–8. 10.1016/0028-3908(90)90076-4. [DOI] [PubMed] [Google Scholar]
- De Simoni M. G.; De Luigi A.; Clavenna A.; Manfridi A. In Vivo Studies on the Enhancement of Serotonin Reuptake by Tianeptine. Brain Res. 1992, 574, 93–97. 10.1016/0006-8993(92)90804-I. [DOI] [PubMed] [Google Scholar]
- Watanabe Y.; Sakai R. R.; McEwen B. S.; Mendelson S. Stress and Antidepressant Effects on Hippocampal and Cortical 5-HT1A and 5-HT2 Receptors and Transport Sites for Serotonin. Brain Res. 1993, 615, 87–94. 10.1016/0006-8993(93)91117-B. [DOI] [PubMed] [Google Scholar]
- Malagié I.; Deslandes A.; Gardier A. M. Effects of Acute and Chronic Tianeptine Administration on Serotonin Outflow in Rats: Comparison with Paroxetine by Using in Vivo Microdialysis. Eur. J. Pharmacol. 2000, 403, 55–65. 10.1016/S0014-2999(00)00486-6. [DOI] [PubMed] [Google Scholar]
- Datla K. P.; Curzon G. Behavioural and Neurochemical Evidence for the Decrease of Brain Extracellular 5-HT by the Antidepressant Drug Tianeptine. Neuropharmacology 1993, 32, 839–845. 10.1016/0028-3908(93)90138-S. [DOI] [PubMed] [Google Scholar]
- Whitton P. S.; Sarna G. S.; O’Connell M. T.; Curzon G. The Effect of the Novel Antidepressant Tianeptine on the Concentration of 5-Hydroxytryptamine in Rat Hippocampal Dialysates in Vivo. Neuropharmacology 1991, 30, 1–4. 10.1016/0028-3908(91)90035-A. [DOI] [PubMed] [Google Scholar]
- Piñeyro G.; Deveault L.; Blier P.; Dennis T.; de Montigny C. Effect of Acute and Prolonged Tianeptine Administration on the 5-HT Transporter: Electrophysiological, Biochemical and Radioligand Binding Studies in the Rat Brain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995, 351, 111–118. 10.1007/BF00169324. [DOI] [PubMed] [Google Scholar]
- Deslandes A.; Spedding M.. Use of Tianeptine in the Production of Medicaments to treat Neurodegenerative Pathologies. FR2791891A1, 2000.
- Reagan L. P.; Rosell D. R.; Wood G. E.; Spedding M.; Muñoz C.; Rothstein J.; McEwen B. S. Chronic Restraint Stress Up-Regulates GLT-1 mRNA and Protein Expression in the Rat Hippocampus: Reversal by Tianeptine. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 2179–2184. 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole M. H. P.; Swan L.; Fuchs E. The Antidepressant Tianeptine Persistently Modulates Glutamate Receptor Currents of the Hippocampal CA3 Commissural Associational Synapse in Chronically Stressed Rats. Eur. J. Neurosci. 2002, 16, 807–816. 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- Szegedi V.; Juhász G.; Zhang X.; Barkóczi B.; Qi H.; Madeira A.; Kapus G.; Svenningsson P.; Spedding M.; Penke B. Tianeptine Potentiates AMPA Receptors by Activating CaMKII and PKA via the p38, p42/44 MAPK and JNK Pathways. Neurochem. Int. 2011, 59, 1109–1122. 10.1016/j.neuint.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Uzbay T. I.; Kayir H.; Ceyhan M. Effects of Tianeptine on Onset Time of Pentylenetetrazole-Induced Seizures in Mice: Possible Role of Adenosine A1 Receptors. Neuropsychopharmacology 2007, 32, 412–416. 10.1038/sj.npp.1301143. [DOI] [PubMed] [Google Scholar]
- Gassaway M. M.; Rives M.-L.; Kruegel A. C.; Javitch J. A.; Sames D. The Atypical Antidepressant and Neurorestorative Agent Tianeptine Is a μ-Opioid Receptor Agonist. Transl. Psychiatry 2014, 4, e411 10.1038/tp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E. J.; Nassehi N.; Bow E. W.; Chambers D. R.; Gutman E. S.; Jacobson A. E.; Lutz J. A.; Marsh S. A.; Rice K. C.; Sulima A.; Selley D. E.; Negus S. S. Role of Efficacy as a Determinant of Locomotor Activation by Mu-Opioid Receptor (MOR) Ligands in Female and Male Mice. II. Effects of novel MOR-Selective Phenylmorphans with High-to-Low MOR Efficacy. Pharmacol. Res. Perspect. 2023, 11, e01111 10.1002/prp2.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B. A.; Nautiyal K. M.; Kruegel A. C.; Levinstein M. R.; Magalong V. M.; Gassaway M. M.; Grinnell S. G.; Han J.; Ansonoff M. A.; Pintar J. E.; Javitch J. A.; Sames D.; Hen R. The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology 2017, 42, 2052–2063. 10.1038/npp.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird T. R.; Akbarali H. I.; Dewey W. L.; Elder H.; Kang M.; Marsh S. A.; Peace M. R.; Poklis J. L.; Santos E. J.; Negus S. S. Opioid-Like Adverse Effects of Tianeptine in Male Rats and Mice. Psychopharmacology 2022, 239, 2187–2199. 10.1007/s00213-022-06093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government Department of Health and Aged Care Therapeutic Goods Administration. 3.6 Tianeptine Scheduling medicines and poisons, 2017; https://www.tga.gov.au/resources/publication/scheduling-decisions-interim/scheduling-delegates-interim-decisions-and-invitation-further-comment-accsacms-november-2016/36-tianeptine (accessed 2024–03–22).
