Abstract
Spinal muscular atrophy (SMA) is a rare genetic disorder that unequivocally results in the degeneration of motor neurons, leading to muscle weakness and atrophy. This condition is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which inevitably results in a deficiency of the SMN protein. In present study, we investigated the potential role of telomere attrition in SMA patients. Relative telomere length in peripheral blood lymphocytes was measured by Monochrome Multiplex Quantitative Polymerase Chain Reaction (MMQPCR) in 98 subjects and we conclusively found that SMA cases exhibit telomere attrition compared to healthy controls (P = 4 × 10− 2). Moreover, significant attrition was also observed in severe form of SMA, i.e. SMA type 0 (P = 0.04) as well.Although, the exact mechanism through which telomere shortening contributes to the pathogenesis of SMA is not fully understood and is yet to be delineated. However, one possibility is that telomere shortening leads to genomic instability and DNA damage, which can contribute to motor neuron degeneration. Another possibility is that telomere shortening leads to cellular senescence, which can impair the ability of motor neurons to regenerate and repair themselves. Recent studies have suggested that telomere shortening may be a potential therapeutic target in SMA. Thus, understanding the role of SMN1 gene in disease pathogenesis & its effect on telomere length will aid in estimating the risk & prognosis of SMA in genetically less explored & highly inbred region of Kashmir, Northern India.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12920-024-01980-x.
Keywords: Spinal muscular atrophy (SMA), Telomere attrition, Telomere length, Survival Motor Neuron
Introduction
Spinal muscular atrophy is undoubtedly, one of most severe neurodegenerative disorder &is second leading cause of infant mortality after Cystic Fibrosis (CF) with an autosomal recessive pattern of inheritance. SMA is characterized by the deterioration of alpha (α) motor neurons in the brain & spinal cord leading to progressive muscle weakness, hypotonia, recurrent chest infection, paralysis & even death in most severe cases. The global prevalence of SMA varies among various ethnic groups with overall estimated incidence of 1 in 10,000 to 11,000 individuals. Phenotypically, SMA is classified into 5 five types based on age of onset & severity of symptoms: Type 0 (the most severe, with prenatal onset), type 1 (the most common & occurs within few months after birth), type 2 (the intermediate form, able to sit independently), type 3 (mild, able to walk independently) & type 4 (mild & adult onset form) [1–6] In SMA, the deletion of SMN1 genes affects SMN protein production which is critical for biogenesis of heterogeneous nuclear ribonucleoprotein, axonal transport (a motor neuron-specific function) transcriptional regulation, cellular trafficking &telomerase regeneration [7, 8] Humans have another paralog of SMN gene, which is SMN2 gene and it also produces the SMN protein. However, only 10–20% of this protein is functional, because of the single base pair substitution (C→T) at position 6 in exon 7, almost 90% of SMN2 mRNA transcripts lacks exon 7, and protein translated from this transcript (named SMND7) is shorter and not fully functional. Thus, only 10–20% of normal SMN protein comes from the SMN2 gene which is not able to compensate the loss caused due the deletion of SMN1 gene [8]. The lack of which results in degeneration of motor neurons in spinal cord, which leads to an inappropriate innervations, muscle wasting with axial & bulbar muscles severely affected [1, 9] and ultimately results in SMA phenotype. More than 90% of SMA cases are caused due to the homozygous absence of SMN1 gene & remaining 5-10% of SMA patients are compound heterozygotes having intragenic mutation within SMN1 gene like deletions, duplications, insertions & frameshift mutations [10]. Spinal Muscular Atrophy (SMA) primarily results from the biallelic deletion of SMN1 exon 7, accounting for the majority of cases. Approximately 5 to 10% of SMA cases involve compound heterozygotes displaying intragenic mutations within the SMN1 gene, such as nonsense mutations, splice site mutations, insertions, deletions, duplications, and missense mutations. Exon 3 and exon 6 are identified as common hotspots for small mutations and missense mutations, respectively. The most frequently reported mutation in the SMN1 gene among SMA patients is the exon 6 p.Tyr272Cys missense mutation. This specific exon encodes a critical protein domain essential for protein oligomerization. Consequently, patients with missense mutations in exon 6 exhibit reduced self-oligomerization capacity of the SMN protein [11]. The SMA causing gene is mapped on chromosome 5q13 [7, 12], which is split up into 2 parts: telomeric part & centromeric part. The gene located on telomeric part of SMA locus is the SMN1( survival of motor neuron) gene, the disease determining gene & gene on centromeric part of SMA locus is the SMN2, the disease modifying gene. Both these genes are required for proper survival & functioning of motor neurons [10, 13, 14]. The number of SMN2 gene copies varies from 1 to even 8 in some individuals. It is known that the higher SMN2 copy number, less is the severity of disease and slower is the progression of disease [15]. Patients with type 1 SMA usually have 2 copies of SMN2, while type 2 & type 3 SMA patients have 3 copies or more of SMN2 [16, 17]. However, it is not always true and sometimes the patients with type 1 SMA may have 4 SMN2 copies [18].
