Abstract
Background
Patients with light-chain (AL) amyloidosis and concomitant multiple myeloma (MM) are known to have a worse prognosis, while the prognostic implication of cytogenetic abnormalities (CA) and optimal treatment schemes are not well-established. By comparing patients with MM or AL amyloidosis (AL) alone, this study aimed to evaluate the clinical characteristics, CA, and outcomes of patients with AL amyloidosis and concomitant symptomatic MM (MM-AL) and sought to provide evidence for their management.
Methods
In total, 915 consecutive patients with newly diagnosed AL amyloidosis or MM were retrospectively analyzed. Patients were classified as MM-alone, MM-AL or AL-alone. The presence of symptomatic MM was based on the International Myeloma Working Group criteria, and the diagnosis of AL amyloidosis was confirmed by Congo-red-positive biopsy and immunoelectron microscopy.
Results
Of 915 patients, 658, 106, and 151 were in the MM-alone group, MM-AL group, and AL-alone group, respectively. The three groups shared a similar incidence rate of CA, while the prevalence of t(11;14) was significantly higher in the AL-alone group than in the MM-AL and MM-alone group (40.7% vs. 25.7% vs. 16.6%, p < 0.001), and the prevalence of del13q, gain1q21 and high-risk CA (HRCA) decrease in turn in MM-alone, MM-AL and AL-alone group (del13q, 46.5% vs. 39.4% vs. 28.5%, p < 0.001; gain1q21, 52.6% vs. 45.2% vs. 27.3%, p < 0.001; HRCA, 27.5% vs. 16.0 vs. 7.3%, p < 0.001). The progression-free survival (PFS) and overall survival (OS) of MM-AL patients (median, 12.8, and 25.2 months) were significantly inferior to patients with MM-alone and AL-alone. No significant difference in PFS and OS was found between MM-AL patients with and without HRCA. When stratified by the type of plasma cell disease and status of t(11;14), patients with MM-AL and t(11;14) presented the worst OS (median, 8.2 months, p < 0.001). Regarding the management of MM-AL, extended cycles of induction therapy and the use of maintenance therapy contributed to a better prognosis.
Conclusions
There was an apparent discrepancy in the distribution and prognostic implication of CA among different plasma cell diseases. Patients with MM-AL had the worst clinical outcomes, requiring extended duration of induction therapy and maintenance therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13219-0.
Keywords: Light-chain amyloidosis, Multiple myeloma, Cytogenetic abnormality, Prognosis
Background
Light chain (AL) amyloidosis and multiple myeloma (MM) are both plasma cell dyscrasias (PCDs), while the two disorders present with different phenotypes. AL amyloidosis is characterized by multiorgan damage resulting from the toxic effects of circulating light chains and their deposition as amyloid [1]. When patients with AL amyloidosis have myeloma-defining events, including hypercalcemia, renal insufficiency, anemia, and lytic bone lesions (CRAB criteria) [2], they are considered to have concomitant MM. The relationship between MM and AL amyloidosis is complex. AL amyloidosis can occur with the initial diagnosis of MM [3] and can also develop later among as many as 12–30% of MM patients through the course of their disease [4, 5]. Conversely, MM has been estimated to coexist in about 10–20% of patients with biopsy-proven AL amyloidosis [6–8].
Although patients with MM and AL amyloidosis share an identical spectrum of cytogenetic abnormalities (CA), the distribution and prognostic value of these aberrations differ. AL amyloidosis has a notably higher prevalence of t(11;14) than MM, with a frequency ranging from 40 to 60%, while the incidence rates of gain1q21, t(4;14), del17p and del13q seem to be lower in AL amyloidosis [9, 10]. The pathogenetic and prognostic role of fluorescence in situ hybridization (FISH) results has been well established in MM and was incorporated into the staging and risk stratification of MM: t(11;14) and del13q were found as standard prognostic factors, t(4;14), t(14;16) and del17p were established as high-risk cytogenetic abnormalities (HRCA) [11]. Meanwhile, their prognostic relevance in AL amyloidosis is still evolving. So far, AL patients with t(11;14) have been reported to have less favorable hematologic response and inferior survival with proteasome inhibitors (PIs, particularly bortezomib) [9, 12, 13] and immunomodulatory agents (iMiDs) [9], but tend to respond more favorably to alkylator therapy (including autologous stem cell transplantation (ASCT)) [14, 15] and Daratumumab [16]. Besides, gain1q21 [17], del17p [18], and trisomies [9, 19] also appeared to have an adverse prognostic effect on AL amyloidosis. Nevertheless, most studies about FISH abnormalities in AL amyloidosis were analyzed in patients without concurrent MM. Limited investigations revealed the distribution and prognostic implication of CA in AL patients with concomitant symptomatic MM (MM-AL).