- Wagstaff A. J.; Ormrod D.; Spencer C. M. Tianeptine: A Review of Its Use in Depressive Disorders. CNS Drugs 2001, 15, 231–259. 10.2165/00023210-200115030-00006. [DOI] [PubMed] [Google Scholar]
- Saletu B.; Grünberger J.; Anderer P.; Linzmayer L.; Zyhlarz G. Comparative Pharmacodynamic Studies with the Novel Serotonin Uptake-Enhancing Tianeptine and -Inhibiting Fluvoxamine Utilizing EEG Mapping and Psychometry. J. Neural. Transm. (Vienna) 1996, 103, 191–216. 10.1007/BF01292627. [DOI] [PubMed] [Google Scholar]
- Poirier M.; Galinowski A.; Amado-Boccara I.; Delalleau B.; Gougoulis N.; Lôo H. Effects of Tianeptine on Attention, Memory and Psychomotor Performances Using Neuropsychological Methods in Young Healthy Volunteers. Eur. Psychiatry. 1993, 8, 95s–102s. 10.1017/S0924933800005459. [DOI] [Google Scholar]
- Von Frenckell R.; Ansseau M.; Dulcire C.; Waintraub L. Effects of Tianeptine on Vigilance and Memory in Young Healthy Volunteers. Psychiatr. & Psychobiol. 1990, 5, 375–380. 10.1017/S0767399X00003631. [DOI] [Google Scholar]
- Brion S.; Audrain S.; de Bodinat C. Major depressive episodes in patients over 70 years of age. Evaluation of the efficiency and acceptability of tianeptine and mianserin. Presse Med. 1996, 25, 461–468. [PubMed] [Google Scholar]
- Ridout F.; Hindmarch I. Effects of Tianeptine and Mianserin on Car Driving Skills. Psychopharmacology (Berl) 2001, 154, 356–361. 10.1007/s002130000662. [DOI] [PubMed] [Google Scholar]
- Vandel P.; Regina W.; Bonin B.; Sechter D.; Bizouard P. Abuse of tianeptine. A case report. Encephale 1999, 25, 672–673. [PubMed] [Google Scholar]
- Leterme L.; Singlan Y.-S.; Auclair V.; Le Boisselier R.; Frimas V. Misuse of tianeptine: five cases of abuse. Ann. Med. Interne. (Paris) 2003, 154 (2), S58–S63. [PubMed] [Google Scholar]
- Pélissier-Alicot A.-L.; Gavaudan G.; Bartoli C.; Kintz P.; Piercecchi-Marti M.-D.; Desfeux J.; Leonetti G. Planned Complex Suicide: An Unusual Case. J. Forensic Sci. 2008, 53 (4), 968–970. 10.1111/j.1556-4029.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- Proença P.; Teixeira H.; Pinheiro J.; Monsanto P. V.; Vieira D. N. Fatal Intoxication with Tianeptine (Stablon). Forensic Sci. Int. 2007, 170, 200–203. 10.1016/j.forsciint.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Schildkraut J. J. The Catecholamine Hypothesis Of Affective Disorders: A Review Of Supporting Evidence. AJP 1965, 122, 509–522. 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Haute Autorité de Santé. Transparency Committee Opinion, 2012; https://www.has-sante.fr/upload/docs/application/pdf/2013-08/stablon_ct_10411_12029.pdf (accessed 2024–03–20).