Telomeres are highly specialized structures that play a pivotal role in protecting chromosome ends from deterioration. These structures consist of hexanucleotide (TTAGGG) repeats and have significant impact on maintaining genomic stability and regulating both cellular and tissue functions [19]. The length of telomeres varies with age, being longest at birth and shortening over time. When telomeres become critically short, the cell ceases to divide and enters a state of senescence [20, 21]. Senescent cells are typically cleared by the immune system, but in aged tissues, this process can become impaired, leading to the accumulation of senescent cells that contribute to further tissue dysfunction and aging [22]. Telomere length serves as a biological clock indicating cellular age and is considered a potential biomarker of aging and age-related diseases. Studies have estimated that leukocyte telomeres in adult humans shorten at a rate of 24.7 base pairs per year [23]. Although telomere length is synchronous within organs at the time of birth, however, it can vary within different organs of the same person later in life [24]. Telomere attrition is also influenced by several factors apart from cellular senescence. Various studies have observed that progressive telomere shortening is associated with chromosomal unreliability. Which contributes to the development of lifelong diseases such as cardiovascular diseases, hypertension, arthritis, osteoporosis, diabetes, cancer, and neurological disorders [25] Fig. 1. Although the precise mechanism by which telomere shortening contributes to the pathogenesis of SMA is not fully understood. One possibility is that telomere shortening leads to genomic instability and DNA damage, which, in turn, can contribute to motor neuron degeneration. Another possibility is that telomere shortening leads to cellular senescence, which can impair the ability of motor neurons to regenerate and repair themselves. Recent studies have suggested that telomere shortening may be a potential therapeutic target in SMA [26]. Therefore, understanding the role of SMN1 gene in disease pathogenesis & its effect on telomere length will aid us in estimating the risk & prognosis of SMA in genetically less explored & highly inbred regions of Kashmir.