It has been reported that AL patients with concomitant symptomatic MM have a worse prognosis [8, 20, 21]. However, little is known about the management of these patients, including the optimal duration of induction therapy and maintenance therapy, as they are often unrecognized or are commonly excluded from clinical trials. Therefore, this study aimed to make up for the vacancy of detailed information on patients with MM-AL. By comparing with patients with MM or AL amyloidosis (AL) alone, we identified and evaluated the clinical characteristics, CA, outcomes, and prognostic factors of patients with MM-AL and sought to provide evidence for the management of these patients.
Methods
Subjects
This study retrospectively enrolled consecutive patients with newly diagnosed AL amyloidosis between January 2007 and September 2023 or newly diagnosed MM (NDMM) between January 2007 and October 2021 in Zhongshan Hospital Fudan University. A total of 915 patients with available FISH profiles were ultimately analyzed. The diagnosis of symptomatic MM was in accordance with International Myeloma Working Group (IMWG) criteria [2]: bone marrow plasma cell (BMPC) > 10% or biopsy-proven bony or extramedullary plasmacytoma, and evidence of myeloma-defining events, including the CRAB-SLiM criteria. The diagnosis of AL amyloidosis was based on a Congo-red-positive biopsy and immunoelectron microscopy study of the amyloid deposition in the involved organs (including kidney, liver, heart, nerve, digestive tract, and others), abdominal fat, and/or labial salivary. Immunohistochemistry and immunofluorescence further confirmed the typing of kappa or lambda light chain. In patients with AL amyloidosis, those who had > 10% BMPC and any myeloma-defining events (CRAB-SLiM criteria) were classified as MM-AL. Otherwise, patients were classified as AL-alone or MM-alone. Besides, patients with > 10% BMPC but without CRAB-SLiM criteria were also assigned to the AL-alone group. The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Zhongshan Hospital Fudan University (approval number: B2023-185R), Shanghai, China. All patients authorized the use of their electronic medical record data for research.
Clinical Data
Data were collected from patients’ electronic medical records. The FISH test was performed in the central laboratory according to standard protocols with purified CD138(+) BMPC, using probes including del17p, del13q14, gain1q21, t(11;14), t(4;14) and t(14;16). The definition of high-risk cytogenetic abnormalities followed the Mayo Clinic mSMART [22]. The definition of organ involvement in AL amyloidosis was based on consensus criteria in the National Comprehensive Center Network (NCCN) guideline [23]. Cardiac involvement was assessed by the European 2015 modification of the Mayo 2004 model [24] and the revised Mayo 2012 model [25].
Outcomes and endpoints
Outcomes assessed in this study included the best hematological response rate to first-line therapy, minimal resident disease (MRD), progression-free survival (PFS) and overall survival (OS) of all patients, and organ response rate and event-free survival (EFS) of patients with AL amyloidosis with or without contaminant MM. First-line therapy was considered as the first-line therapy given regardless of subsequent modifications. The definition of hematological responses in patients with multiple myeloma followed the IMWG consensus criteria for response in 2016 [26], and the definition of hematological response and organ response in patients with AL amyloidosis was based on the AL consensus criteria [27]. MRD detection was performed with the flow cytometry method (FCM) every two cycles of induction therapy. After July 2017, eight-color antibody combinations (CD38/CD138/CD19/CD56/CD45/CD81/CD117 and κ/λ light chain) were adopted for MRD assessment with sensitivity at 10− 6. The flow cytometry was conducted and interpreted at an independent laboratory by two experienced professionals. PFS was defined as the duration from diagnosis to hematologic progression or death, whichever came first. OS was defined as the duration from diagnosis to the last follow-up date or death. EFS, which applied only to patients with AL amyloidosis, was defined as the duration from diagnosis to major organ deterioration, subsequent treatment, hematologic progression, or death.
Statistical analysis
Patient demographics and other disease characteristics were summarized using frequencies and relative frequencies for categorical variables; medians and ranges were computed for continuous numerical variables. The Student’s t-tests were conducted to compare continuous variables if the variance was homogeneous or Mann–Whitney was applied. The categorical variables were assessed by Fisher’s exact test or Pearson’s chi-squared test. Survival outcomes, including PFS, EFS, and OS, were evaluated using the Kaplan-Meier (KM) method, and comparisons among subgroups were performed via log-rank tests. Besides, when investigating the necessity of maintenance in patients with MM-AL, we estimated EFS and OS through the KM method using a landmark survival analysis, which was restricted to patients surviving for over three months. Optimal cut points for continuous variables associated with survival were identified by examination of receiver operating characteristics (ROC) analyses. Univariate and multivariate Cox regression analyses were applied to assess the EFS and OS of patients with MM-AL. Factors with p-value ≤ 0.10 in univariate analyses were included in the multivariate model. The p < 0.05 on two sides was considered statistically significant. All data were analyzed by SPSS (version 27.0) and STATA (version 17.0).
