Abstract
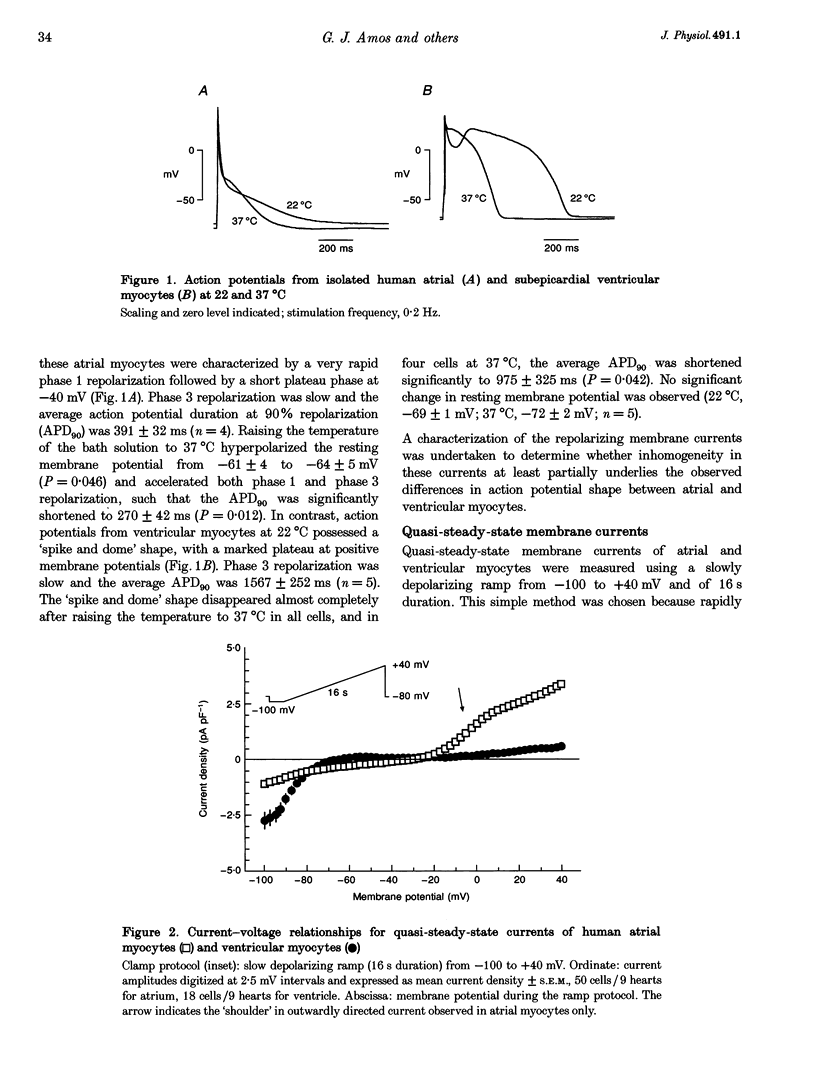
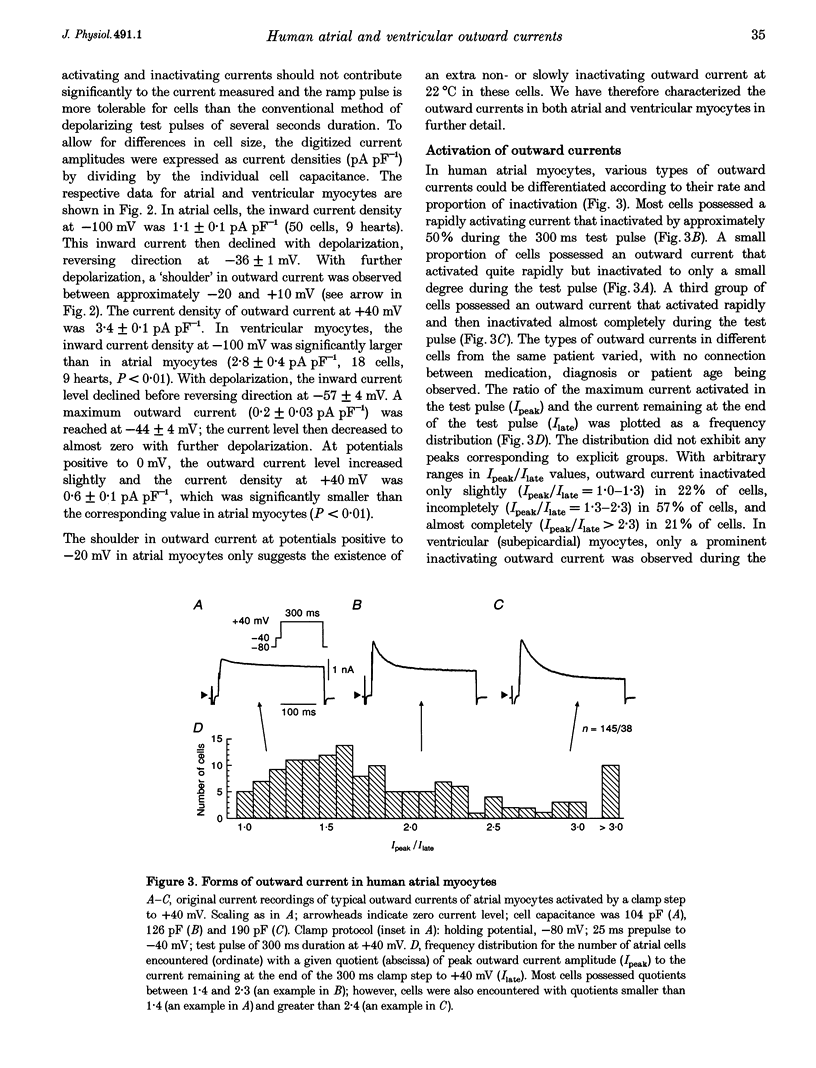
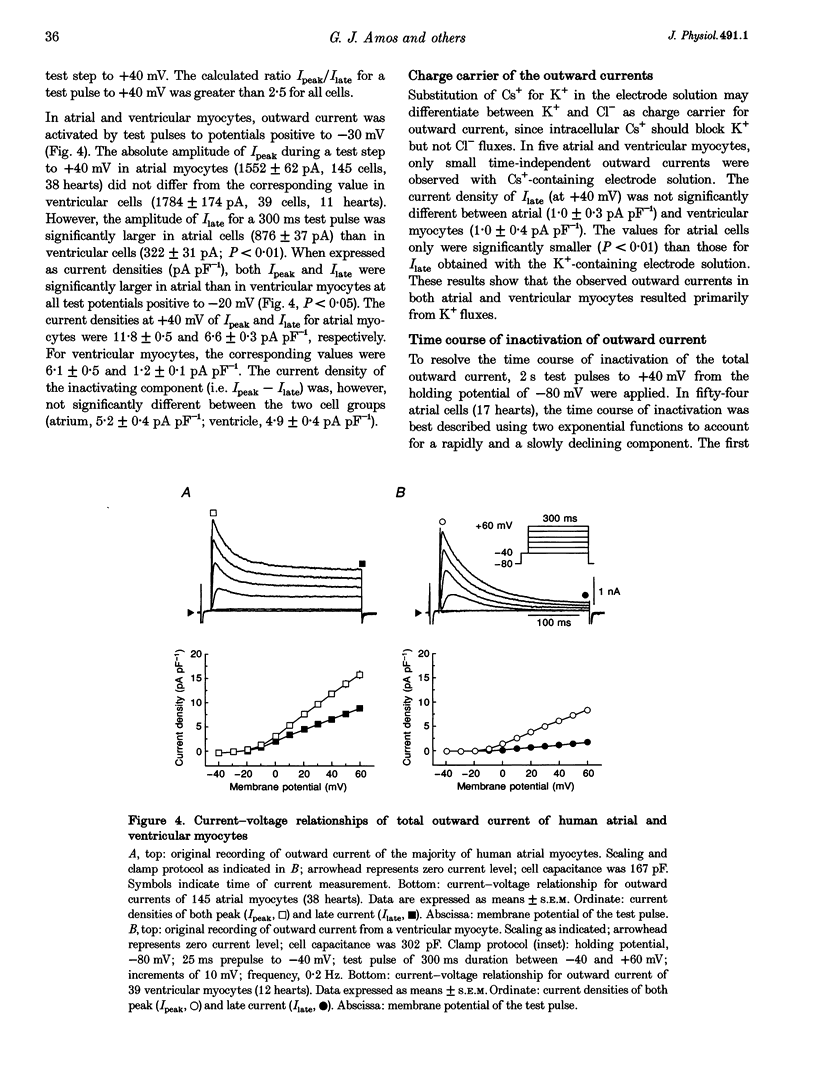
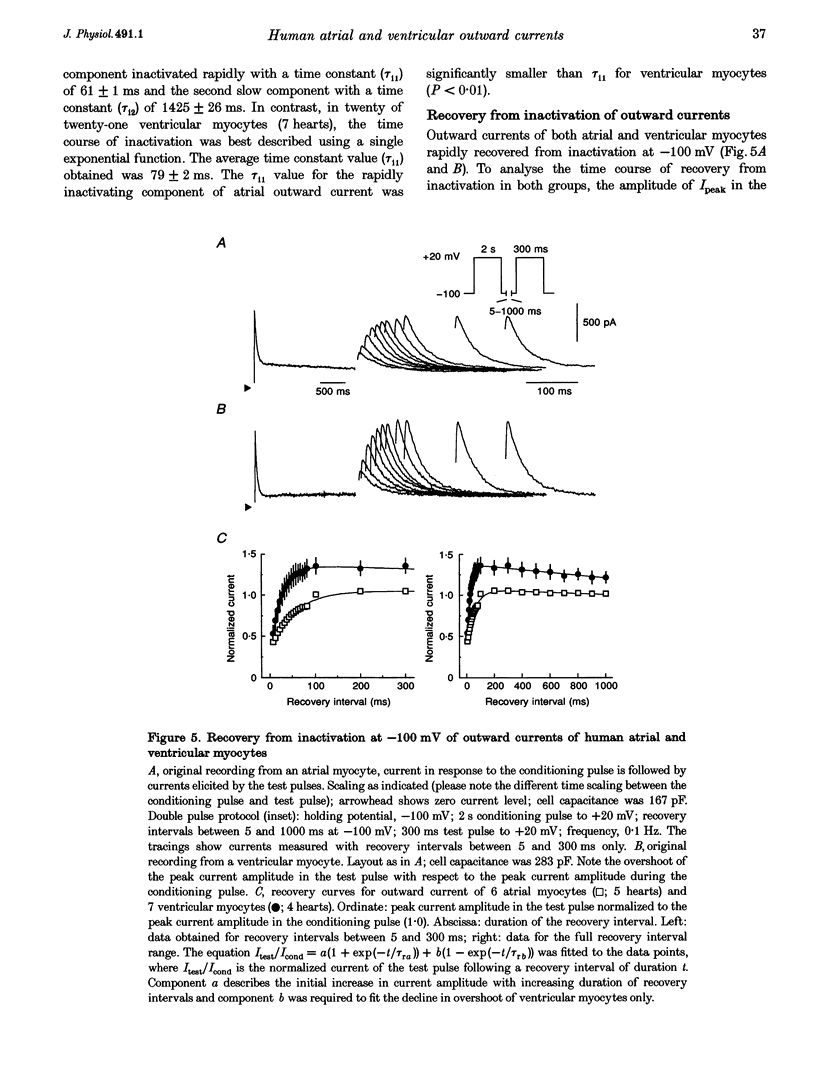
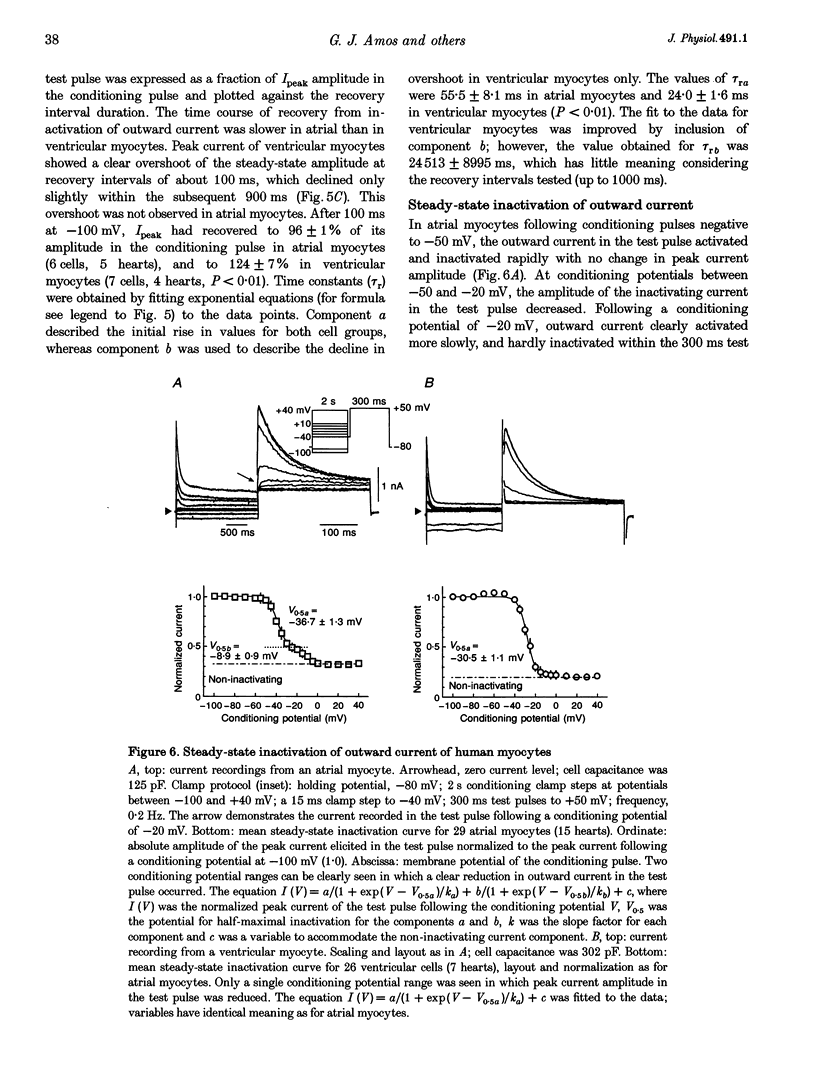
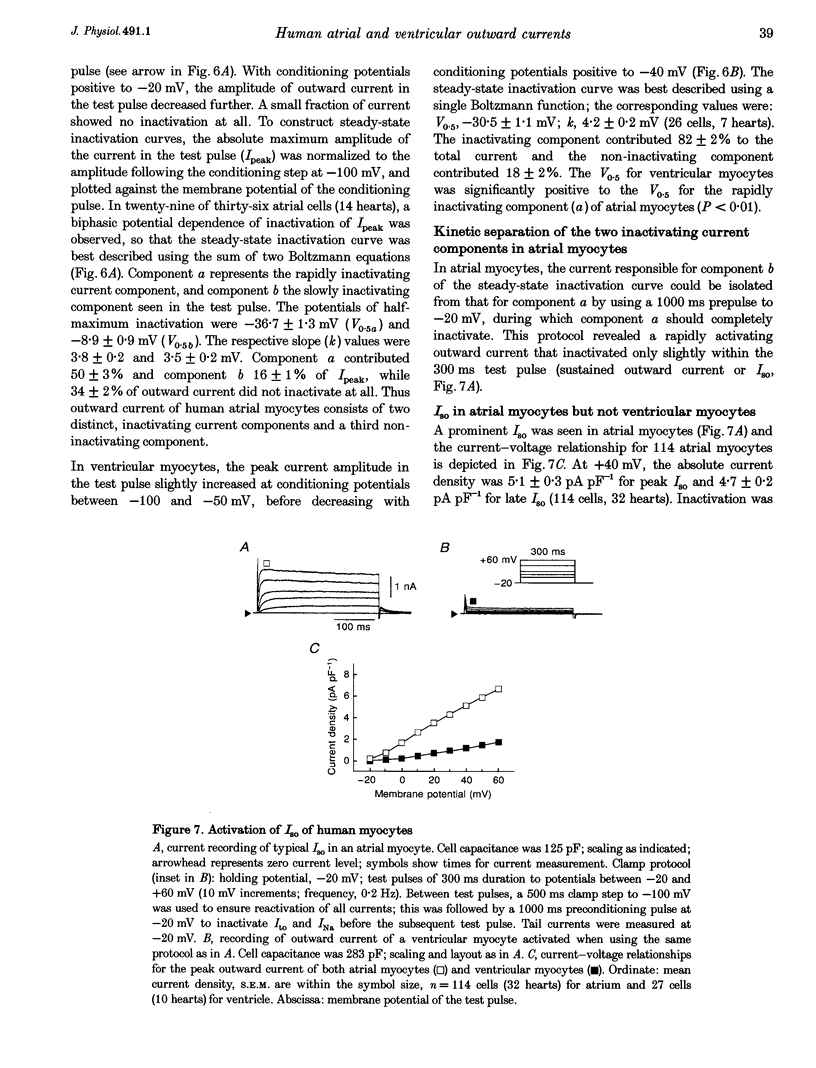
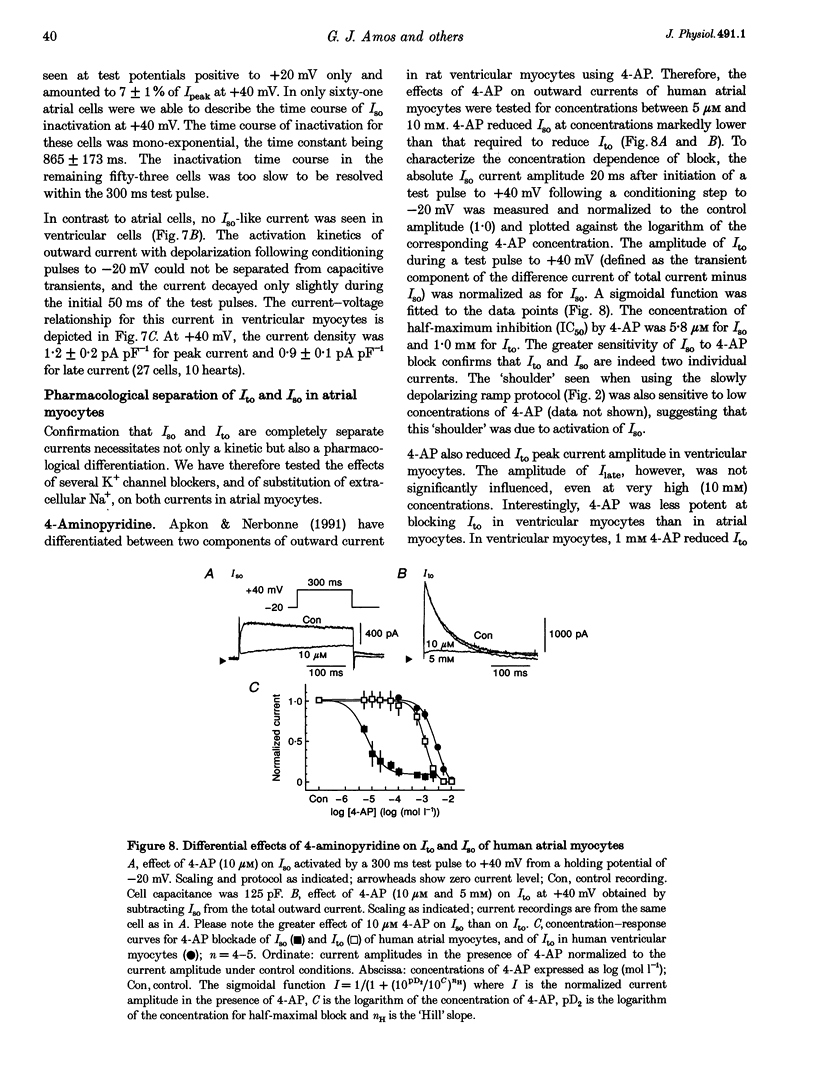
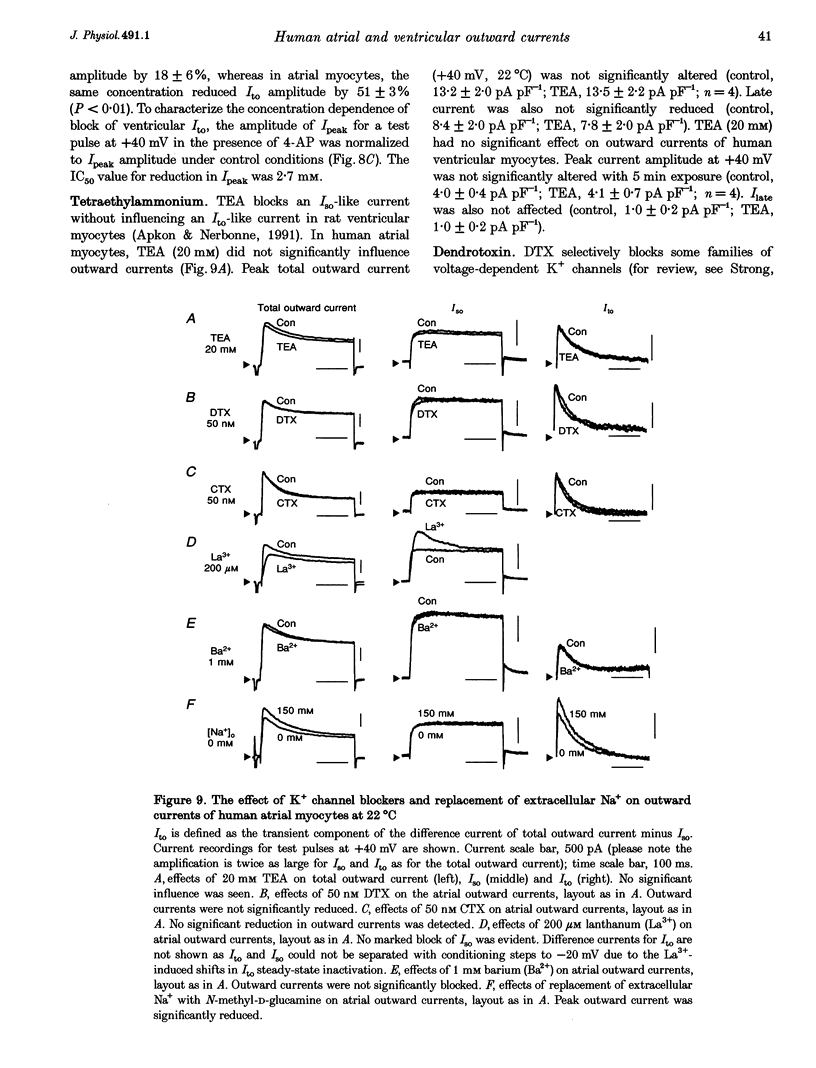
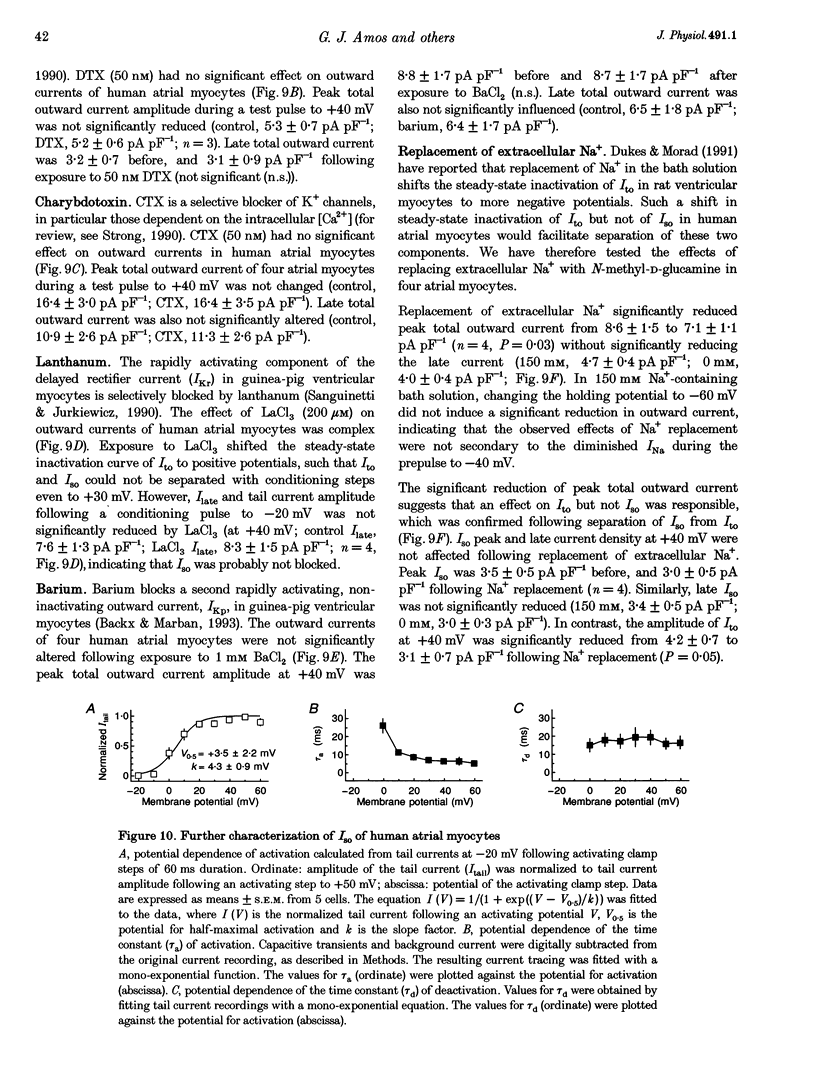
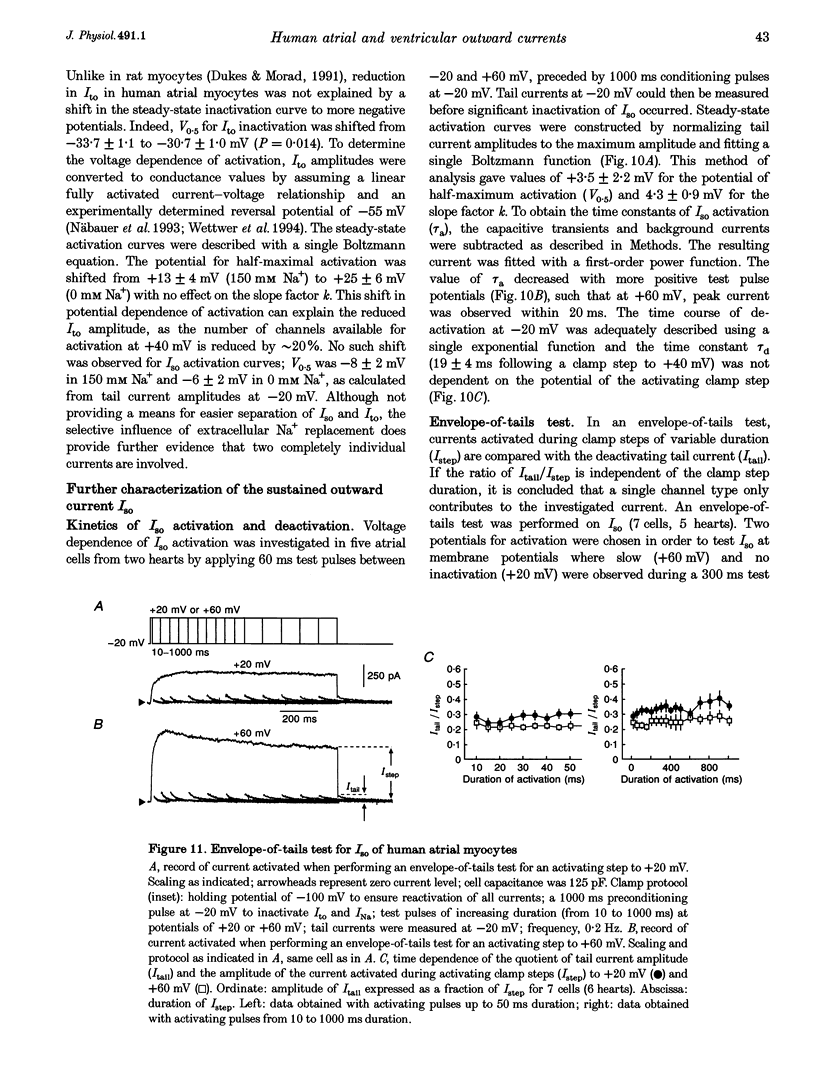
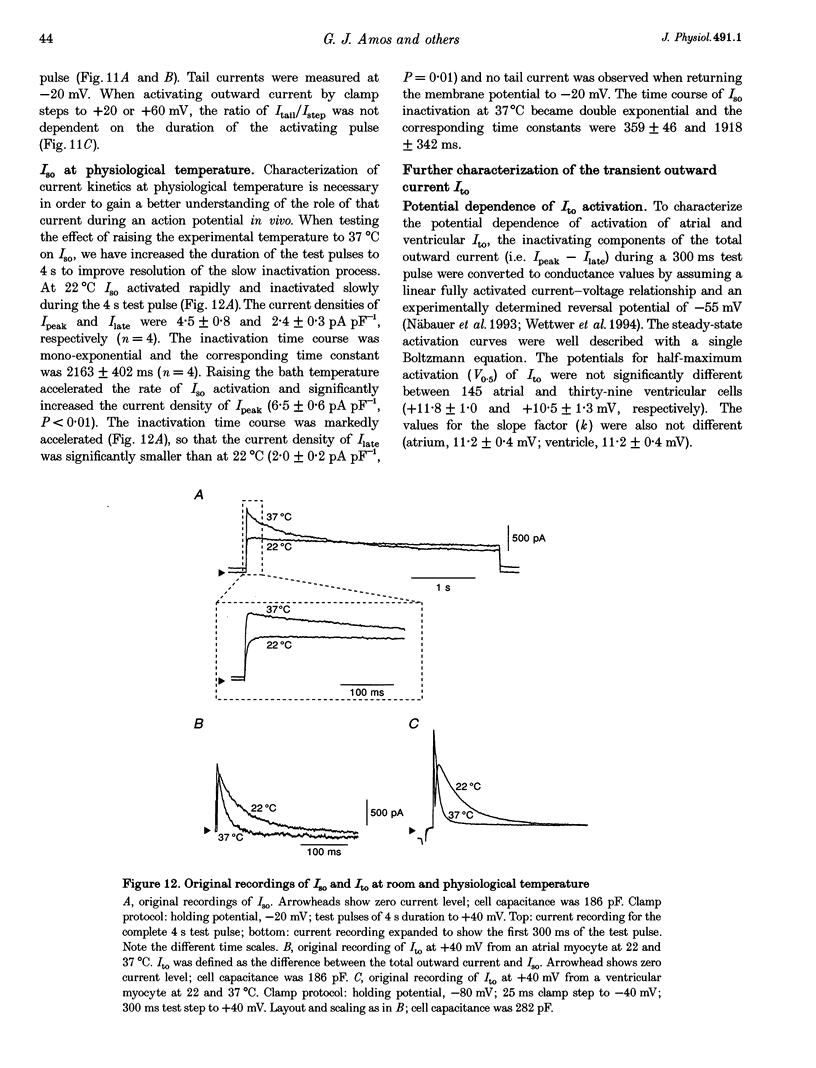
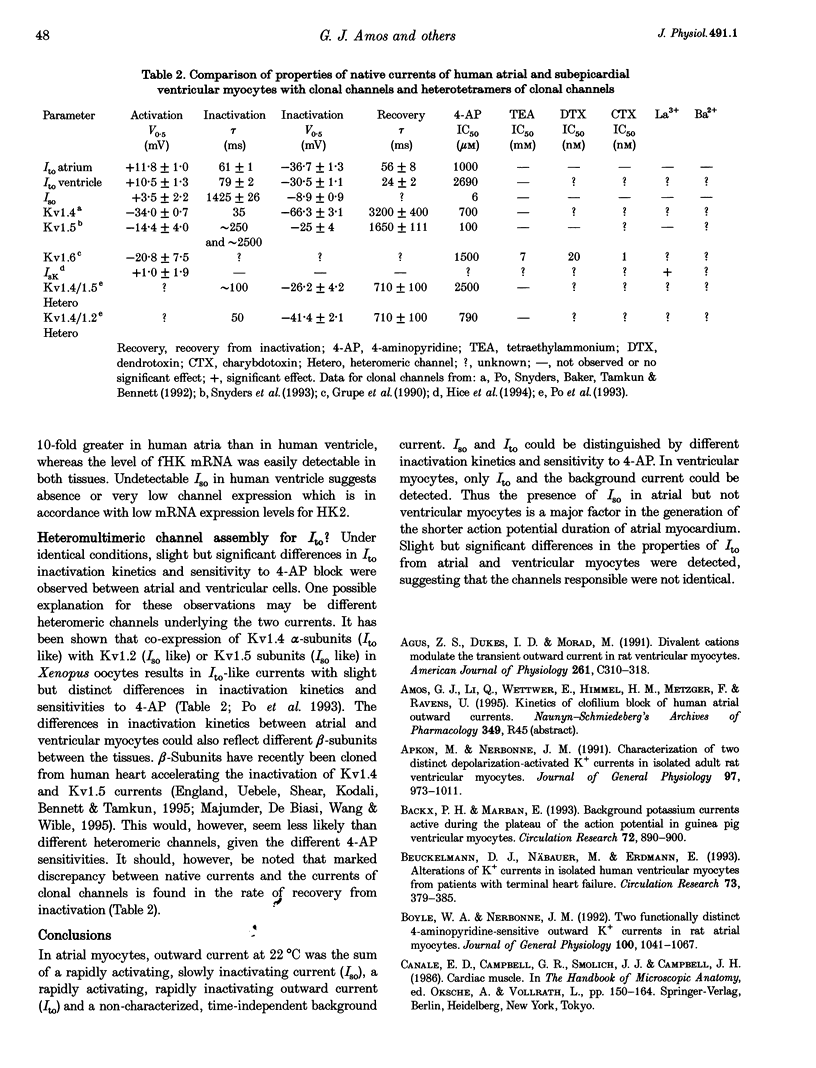
1. Outward currents were studied in myocytes isolated from human atrial and subepicardial ventricular myocardium using the whole-cell voltage clamp technique at 22 degrees C. The Na+ current was inactivated with prepulses to -40 mV and the Ca2+ current was eliminated by both reducing extracellular [Ca2+] to 0.5 mM and addition of 100 microM CdCl2 to the bath solution. 2. In human myocytes, three different outward currents were observed. A slowly inactivating sustained outward current, I(so), was found in atrial but not ventricular myocytes. A rapidly inactivating outward