Fig. 1.
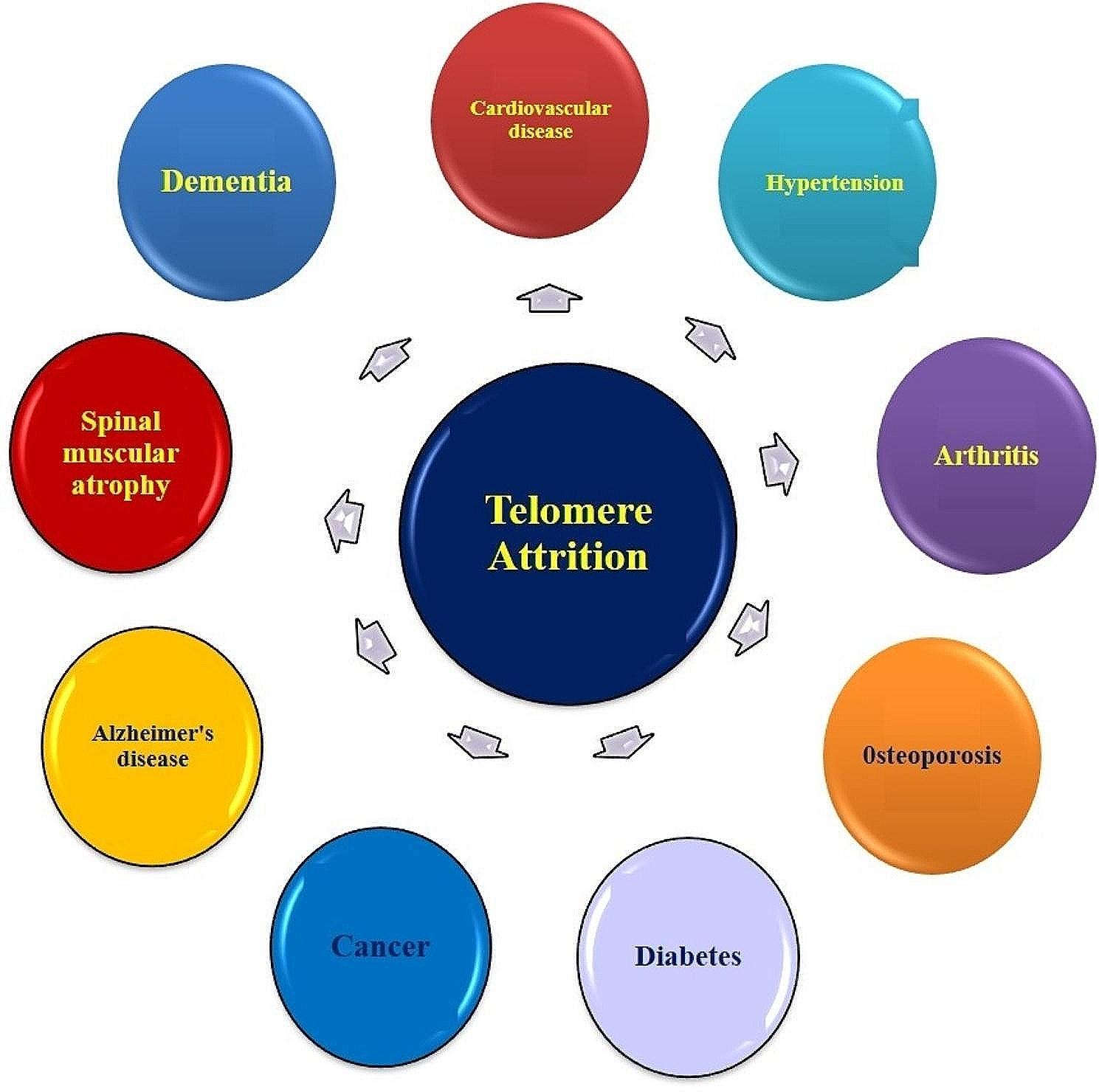
Progressive telomere attrition associated with various diseases
Materials and methods
Sample collection & research subjects
A total of 98 samples of which (40 Spinal Muscular Atrophy (SMA) cases and 58 age and gender matched healthy controls) were recruited in the study. The diagnosis of patients was confirmed in accordance with guidelines established by the International SMA Consortium. All the SMA patients were classified into 5 phenotypes based on criteria set up by International SMA Consortium [27]. The patients participated in the current study were referred from the Department of Pediatrics& Neonatology and Department of Neurology, Sher-i-Kashmir Institute of Medical Sciences (SKIMS) Soura Hospital, Srinagar. The clinical details from all patients including detailed family history, obstetric history and other clinical details were recorded in a proper questionnaire with patients/ guardians (in case of minors) and controls informed consent to take part in the study. The study was approved by the ethics committee of the Sher-i-Kashmir Institute of Medical Sciences (SKIMS) under notification no. #RP-250/2022.
DNA extraction
About 1 -2mL of peripheral blood was collected from all SMA patients and healthy controls in EDTA (ethylene diamine tetra acetic acid) tubes and genomic DNA was extracted manually by Phenol /Chloroform method or by using DNA extraction kit (DNA(HIMEDIA; MB504). quality & quantity of extracted DNA samples were assessed by the ratio of absorbance at 260 nm divided by the absorbance at 280 nm by using a spectrophotometer (Eppendorf Biospectrometer, Hamburg, Germany) or by 0.8% agarose gel electrophoresis. Pure DNA samples with absorbance (A260/A280) ratio of 1.8 -2.0 were processed for the study.