Results
Baseline clinical characteristics
A total of 915 consecutive patients were analyzed in this study, including 106 patients with MM-AL, 658 patients with MM-alone, and 151 patients with AL-alone. The median follow-up of the MM-alone, MM-AL, and AL-alone groups was 31.1 months, 33.0 months, and 21.8 months, respectively. Baseline clinical characteristics are reported in Table 1. In the MM-AL group, the median age at diagnosis was 65 years old (range 41–82), similar to the MM-alone group (median, 65; p = 0.661) but older than the AL-alone group (median, 63; p = 0.004). There was no significant difference in the proportions of males among the MM-alone, MM-AL, and AL-alone groups (61.6% vs. 68.9% vs. 69.5%, p = 0.909). An ECOG PS > 2 was found in 25.5% of patients with MM-AL, which was higher than in patients with MM-alone (10.2%, p < 0.001) and AL-alone (18.5%, p = 0.182). The level of BMPC decreased significantly in turn in patients with MM-alone, MM-AL, and AL-alone (median, 37.5% vs. 15% vs. 8%; p < 0.001). As for immunoglobulin subtypes, 40.5%/19.8%/4.7%/0.9%/17.0% of patients with MM-AL had IgG/IgA/IgM/IgD/light-chain subtype, and 16.0% had negative IFE results. Lambda restriction was dominant in both MM-AL and AL-alone groups (71.7% vs. 77.5%, p = 0.176), and the two groups shared a similar distribution of organ involvement, with 91.5% and 86.8% having cardiac involvement, 30.2% and 40.4% having renal involvement, and 11.3% and 9.9% having hepatic involvement, respectively. Laboratory testing significantly differed among the three groups. Compared to patients with MM-alone and AL-alone, patients with MM-AL showed higher levels of involved and uninvolved free light-chain (dFLC), alkaline phosphatase, and creatinine, and lower levels of albumin and estimated glomerular filtration rate. Notably, levels of N-Terminal pro-brain natriuretic peptide (NT-proBNP) and Revised 2012 Mayo AL amyloidosis stage in the MM-AL group were significantly worse than the AL-alone group, indicating the higher severity of cardiac involvement at baseline in AL patients with concomitant symptomatic myeloma. The majority of patients in the MM-alone group were treated with PI-based and PI + IMiD-based therapies. Over 80% of patients with MM-AL or AL-alone received a PI-based regimen, as the use of IMiDs in AL amyloidosis was typically challenging due to poor tolerability in patients with significant cardiac involvement. (Details about the first-line therapeutic regimens are listed in Supplementary Table 1).
Table 1.
Demographics and clinical characteristics of patients with MM-alone, MM-AL and AL-alone
| MM-alone N = 658 |
MM-AL N = 106 |
AL-alone N = 151 |
P value (MM-alone vs. MM-AL) |
P value (MM-AL vs. AL-alone |
P value (overall) |
|
|---|---|---|---|---|---|---|
| Male, n(%) | 405 (61.6) | 73 (68.9) | 105 (69.5) | 0.149 | 0.909 | 0.092 |
| Age (years), median(range) | 65 (32–87) | 65 (41–82) | 63 (38–83) | 0.661 | 0.004 | 0.002 |
| ECOG performance status, n(%) | ||||||
| 0–2 | 591 (89.8) | 79 (74.5) | 123 (81.5) | < 0.001 | 0.182 | < 0.001 |
| >2 | 67 (10.2) | 27 (25.5) | 28 (18.5) | |||
| IFE, n(%) | < 0.001 | 0.093 | < 0.001 | |||
| IgG | 356 (54.1) | 43 (40.5) | 44 (29.1) | |||
| IgA | 167 (25.4) | 21 (19.8) | 28 (18.5) | |||
| IgM | 5 (0.8) | 5 (4.7) | 5 (3.3) | |||
| IgD | 20 (3.0) | 1 (0.9) | 1 (0.7) | |||
| Light chain | 86 (13.1) | 18 (17.0) | 24 (25.9) | |||