current, I(to), of similar current density was observed in cells from the two tissues. An additional uncharacterized non-inactivating background current of similar size was observed in atrial and in ventricular myocytes. 3. I(to) and I(so) could be differentiated in atrial myocytes by their different kinetics and potential dependence of inactivation, and their different sensitivities to block by 4-amino-pyridine, suggesting that two individual channel types were involved. 4. In atrial cells, inactivation of I(to) was more rapid and steady-state inactivation occurred at more negative membrane potentials than in ventricular cells. Furthermore, the recovery of I(to) from inactivation was slower and without overshoot in atrial myocytes. In addition, 4-aminopyridine-induced block of I(to) was more efficient in atrial than in ventricular cells. These observations suggest that the channels responsible for atrial and ventricular I(to) were not identical. 5. We conclude that the differences in outward currents substantially contribute to the particular shapes of human atrial and ventricular action potentials. The existence of I(so) in atrial cells only provides a clinically interesting target for anti-arrhythmic drug action, since blockers of I(so) would selectively prolong the atrial refractory period, leaving ventricular refractoriness unaltered.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Dukes I. D., Morad M. Divalent cations modulate the transient outward current in rat ventricular myocytes. Am J Physiol. 1991 Aug;261(2 Pt 1):C310–C318. doi: 10.1152/ajpcell.1991.261.2.C310. [DOI] [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx P. H., Marban E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ Res. 1993 Apr;72(4):890–900. doi: 10.1161/01.res.72.4.890. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Boyle W. A., Nerbonne J. M. Two functionally distinct 4-aminopyridine-sensitive outward K+ currents in rat atrial myocytes. J Gen Physiol. 1992 Dec;100(6):1041–1067. doi: 10.1085/jgp.100.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Slawsky M. T. Characterization of 4-aminopyridine block of the transient outward K+ current in adult rat ventricular myocytes. J Pharmacol Exp Ther. 1993 Jun;265(3):1450–1459. [PubMed] [Google Scholar]