Assessment of telomere length
Quantitative real-time PCR was performed on Rotor-Gene (QIAGEN) detection systems for the assessment of relative telomere length by using specific set of primers for single copy gene & telomere. The relative telomere length was evaluated by the T/S ratio (= 2−Δct), which represents the ratio of telomere repeat copy number to single gene copy number. The reaction was executed under standard PCR conditions. The telomere primer sequence for both single copy gene (36b4) & telomere are provided in Table 1. The reaction mixture was set in a final volume of 20 µl containing 2× SYBR Green qPCR Master Mix (Thermo Fisher Scientific). The thermal profile setup for QPCR consisted of the following steps : an initial denaturation at 95 °C for 10-min, 45 cycles of 95° C hold for 15 s, followed by annealing at 60 °C for 30 s, and 72 °C hold for 11 s.
Table 1.
Showing the primer sequence of different genes used during the study
| Gene | Primer sequence |
|---|---|
| Telomere_F | 5 ′ -CGGTTTGTTTGGG TTTGGGTTTGGGTTT GGGTTTGGGTT-3′ |
| Telomere_R | 5′-GGCTTGCCTTACCCTT ACCCTTACCCTTACCCTT-ACCCT-3′, |
| 36B4_F | 5′-CAGCAAGTGGGAAGGTGTAATCC-3′ |
| 36B4_R | 5′-CCC ATTCTATCATCAACGGGTACAA-3′ |
Statistical analysis
Clinical characteristics between cases and controls were compared by t-test. Statistical analysis was performed on IBM SPSS statistics 20 software.
Results
The clinical characteristic distribution of the cases and controls are given in Table 2. The mean age of cases was 33.76 months and that of controls was 98.28 months cases with significant difference (p = 0.003). However, 47.5% were males, 52.5% were females in cases & 62% were males, 38% were males in controls. Consanguinity was observed in 52% in Cases and 21.5% in Controls respectively.
Table 2.
Represents the clinical characteristic distribution of the cases and controls
| PARAMETERS OF SMA | CASES | CONTROLS | P- Value |
|---|---|---|---|
| Gender (in %) | |||
|
Males Females |
19 (47.5%) 21 (52.5%) |
36 (62%) 22 (38%) |
0.2139 |
| Age (in months) (in %) | |||
|
≤ 36 months ≥ 36 months |
33 (82%) 07 (18%) |
18 (30%) 40 (70%) |
0.003 |
| Achieved Milestones (in %) | |||
|
Non-Sitter Sitter Walker |
29 (72.5%) 07 (17.5%) 04 (10%) |
08 (14%) 07 (12%) 43 (74%) |
0.00001 |
| Consanguinity (in %) | |||
|
Yes No |
21 (52.5%) 19 (47.5%) |
12 (21%) 46 (79%) |
0.0021 |
| Maternal Age (in %) | |||
|
≤ 30 Years ≥ 31 Years |
25 (62.5%) 15(37.5%) |
14(24%) 44 (76%) |
0.00001 |
| Dwelling Are a(in %) | |||
|
Rural Urban |
19 (47.5%) 21 (52.5%) |
39 (67%) 19 (33%) |
0.061 |
| Family History (in %) | |||
|
Yes No |
05 (12.5%) 35 (87.5%) |