| Negative | 24 (3.6) | 17 (16.0) | 49 (32.5) | |||
| Missing | 0 (0) | 1 (0.9) | 0 (0) | |||
| BMPCs(%),median(range) | 37.5 (0–99) | 15 (1–80) | 8 (1–40) | < 0.001 | < 0.001 | < 0.001 |
| BMPC ≥ 10%, n(%) | 575 (87.7) | 87 (82.1) | 71 (47.7) | 0.115 | < 0.001 | < 0.001 |
| Organ involvement, n(%) | ||||||
| Heart | - | 97 (91.5) | 131 (86.8) | - | 0.236 | - |
| Kidney | - | 32 (30.2) | 61 (40.4) | - | 0.094 | - |
| Liver | - | 12 (11.3) | 15 (9.9) | - | 0.721 | - |
| Soft tissues | - | 73 (68.9) | 116 (76.8) | - | 0.155 | - |
| Lung | - | 0 (0) | 2 (1.3) | - | 0.234 | - |
| Peripheral neuropathy | - | 1 (0.9) | 3 (2.0) | - | 0.506 | - |
| Gastrointestinal tract | - | 3 (2.8) | 2 (1.3) | - | 0.390 | - |
| Spleen | - | 0 (0) | 1 (0.7) | - | 0.401 | - |
| Number of involved organs, median (range) | - | 2 (1–4) | 2 (1–5) | - | 0.138 | - |
| Lambda restriction, n(%) | - | 76 (71.7) | 117 (77.5) | - | 0.176 | - |
| dFLC (mg/L), median(range) | 236.9 (0.2-34886.9) | 253.25 (3.7-3500.3) | 125.1 (1.0-1691.4) | 0.566 | < 0.001 | < 0.001 |
| Serum hemoglobin(g/L), median(range) | 97.5 (35–168) | 109.5 (60–168) | 131 (71–179) | < 0.001 | < 0.001 | < 0.001 |
| Serum albumin(g/L), median(range) | 37 (16–51) | 35 (14–49) | 36 (14–62) | < 0.001 | 0.317 | < 0.001 |
| Serum alkaline phosphatase(U/L), median(range) | 72 (18–791) | 81.5 (35–700) | 80 (35-1054) | < 0.001 | 0.734 | < 0.001 |
| Serum creatine(µmol/L), median(range) | 91 (34-1185) | 94.5 (47–597) | 83 (41–515) | 0.749 | 0.027 | 0.045 |
| eGFR(mL/min/1.73m2), median(range) | 71 (3-120) | 65.5 (7-120) | 79 (7-111) | 0.952 | 0.008 | 0.003 |
| LDH(U/L), median(range) | 169 (58-3542) | 215 (98–429) | 223.5 (98-2967) | < 0.001 | 0.237 | < 0.001 |
| β2-microglobulin(mg/L), median(range) | 4.80 (1.14–58.38) | 4.45 (1.43–26.09) | 3.42 (1.51–15.14) | 0.198 | 0.003 | < 0.001 |
| NT-proBNP(pg/mL), median(range) | 187.5 (1.4->35000) | 3976.0 (42.0->35000) | 3099.0 (28.5->35000) | < 0.001 | 0.070 | < 0.001 |
| cTNT(ng/mL), median(range) | 0.011 (0.001–0.962) | 0.0815 (0.010–0.448) | 0.067 (0.003–0.655) | < 0.001 | 0.119 | < 0.001 |
| Proteinuria (g/24 h), median(range) | - | 0.72 (0.06–36.33) | 0.62 (0.03–13.88) | - | 0.690 | - |
| European 2015 modification of 2004 Mayo model, I/II/IIIa/IIIb (%) | - | 7.5/ 12.3/ 55.7/ 24.5 | 12.6/ 11.9/ 60.3/ 15.2 | - | 0.207 | - |
| Revised 2012 Mayo model, I/II/III/IV (%) | - | 3.8/ 12.3/ 37.7/ 46.2 | 13.5/ 14.9/ 40.5/ 31.1 | - | 0.015 | - |
| First-line induction therapy, n(%) | ||||||
| PI-based | 364 (55.3) | 93 (87.7) | 127 (84.1) | < 0.001 | 0.415 | < 0.001 |
| IMiD-based | 32 (4.9) | 0 (0.0) | 3 (2.0) | 0.020 | 0.144 | 0.023 |
| PI + IMiD-based | 261 (39.7) | 9 (8.5) | 9 (6.0) | < 0.001 | 0.434 | < 0.001 |
| Dara-baseda | 1 (0.2) | 3 (2.8) | 10 (6.6) | < 0.001 | 0.172 | < 0.001 |
| Othersb | 0 (0.0) | 1 (0.9) | 2 (1.3) | 0.013 | 0.779 | 0.018 |
| Cycles of induction therapy, median(range) | 8 (0.25-26) | 2 (0.25-12) | 4 (0.25-12) | < 0.001 | 0.235 | < 0.001 |
| ASCT, n(%) | 95 (14.4) | 0 (0) | 2 (1.3) | < 0.001 | 0.234 | < 0.001 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IFE, immunofixation electrophoresis; iFLC, involved free light chain; dFLC, difference in involved and uninvolved free light chain; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; BMPC, bone marrow plasmacytosis; PI, protease inhibitor; IMiD, immunomodulatory drug; Dara, daratumumab; ASCT, autologous stem cell transplantation