- Dukes I. D., Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol. 1991 Apr;435:395–420. doi: 10.1113/jphysiol.1991.sp018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S. K., Uebele V. N., Shear H., Kodali J., Bennett P. B., Tamkun M. M. Characterization of a voltage-gated K+ channel beta subunit expressed in human heart. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6309–6313. doi: 10.1073/pnas.92.14.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Fedida D., Wible B., Wang Z., Fermini B., Faust F., Nattel S., Brown A. M. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993 Jul;73(1):210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Koumi S., Sakakibara Y., Singer D. H., Jia H., Arentzen C. E., Backer C. L., Wasserstrom J. A. An analysis of lidocaine block of sodium current in isolated human atrial and ventricular myocytes. J Mol Cell Cardiol. 1995 Feb;27(2):831–846. doi: 10.1016/0022-2828(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Grajek S., Lesiak M., Pyda M., Zajac M., Paradowski S., Kaczmarek E. Hypertrophy or hyperplasia in cardiac muscle. Post-mortem human morphometric study. Eur Heart J. 1993 Jan;14(1):40–47. doi: 10.1093/eurheartj/14.1.40. [DOI] [PubMed] [Google Scholar]
- Grupe A., Schröter K. H., Ruppersberg J. P., Stocker M., Drewes T., Beckh S., Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990 Jun;9(6):1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hice R. E., Folander K., Salata J. J., Smith J. S., Sanguinetti M. C., Swanson R. Species variants of the IsK protein: differences in kinetics, voltage dependence, and La3+ block of the currents expressed in Xenopus oocytes. Pflugers Arch. 1994 Jan;426(1-2):139–145. doi: 10.1007/BF00374681. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Furukawa T., Singer D. H., Sakakibara Y., Eager S., Backer C., Arentzen C., Wasserstrom J. A. Characteristics of lidocaine block of sodium channels in single human atrial cells. J Pharmacol Exp Ther. 1993 Mar;264(3):1275–1284. [PubMed] [Google Scholar]