||
| Types of SMA (in %) | |||
|
Type 0 Type 1 Type 2 Type 3 Type 4 |
04 (10%) 13 (32.5%) 14 (35%) 07 (17.5%) 02 (5%) |
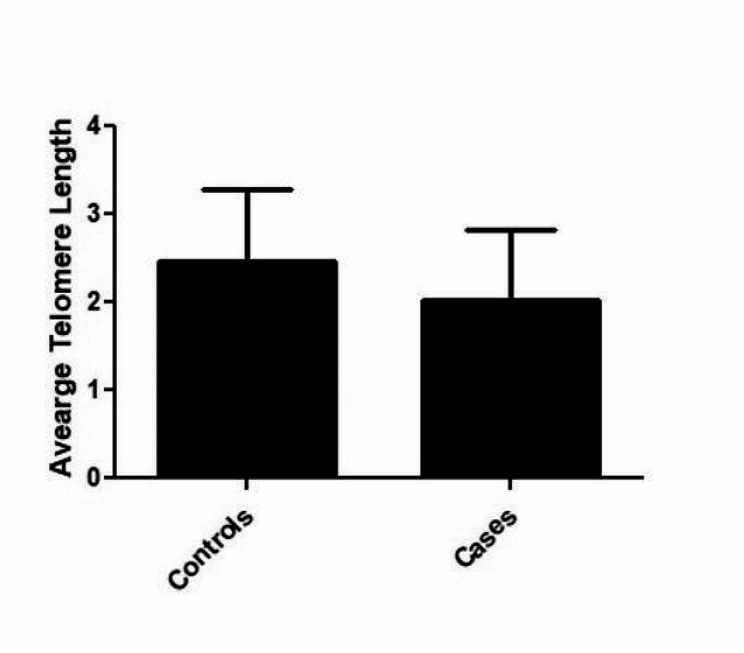
Relative telomere length in peripheral blood lymphocytes was measured by monochrome multiplex quantitative polymerase chain reaction in 40 spinal muscular atrophy patients and 58 healthy controls (triplicates) that were matched for age and gender. Telomere length was significantly shorter in SMA patients than in controls (p = 4 × 10− 2 Fig. 2). Furthermore, we categorized our data into various subtypes like type0, type1, type2, type3, type4 and performed the sub group analysis. It was observed that type0, type1& type2 showed the significant telomere attrition (P = 0.04; P = 0.01; P = 0.0004) than the subtypes type3 and type 4 (P = 0.40; P = 0.17) respectively. The possibility is that telomere shortening leads to genomic instability and DNA damage, which can contribute to motor neuron degeneration. Another possibility is that telomere shortening leads to cellular senescence, which can impair the ability of motor neurons to regenerate and repair themselves. Recent studies have suggested that telomere shortening may be a potential therapeutic target in SMA.
Fig. 2.
Showing the average telomere length among the cases and controls with P=4x10-2
Discussion
Spinal muscular atrophy (SMA) is a rare genetic disorder characterized by the degeneration of motor neurons, resulting in muscle weakness and atrophy. SMA is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which leads to a deficiency of the SMN protein [10]. Telomeres are repetitive DNA sequences located at the ends of chromosomes that protect them from deterioration. and fusion at their ends. Telomerase, a ribonucleoprotein complex, plays a crucial role in elongating telomeres and reducing the shortening of telomeres via reverse transcription activity. Telomerase is comprised of two primary subunits: TERT (telomerase reverse transcriptase), the catalytic subunit, and TERC (telomerase RNA component). Telomerase prevents the accumulation of short telomeres, which can lead to telomere dysfunction, through the process of reverse transcription. Telomerase is present in both the developing and adult brain where telomerase deficiency and telomere shortening leads to impaired neurogenesis, neuronal differentiation, and an increased susceptibility to neurodegenerative disorders [28]. A study by Rossiello et al. [29] demonstrated the association between telomere shortening and various neurodegenerative disorders including SMA, which has also been observed in various mouse models.