aDara-based: the therapeutic regimens in one patient with AL-alone was Dara plus dexamethasone, the others were combined with PI-based therapies
bIncluding supportive treatment and chemo-only therapy
Cytogenetic abnormalities
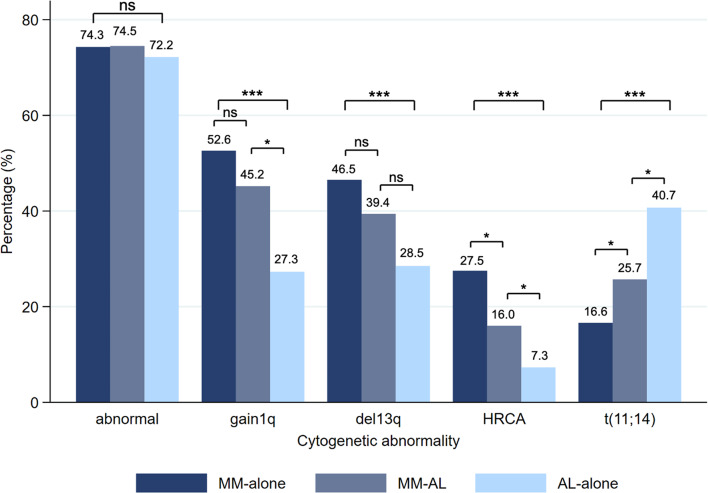
The landscape of CA in patients with MM-alone, MM-AL, and AL-alone is shown in Fig. 1. The three groups shared a similar incidence rate of cytogenetic abnormalities (74.3% vs. 74.5% vs. 72.2%, p = 0.857). The prevalence of t(11;14) was significantly higher in the AL-alone group than MM-AL and MM-alone group (40.7% vs. 25.7% vs. 16.6%, p < 0.001), while the incidence rate of del13q, gain1q21 and IMWG HRCA (including t(4;14), t(14;16) and del17p) decreased in turn in MM-alone, MM-AL and AL-alone group (del13q, 46.5% vs. 39.4% vs. 28.5%, p < 0.001; gain1q21, 52.6% vs. 45.2% vs. 27.3%, p < 0.001; HRCA, 27.5% vs. 16.0 vs. 7.3%, p < 0.001). Regarding HRCA, we observed a paucity of t(4;14)-7 cases, del17p-5 cases, and t(14;16)-0 cases in AL-alone group. In general, the distribution of CA in patients with MM-AL lay somewhere between those in patients with MM-alone or AL-alone.
Fig. 1.
Distributions of cytogenetic abnormalities in patients with MM-alone, MM-AL, and AL-alone. ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001. HRCA, high-risk cytogenetic abnormalities, including t(4;14), t(14;16) and del17p
Clinical outcomes
Hematologic response, organ response, and MRD status
The best hematological response to first-line induction therapy was available for 570 (86.6%) patients of the MM-alone group, 75 (70.8%) of the MM-AL group, and 114 (75.5%) of the AL-alone group. When comparing the MM-AL group with the MM-alone group and the AL-alone group, we evaluated the hematological response based on IMWG consensus criteria and AL consensus criteria, respectively. The overall response rate (ORR, partial response or better) of patients with MM-AL was significantly inferior to patients with MM-alone (72.4% vs. 90.4%, p < 0.001), as well as very good partial response or better (≥ VGPR, 48.0% vs. 68.9%, p < 0.001), and complete response (CR, 22.7% vs. 41.8%, p < 0.001). MM-AL patients had similar ORR (77.3% vs. 82.5%, p = 0.385) compared with the AL-alone group, while a far smaller proportion of them achieved deeper hematologic response rate (≥ VGPR, 58.7% vs. 72.8%, p = 0.043; CR, 18,7% vs. 41.2%, p = 0.001). In terms of organ response, no significant difference in rates of cardiac response (36.2% vs. 31.8%, p = 0.493) and renal response (43.3% vs. 39.7%, p = 0.739) was found between patients with MM-AL and AL-alone. Meanwhile, patients in the AL-alone group achieved higher hepatic response rates (5/14, 35.7%) than the MM-AL group (0/11, 0%; p = 0.027). Besides, the overall MRD negative rate of patients with MM-AL (10/43, 23.3%) during first-line therapy was lower than that in patients with MM-alone (217/602, 36.0%; p = 0.090) and AL-alone (36/86, 41.9%; p = 0.038).
Moreover, we compared the treatment response and MRD negative rate among patients receiving the triple regimen of bortezomib, cyclophosphamide, and dexamethasone (CyBorD) (Supplementary Table 2), which was received by 37.1%, 50.0%, and 60.9% of patients in the MM-alone, MM-AL, and AL-alone group, respectively. Similarly, patients with MM-AL showed an inferior hematologic response and MRD negative rate compared to patients with MM-alone or AL-alone.