- Shim Clinic. SIN11182P Medication Singapore|Shim Clinic, 1999; https://www.shimclinic.com/singapore/meds/sin11182p (accessed 2024–03–20).
- Gupta S.; Wallace R.; Sloshower J. Online Sales of Unscheduled Pharmaceutical Agents: A Case Report of Tianeptine Use in the United States. J. Addict. Med. 2017, 11, 411–412. 10.1097/ADM.0000000000000342. [DOI] [PubMed] [Google Scholar]
- National Library of Medicine, National Center for Biotechnology Information. Tianeptine; https://clinicaltrials.gov/search?term=tianeptine (accessed 2024–07–15).
- Schruers K.; Griez E. The Effects of Tianeptine or Paroxetine on 35% CO2 Provoked Panic in Panic Disorder. J. Psychopharmacol. 2004, 18, 553–558. 10.1177/0269881104047283. [DOI] [PubMed] [Google Scholar]
- Lechin F.; van der Dijs B.; Lechin A.E. Treatment of Bronchial Asthma with Tianeptine. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 697–701. 10.1358/mf.2004.26.9.872567. [DOI] [PubMed] [Google Scholar]
- Sohn W.; Lee O. Y.; Kwon J. G.; Park K. S.; Lim Y. J.; Kim T. H.; Jung S. W.; Kim J. I. Tianeptine vs Amitriptyline for the Treatment of Irritable Bowel Syndrome with Diarrhea: A Multicenter, Open-Label, Non-Inferiority, Randomized Controlled Study. Neurogastroenterol. Motil. 2012, 24, 860–e398. 10.1111/j.1365-2982.2012.01945.x. [DOI] [PubMed] [Google Scholar]
- Uzbay T. I. Tianeptine: Potential Influences on Neuroplasticity and Novel Pharmacological Effects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 915–924. 10.1016/j.pnpbp.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Fructuoso F. J.Tianeptine for the treatment of fibromyalgia: a prospective double-bind, randomized, single-centre, placebo-controlled, parallel group study. https://web.archive.org/web/20100721093453/http://www.controlled-trials.com/ISRCTN16400909/ (accessed 2024–07–15).
- Niederhofer H. Tianeptine as a Slightly Effective Therapeutic Option for Attention-Deficit Hyperactivity Disorder. Neuropsychobiology 2004, 49, 130–133. 10.1159/000076721. [DOI] [PubMed] [Google Scholar]
- Uzbay I. T.; Cinar M. G.; Aytemir M.; Tuglular I. Analgesic Effect of Tianeptine in Mice. Life Sci. 1999, 64, 1313–1319. 10.1016/S0024-3205(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Contet C.; Kieffer B. L.; Befort K. Mu Opioid Receptor: A Gateway to Drug Addiction. Curr. Opin. Neurobiol. 2004, 14, 370–378. 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rouby F.; Pradel V.; Frauger E.; Pauly V.; Natali F.; Reggio P.; Thirion X.; Micallef J. Assessment of Abuse of Tianeptine from a Reimbursement Database Using ‘Doctor-shopping’ as an Indicator. Fundam. Clin. Pharmacol. 2012, 26, 286–294. 10.1111/j.1472-8206.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- Hoffman J.‘Gas-Station Heroin’ Sold as Dietary Supplement Alarms Health Officials. The New York Times (Digital Edition), 2024; https://www.nytimes.com/2024/01/10/health/gas-station-heroin-tianeptine-addiction.html (accessed 2024–07–15).
- Tennessee General Assembly Bill. HB 2043; https://wapp.capitol.tn.gov/apps/Billinfo/default.aspx?BillNumber=HB2043&ga=112 (accessed 2024–07–24).
- Sen A.; Ilhan G.; Tomak Y.; Erdivanli B.; Ersoz T.; Ergene M. S. Role of Cultural Interaction in Tianeptine Abuse and Different Application Methods. Turk. J. Anesth. Reanim. 2013, 41, 229–231. 10.5152/TJAR.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman-Pennesi D.; Ou O.; Atoigue A.; Kenez S.; Oladipo T.; Nolan N.. Tianeptine Product Adverse Event Reports from the FDA CFSAN Adverse Event Reporting System (CAERS), 2015–2022; https://www.fda.gov/media/168811/download (accessed 2024–03–24).