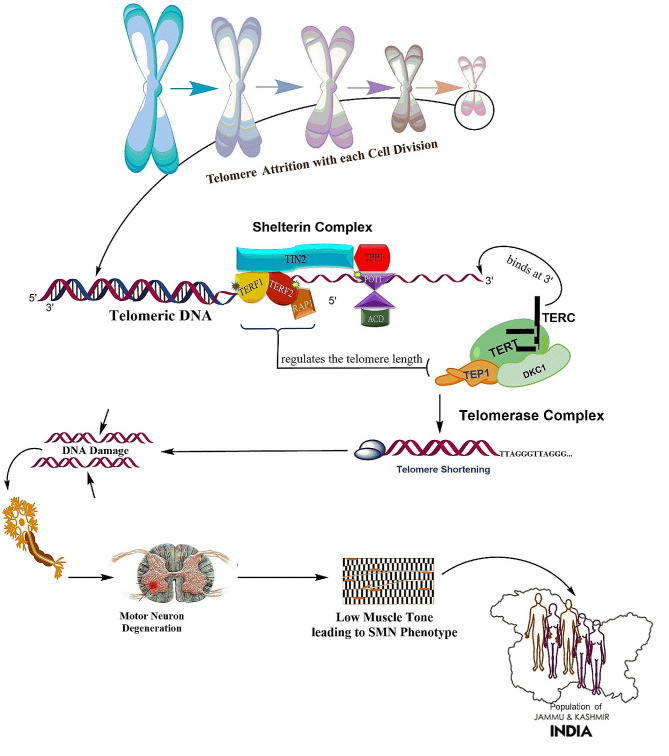
The present study discusses the role of telomere shortening in SMA patients. Telomere shortening is a natural process that occurs with each cell division. Telomeres become shorter with each division until they reach a critical length and induces DNA damage and other molecular mechanisms that contributes to cellular senescence. In SMA patients, telomere shortening has been observed in both motor neurons and peripheral blood cells. Relative telomere length in peripheral blood lymphocytes was measured by monochrome multiplex quantitative polymerase chain reaction and telomere attrition was observed in SMA cases compared to healthy controls (p = 4 × 10− 2)& significant attrition was observed in severe form of SMA i.e. SMA type0. So, our result are consistent with the previous studies. A study by Monani et al. (1999) found that telomeres were significantly shorter in motor neurons from SMA patients compared to controls. This shortening was more pronounced in severe forms of the disease [30]. The exact mechanism by which telomere shortening contributes to the pathogenesis of SMA is not fully understood. However, studies have revealed the disruption of neuronal differentiation and neurogenesis due to telomere shortening induces oxidative stress, DNA damage, and impaired DNA repair mechanisms may all play a role in accelerating telomere shortening in SMA patients and can contribute to motor neuron degeneration as shown in Fig. 3 leading to a more severe disease course and poorer outcomes for affected individuals [30–32]. Another possibility is that telomere shortening leads to cellular senescence, which can impair the ability of motor neurons to regenerate and repair themselves. Recent studies have suggested that telomere shortening may be a potential therapeutic target in SMA. A study by Sareen et al. found that treatment with a telomerase activator, which can prevent telomere shortening, improved the survival and function of motor neurons in a mouse model of SMA [33]. Another study Eitan et al. by found that treatment with a telomerase activator improved the motor function and survival of SMA mice [34]. Understanding how telomeres are affected in SMA can provide valuable insights into the disease process and may lead to the development of targeted therapies aimed at preserving telomere length and improving outcomes for SMA patients.
Fig. 3.
Mechanism of Telomere shortening in motor neuron degeneration
Limitation of description
The study on telomere attrition in spinal muscular atrophy (SMA) conducted in highly inbred regions of Jammu and Kashmir with a sample size of 40 cases and 58 controls may face several potential limitations. Firstly, the small sample size could compromise the generalizability of the findings due to limited representation of the diverse population. In highly inbred regions, the homogeneity of the population might restrict the variation in genetic and environmental factors, affecting the extrapolation of results to more diverse populations. Additionally, the limited sample size may enhance the risk of Type I and Type II errors, leading to inaccurate conclusions. Furthermore, the potential confounding effects of unmeasured variables in the inbred population could introduce bias and could impact the validity of the results. However, addressing these limitations through meticulous study design, rigorous statistical analysis, and cautious interpretation of findings is crucial to enhance the reliability and applicability. We are taking quest to perform the study on large sample cohort to address all the above potential limitations in future course.
Novelity
The novel findings from this study provide valuable insights into the potential impact of telomere attrition on the progression of SMA and lay the foundation for future research in this domain. However, further research is needed to explore the underlying mechanisms and unravel the mechanistic pathways to slow down telomere shortening in SMA patients that can act as predictive or prognostic biomarkers and will be a step towards a personalized medicine.