Survival
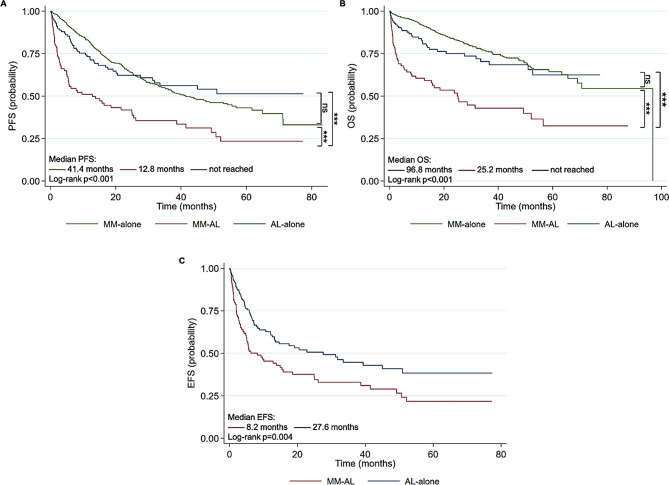
During a median follow-up of 30.8 months among all patients, the probability of PFS was significantly shorter in patients with MM-AL (median, 12.8 months) compared to patients with MM-alone (median, not reached; p < 0.001) and AL-alone (median, 41.4 months; p < 0.001)(Fig. 2A). Likewise, the possibility of OS of the MM-AL group (median, 25.2 months) was significantly inferior to the MM-alone group (median, 96.8 months; p < 0.001) and AL-alone group (median, not reached; p < 0.001) (Fig. 2B). Furthermore, taking into account survival free from organ deterioration and hematologic progression, which was defined as EFS in this study, the MM-AL group also had poorer EFS than the AL-alone group (median, 8.2 months vs. 27.6 months; p = 0.004)(Fig. 2C). In addition, in terms of early mortality, 27.4% and 32.1% of patients in the MM-AL group died within 3 and 6 months of diagnosis, respectively, which was significantly higher than in the MM-alone group (3 months, 2.9%, p < 0.001; 6 months, 4.1%, p < 0.001), and AL-alone group (3 months, 7.9%, p < 0.001; 6 months, 9.9%, p < 0.001).
Fig. 2.
Kaplan-Meier curves for progression-free survival (A) and overall survival (B) of patients with MM-alone, MM-AL, and AL-alone. Kaplan-Meier curved for event-free survival (C) of patients with AL MM-AL and AL-alone
Prognostic factors related to outcomes of MM-AL patients
Cytogenetic abnormalities
Patients with AL-alone harboring t(11;14) had a lower rate of hematologic response (≥ VGPR) to first-line treatment compared with patients who did not (64.0% vs. 81.0%, p = 0.043). Whereas, for patients with MM-AL, no significant difference in ≥ VGPR rate between t(11;14)-negative and -positive groups was observed (63.2% vs. 46.7%, p = 0.247). There was also no statistical difference in the rate of ≥ VGPR when MM-AL patients were stratified by the status of other CA, including HRCA, del3q14, and gain1q21. Besides, Bortezomib-based regimens did not result in significantly different rates of ≥ VGPR in MM-AL patients between t(11;14)-negative and -positive groups (69.2% vs. 58.3, p = 0.484), as well as other CA groups. Detailed information about rates of ≥ VGPR of patients with different PCDs stratified by FISH status and regimen type was listed in Supplementary Table 3.
In terms of survival, stratifications by type of PCDs and CA status were also analyzed. In the MM-alone group, patients harboring HRCA had significantly inferior PFS (median, 23.6 vs. 64.9 months; p < 0.001) and OS (median, 65.6 months vs. not reached; p < 0.001), which has been well-established in previous studies [11]. However, no significant difference in PFS and OS was found between MM-AL patients with HRCA-positivity and -negativity (median PFS, 5.6 vs. 13.8 months, p = 0.671; median OS, 16.4 vs. 25.5 months, p = 0.391), as well as patients with AL-alone (median PFS, not reached vs. not reached, p = 0.538; median OS, not reached vs. not reached, p = 0.079) (Supplementary Fig. 1A and 1B), indicating the distinct prognostic implication of common HRCAs among the three groups. Additionally, compared with MM-alone patients with HRCA-positivity, MM-AL patients with HRCA-positivity had significantly shorter OS (p < 0.001). On the other hand, t(11;14) was a CA that had been reported to strongly correlate to poor outcome of AL [9, 12, 13], whereas in our cohort, no significant difference in PFS (median, not reached vs. 51.0 months; p = 0.320) and OS (median, not reached vs. not reached; p = 0.300) was observed between t(11;14)-positive and t(11;14)-negative patients with AL-alone. Patients with MM-AL and t(11;14)-positivity experienced the worst PFS (median, 5.8 months) and OS (median, 8.2 months), which was significantly inferior to PFS and OS of all the MM-alone and AL-alone subgroups, while there was no statistical difference when they were compared with patients with MM-AL and t(11;14)-negativity (median PFS 15.8 months, p = 0.422; median OS 31.5 months, p = 0.229) (Supplementary Fig. 1C and 1D). PFS and OS classified by type of PCDs and status of other CAs, including presence and absence of all CA, del13q14, and gain1q21, were also analyzed, while no significant difference in MM-AL subgroups was detected (Supplementary Fig. 2).
Organ involvement
MM-AL patients with cardiac involvement had worse survival compared to their non-cardiac counterparts, with EFS (median, 5.6 vs. 41.5 months; p = 0.028) and OS (median, 18.6 vs. not reached; p = 0.017). There was no statistical difference in EFS (median, 15.5 vs. 5.8 months; p = 0.214) and OS (median, 24.9 vs. 25.2 months; p = 0.468) of patients with and without renal involvement. Besides, since all the MM-AL patients with hepatic involvement in our cohort had coexistent cardiac involvement, we further classified them into four groups based on involved organs (i.e., liver + heart, heart, kidney, and others). Patients with both hepatic and cardiac involvement had numerically shorter EFS than patients with cardiac involvement only (median, 4.3 vs. 9.7 months; p = 0.147), as well as OS (median, 4.3 months vs. 25.2 months; p = 0.061). Whereas the EFS and OS of cardiac amyloidosis patients, with or without coexisting hepatic involvement, were significantly inferior to those with renal involvement (median EFS, not reached; median OS, not reached) or any other organ involvement (median EFS, 9.3 months; median OS, 56.5 months).