- Dempsey S. K.; Poklis J. L.; Sweat K.; Cumpston K.; Wolf C. E. Acute Toxicity From Intravenous Use of the Tricyclic Antidepressant Tianeptine. J. Anal. Toxicol. 2017, 41, 547–550. 10.1093/jat/bkx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ari M.; Oktar S.; Duru M. Amitriptyline and Tianeptine Poisoning Treated by Naloxone. Hum. Exp. Toxicol. 2010, 29, 793–795. 10.1177/0960327110372403. [DOI] [PubMed] [Google Scholar]
- Trowbridge P.; Walley A. Y. Use of Buprenorphine-Naloxone in the Treatment of Tianeptine Use Disorder. J. Addict. Med. 2019, 13, 331–333. 10.1097/ADM.0000000000000490. [DOI] [PubMed] [Google Scholar]
- Lederman S.; Fogarty S. J.. Tianeptine Oxalate and Naloxone Combination for the Treatment of Major Depressive Disorder. WO2022/197719A1.
- Precedence Research . Nootropics Market Size, Share and Trends 2024 to 2033, last updated April 2024; https://www.precedenceresearch.com/nootropics-market#:~:text=The%20U.S.%20nootropics%20market%20size,the%20nootropics%20market%20in%202023 (accessed 2024–07–15).
- U.S. Food & Drug Administration . FDA warns consumers not to purchase or use Neptune’s Fix or any tianeptine product due to serious risks, February 15, 2024; https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-neptunes-fix-or-any-tianeptine-product-due-serious-risks (accessed 2024–07–15).
- Smith K. E.; Rogers J. M.; Strickland J. C.; Epstein D. H. When an Obscurity Becomes Trend: Social-Media Descriptions of Tianeptine Use and Associated Atypical Drug Use. AJDAA 2021, 47, 455–466. 10.1080/00952990.2021.1904408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauhan R.; Hsu A.; Alam A.; Beizai K. Tianeptine Abuse and Dependence: Case Report and Literature Review. Psychosomatics 2018, 59, 547–553. 10.1016/j.psym.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Bakota E. L; Samms W. C; Gray T. R; Oleske D. A; Hines M. O Case Reports of Fatalities Involving Tianeptine in the United States. J. Anal. Toxicol. 2018, 42, 503–509. 10.1093/jat/bky023. [DOI] [PubMed] [Google Scholar]
- Tobe E. H.; Rybakowski J. K. Possible Usefulness of Tianeptine in Treatment-Resistant Depression. Int. J. Psychiatry Clin. Pract. 2013, 17, 313–316. 10.3109/13651501.2013.798418. [DOI] [PubMed] [Google Scholar]
- Ehrich E.; Turncliff R.; Du Y.; Leigh-Pemberton R.; Fernandez E.; Jones R.; Fava M. Evaluation of Opioid Modulation in Major Depressive Disorder. Neuropsychopharmacology 2015, 40, 1448–1455. 10.1038/npp.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-N.; Ghosh B.; Tyler M.; Lalonde J.; Joseph N. F.; Kosaric N.; Fass D. M.; Tsai L.-H.; Mazitschek R.; Haggarty S. J. Class I Histone Deacetylase Inhibition by Tianeptinaline Modulates Neuroplasticity and Enhances Memory. ACS Chem. Neurosci. 2018, 9, 2262–2273. 10.1021/acschemneuro.8b00116. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I.; Yeomans L.; Anand J. P.; Porter V.; Sobczyk-Kojiro K.; Traynor J. R.; Jutkiewicz E. M. Development of a Bioavailable μ Opioid Receptor (MOPr) Agonist, δ Opioid Receptor (DOPr) Antagonist Peptide That Evokes Antinociception without Development of Acute Tolerance. J. Med. Chem. 2014, 57, 3148–3153. 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. M.; Griggs N. W.; Anand J. P.; Traynor J. R.; Jutkiewicz E. M.; Mosberg H. I. Asymmetric Synthesis and in Vitro and in Vivo Activity of Tetrahydroquinolines Featuring a Diverse Set of Polar Substitutions at the 6 Position as Mixed-Efficacy μ Opioid Receptor/δ Opioid Receptor Ligands. ACS Chem. Neurosci. 2015, 6, 1428–1425. 10.1021/acschemneuro.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. W.; Fundytus M. E.; Merovitz L.; Weltrowska G.; Nguyen T. M. D.; Lemieux C.; Chung N. N.; Coderre T. J. The Opioid μ Agonist/δ Antagonist DIPP-NH2[ψ] Produces a Potent Analgesic Effect, No Physical Dependence, and Less Tolerance than Morphine in Rats. J. Med. Chem. 1999, 42, 3520–3526. 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]