- Liu D. W., Gintant G. A., Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993 Mar;72(3):671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- McCullough J. R., Chua W. T., Rasmussen H. H., Ten Eick R. E., Singer D. H. Two stable levels of diastolic potential at physiological K+ concentrations in human ventricular myocardial cells. Circ Res. 1990 Jan;66(1):191–201. doi: 10.1161/01.res.66.1.191. [DOI] [PubMed] [Google Scholar]
- Mewes T., Ravens U. L-type calcium currents of human myocytes from ventricle of non-failing and failing hearts and from atrium. J Mol Cell Cardiol. 1994 Oct;26(10):1307–1320. doi: 10.1006/jmcc.1994.1149. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Beuckelmann D. J., Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- Po S., Roberds S., Snyders D. J., Tamkun M. M., Bennett P. B. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res. 1993 Jun;72(6):1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Po S., Snyders D. J., Baker R., Tamkun M. M., Bennett P. B. Functional expression of an inactivating potassium channel cloned from human heart. Circ Res. 1992 Sep;71(3):732–736. doi: 10.1161/01.res.71.3.732. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Furukawa T., Singer D. H., Jia H., Backer C. L., Arentzen C. E., Wasserstrom J. A. Sodium current in isolated human ventricular myocytes. Am J Physiol. 1993 Oct;265(4 Pt 2):H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995 Apr 21;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Drury T., Refsum H., Aldrete V., Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989 Dec;257(6 Pt 2):H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Snyders D. J., Tamkun M. M., Bennett P. B. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993 Apr;101(4):513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G., NELSON R. M., HECHTHH Electrophysiological study of human heart muscle. Circ Res. 1962 Mar;10:306–312. doi: 10.1161/01.res.10.3.306. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Knoth K. M., Walbridge J. A., Kroemer H., Roden D. M., Glover D. M. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991 Mar 1;5(3):331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- Ten Eick R. E., Singer D. H. Electrophysiological properties of diseased human atrium. I. Low diastolic potential and altered cellular response to potassium. Circ Res. 1979 Apr;44(4):545–557. doi: 10.1161/01.res.44.4.545. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Lauribe P., Escande D. A hyperpolarization-activated inward current in human myocardial cells. J Mol Cell Cardiol. 1992 May;24(5):451–455. doi: 10.1016/0022-2828(92)91833-q. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993 Aug;73(2):276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993 Dec;73(6):1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Wettwer E., Amos G. J., Posival H., Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994 Sep;75(3):473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Wettwer E., Amos G., Gath J., Zerkowski H. R., Reidemeister J. C., Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovasc Res. 1993 Sep;27(9):1662–1669. doi: 10.1093/cvr/27.9.1662. [DOI] [PubMed] [Google Scholar]