Conclusion
In conclusion, telomere shortening has been implicated in the pathogenesis of SMA. Telomere shortening has been observed in both motor neurons and peripheral blood cells from SMA patients. Although the exact mechanism by which telomere shortening contributes to the pathogenesis of SMA is not fully understood, but it may involve genomic instability, DNA damage, and cellular senescence. Telomere shortening may be a potential therapeutic target in SMA, and telomerase activators may be a promising treatment option. Moreover, the critical reason to identify these associations is to understand the genetic heterogeneity of spinal muscular atrophy in highly inbred region and possibility of using telomere length variation as prognostic biomarker for diagnosis of SMA. Further research is needed to fully understand the role of telomere shortening in SMA and to develop effective therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
RH and DA acknowledge the family members and patient for their contribution in the study.
Abbreviations
- SMA
Spinal Muscular Atrophy
- SMN
Survival of motor neuron
- TL
Telomere length
- TA
Telomere attrition
- MMQPCR
Monochrome Multiplex Quantitative Polymerase Chain Reaction
Author contributions
RH, GRB and DA planned the work, RH carried out work on SMN samples and RH wrote the manuscript and restructured it, HAG, MAB, FAM helped in sampling processes, RH, GRB, IM performed data analysis, RH, GRB, HAG, FAM, MAB, RA, IM, DA finally refined the manuscript. All the authors meet the criteria for authorship. Every author is aware of, has agreed to this paper’s content, and is listed as an author on the paper.
Funding
This research was supported by Grant provided Institutional Intramural grant (SKIMS).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Institutional ethics committee of the Sher-i-Kashmir Institute of Medical Sciences (SKIMS) under notification no. #RP 250/2022. All the information was carried in predesigned consent form. All the experiments were carried under standard guidelines.
Consent to publish
Our manuscript does not contain any individual person’s data in any form (including any individual details, images or videos).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gh Rasool Bhat, Feroze Ahmad Mir authors contributed Equally.
References
- 1.Munsat TL, Davies KE. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul Disorders: NMD. 1992;2(5–6):423–8. [DOI] [PubMed] [Google Scholar]
- 2.Macleod MJ, Taylor JE, Lunt PW, Mathew CG, Robb SA. Prenatal onset spinal muscular atrophy. Eur J Pediatr Neurol. 1999;3(2):65–72. [DOI] [PubMed] [Google Scholar]
- 3.Dubowitz V. Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. Eur J Pediatr Neurology: EJPN: Official J Eur Pediatr Neurol Soc. 1999;3(2):49–51. [DOI] [PubMed] [Google Scholar]
- 4.Sarnat HB, Trevenen CL. Motor neuron degeneration in a 20-week male fetus: spinal muscular atrophy type 0. Can J Neurol Sci. 2007;34(2):215–20. [DOI] [PubMed] [Google Scholar]
- 5.Clermont O, Burlet P, Lefebvre S, Bürglen L, Munnich A, Melki J. SMN gene deletions in adult-onset spinal muscular atrophy. Lancet. 1995;346(8991):1712–3. [DOI] [PubMed] [Google Scholar]
- 6.Brahe C, Servidei S, Zappata S, Ricci E, Tonali P, Neri G. Genetic homogeneity between childhood-onset and adult-onset autosomal recessive spinal muscular atrophy. Lancet (London England). 1995;346(8977):741–2. [DOI] [PubMed] [Google Scholar]
- 7.Brzustowicz L, Lehner T, Castilla L, Penchaszadeh G, Wilhelmsen K, Daniels R, Davies K, Leppert M, Ziter F, Wood D. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q1 1.2–13.3. Nature. 1990;344(6266):540–1. [DOI] [PubMed] [Google Scholar]
- 8.Melki J, Lefebvre S, Burglen L, Burlet P, Clermont O, Millasseau P, Reboullet S, Bénichou B, Zeviani M, Le Paslier D, et al. De novo and inherited deletions of the 5q13 region in spinal muscular atrophies. Science. 1994;264(5164):1474–7. [DOI] [PubMed] [Google Scholar]