Duration of induction therapy and use of maintenance therapy
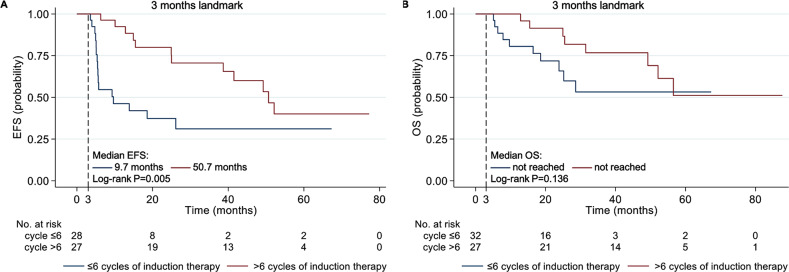
To address the optimal duration of first-line induction therapy, we divided the MM-AL patients into two groups, the cycle ≤ 6 group (n = 40) and the cycle > 6 group (n = 27), and those who had received less than two cycles of induction therapy were excluded. However, the CR rate was comparable between the cycle ≤ 6 group and the cycle > 6 group (13.2% vs. 30.8%, p = 0.085), fewer patients in the cycle ≤ 6 group achieved ≥ VGPR (44.7% vs. 80.8%, p = 0.004), as well as ≥ PR (63.2% vs. 96.2%, p = 0.002). On landmark analysis for patients surviving at least 3 months after diagnosis, the median EFS for patients of the cycle ≤ 6 group and the cycle > 6 group was 9.7 and 50.7 months (p = 0.005, Fig. 3A), respectively; the median OS for the two groups was both not reached (p = 0.136, Fig. 3B). Moreover, in terms of maintenance therapy, the survival rates of patients who received maintenance therapy or not were compared. Among patients with similar clinical characteristics and pre-maintenance treatment response, we found that maintenance therapy contributed to longer EFS in those who had not achieved hematologic CR during first-line induction therapy, while for patients who had achieved CR, maintenance did not bring additional benefits to survival (Supplementary Fig. 3).
Fig. 3.
Kaplan-Meier curves for landmark analysis of event-free survival (A) and overall survival (B) of patients with MM-AL undergoing ≤ 6 or > 6 cycles of induction therapy
MRD status
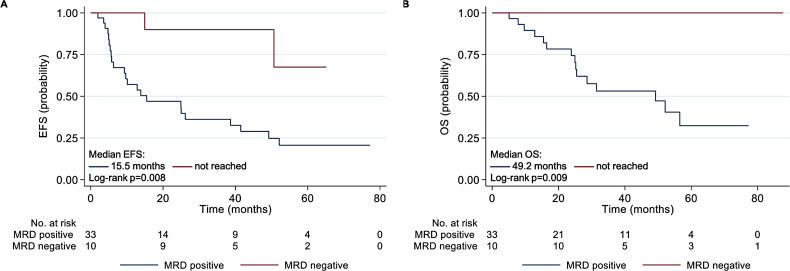
MRD negativity was more likely to be achieved in those who achieved hematologic CR (p < 0.001) or better than VGPR (p = 0.008) (based on AL consensus criteria) during first-line induction therapy. The MRD negative rate amongst patients in hematologic CR was 66.7% (6/9), and in VGPR, 21.1% (4/19). No patients with the best hematologic response less than VGPR achieved MRD eradication. The achievement of MRD negativity was also associated with a significantly higher likelihood of cardiac response (9/9, 100%) than the MRD-positive group (18/30, 60%; p = 0.023). The renal response rate was similar regardless of MRD status (MRD-negative: 7/9, 77.8%; MRD-positive: 4/4, 100%; p = 0.305). Moreover, at a median follow-up of 33.0 months, MRD-negative patients showed significantly longer EFS from diagnosis than MRD-positive patients (15.5 months vs. not reached, p = 0.008), as well as longer OS (49.2 months vs. not reached, p = 0.009) (Fig. 4).
Fig. 4.