- 9.Cano SJ, Mayhew A, Glanzman AM, Krosschell KJ, Swoboda KJ, Main M, Steffensen BF, Bérard C, Girardot F, Payan CA. Rasch analysis of clinical outcome measures in spinal muscular atrophy. Muscle Nerve. 2014;49(3):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65. [DOI] [PubMed] [Google Scholar]
- 11.Hahnen E, Schönling J, Rudnik-Schöneborn S, Raschke H, Zerres K, Wirth B. Missense mutations in exon 6 of the survival motor neuron gene in patients with spinal muscular atrophy (SMA). Hum Mol Genet. 1997;6(5):821–5. [DOI] [PubMed] [Google Scholar]
- 12.Melki J, Abdelhak S, Sheth P, Bachelot M, Burlet P, Marcadet A, Aicardi J, Barois A, Carriere J, Fardeau M. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature. 1990;344(6268):767–8. [DOI] [PubMed] [Google Scholar]
- 13.Lewin B. Genes for SMA: multum in parvo. Cell. 1995;80(1):1–5. [DOI] [PubMed] [Google Scholar]
- 14.Roy N, Mahadevan MS, McLean M, Shutter G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80(1):167–78. [DOI] [PubMed] [Google Scholar]
- 15.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Annals Neurology: Official J Am Neurol Association Child Neurol Soc. 2005;57(5):704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold ES, Fischbeck KH. Spinal muscular atrophy. Handb Clin Neurol. 2018;148:591–601. [DOI] [PubMed] [Google Scholar]
- 17.Cano SJ, Mayhew A, Glanzman AM, Krosschell KJ, Swoboda KJ, Main M, Steffensen BF, Bérard C, Girardot F, Payan CA, et al. Rasch analysis of clinical outcome measures in spinal muscular atrophy. Muscle Nerve. 2014;49(3):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vill K, Kölbel H, Schwartz O, Blaschek A, Olgemöller B, Harms E, Burggraf S, Röschinger W, Durner J, Gläser D, et al. One year of newborn screening for SMA - results of a German pilot project. J Neuromuscul Dis. 2019;6(4):503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–19. [DOI] [PubMed] [Google Scholar]
- 20.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biology Reviews: MMBR. 2002;66(3):407–25. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat GR, Bhat A, Verma S, Sethi I, Shah R, Sharma V, Dar KA, Abrol D, Kaneez S, Kaul S, et al. Association of newly identified genetic variant rs2853677 of TERT with non-small cell lung cancer and leukemia in population of Jammu and Kashmir, India. BMC Cancer. 2019;19(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu GL, Bradley JD, Attardi LD, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344(6262):126–32. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–96. [DOI] [PubMed] [Google Scholar]
- 24.Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, Skurnick J, Bardeguez A, Aviv A. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102(6):640–3. [DOI] [PubMed] [Google Scholar]
- 25.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prev Oncol. 2011;20(6):1238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bär C, Blasco MA. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Research 2016, 5. [DOI] [PMC free article] [PubMed]
- 27.Zerres K, Davies KE. 59th ENMC International Workshop: Spinal Muscular Atrophies: recent progress and revised diagnostic criteria 17-19 April 1998, Soestduinen, The Netherlands. Neuromuscul Disord NMD. 1999;9(4):272–278. [DOI] [PubMed]
- 28.Harley J, Santosa MM, Ng CY, Grinchuk OV, Hor JH, Liang Y, Lim VJ, Tee WW, Ong DST, Ng SY. Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology. 2024;25(2):341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–147. [DOI] [PMC free article] [PubMed]
- 30.Monani UR, De Vivo DC. Neurodegeneration in spinal muscular atrophy: from disease phenotype and animal models to therapeutic strategies and beyond. Future Neurol. 2014;9(1):49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Brettschneider J, Ludolph AC, Lee VM, Trojanowski JQ, Tredici KD. Amyotrophic lateral sclerosis—a model of corticofugal axonal spread. Nat Reviews Neurol. 2013;9(12):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sareen D, Ebert AD, Heins BM, McGivern JV, Ornelas L, Svendsen CN. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS ONE. 2012;7(6):e39113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4(4):313–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.