Kaplan-Meier curves for event-free survival (A) and overall survival (B) of patients with MM-AL classified by MRD status during first-line therapy. MRD, minimal resident disease
Cox regression analysis
Two Cox proportional hazard models were built to screen the prognostic factors for the EFS and OS of patients with MM-AL (Table 2). Multivariate analyses were performed with statistically significant factors determined in univariable analyses. In the multivariate analysis, dFLC reduction ≥ 50% (HR 0.170, 95%CI 0.038–0.763, p = 0.021) and attaining cardiac response (HR 0.248, 95%CI 0.085–0.726, p = 0.011) during first-line treatment were identified as independent positive predictors for EFS. Furthermore, baseline NT-proBNP ≥ 1800pg/mL (HR 3.510, 95%CI 1.011–12.185, p = 0.048) and achieving cardiac response (HR 0.125, 95%CI 0.047–0.333, p < 0.001) were independent negative and positive factors of OS in the multivariate analysis, respectively. The CA status of t(11;14) was not included in the models, as it did not demonstrate a significant effect on EFS or OS in univariate analyses.
Table 2.
Univariate and multivariate Cox regression analysis of prognostic factors for EFS and OS in patients with AL amyloidosis and contaminant MM
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variate | HR | 95% CI | p value | HR | 95% CI | p value | ||
| EFS | ||||||||
| ECOG PS > 2 | 2.410 | 1.406–4.130 | 0.001 | 0.630 | 0.186–2.131 | 0.457 | ||
| Baseline dFLC > 290 mg/L | 1.537 | 0.942–2.508 | 0.085 | 0.865 | 0.251–2.975 | 0.818 | ||
| Baseline BMPC ≥ 30% | 1.768 | 1.047–2.986 | 0.033 | 1.383 | 0.538–3.556 | 0.501 | ||
| Baseline NT-proBNP ≥ 1800 pg/mL | 2.371 | 1.314–4.279 | 0.004 | 1.986 | 0.514–7.669 | 0.320 | ||
| Baseline cTNT ≥ 0.025 ng/mL | 3.054 | 1.102–8.463 | 0.032 | 2.704 | 0.273–26.738 | 0.395 | ||
| Baseline LDH > 250U/L | 1.837 | 1.081–3.121 | 0.025 | 0.709 | 0.198–2.533 | 0.596 | ||
| MRD negativity | 0.176 | 0.041–0.748 | 0.019 | 0.328 | 0.047–2.296 | 0.261 | ||
| dFLC reduction ≥ 50% | 0.365 | 0.187–0.714 | 0.003 | 0.170 | 0.038–0.763 | 0.021 | ||
| Cardiac response | 0.212 | 0.115–0.391 | < 0.001 | 0.248 | 0.085–0.726 | 0.011 | ||
| OS | ||||||||
| ECOG PS > 2 | 2.678 | 1.474–4.868 | 0.001 | 1.541 | 0.597–3.977 | 0.372 | ||
| Baseline NT-proBNP ≥ 1800 pg/mL | 3.418 | 1.634–7.151 | 0.001 | 3.510 | 1.011–12.185 | 0.048 | ||
| Baseline cTNT ≥ 0.025 ng/mL | 4.739 | 1.140-19.701 | 0.032 | 1.521 | 0.162–14.264 | 0.713 | ||
| Baseline β2-MG > 3.5 mg/L | 2.091 | 1.109–3.944 | 0.023 | 2.314 | 0.976–5.488 | 0.057 | ||
| Baseline LDH > 250U/L | 2.503 | 1.405–4.459 | 0.002 | 1.807 | 0.735–4.446 | 0.198 | ||
| dFLC reduction ≥ 50% | 0.450 | 0.203–0.995 | 0.049 | 0.813 | 0.309–2.135 | 0.674 | ||
| Cardiac response | 0.127 | 0.056–0.287 | < 0.001 | 0.125 | 0.047–0.333 | < 0.001 | ||
Discussion
AL patients with concomitant symptomatic myeloma are a special population that has a worse prognosis and has not been offered abundant concern. In this study, we retrospectively described the clinical characteristics, CA, and outcome of MM-AL patients based on horizontal comparison with patients with MM-alone and AL-alone. The distribution of CA in patients with MM-AL lay somewhere between those in patients with MM-alone or AL-alone. Intriguingly, other than the MM-alone group, no significant difference in PFS and OS was found between MM-AL patients with and without HRCA. When stratified by the type of PCDs and status of t(11;14), patients with MM-AL and t(11;14) presented the worst OS, with a median of 8.2 months. Patients with MM-AL were associated with a higher incidence of early mortality, a lower hematological response rate, and an MRD-negative rate during first-line therapy. The PFS and OS of MM-AL patients were significantly inferior to the other two groups. Regarding the management of MM-AL, the extended duration of first-line induction therapy and the use of maintenance therapy contributed to a superior prognosis. To our knowledge, our study provided the largest series of patients with MM-AL and was the first to investigate the prognostic implication of the various CA and provide evidence for the management of these patients.
Although MM and AL amyloidosis share a similar spectrum of CA, limited studies have investigated the cytogenetic features of AL patients with myeloma phenotype. t(11;14) is the most commonly observed CA in AL amyloidosis (range from 40 to 60%), the prevalence of which was significantly higher than in MM [9, 10, 28]. In this study, the incidence rate of t(11;14) in the patients with AL-alone was examined as 40.7%, similar to that reported previously. The prevalence of t(11;14) in MM-AL was 25.7%, which was significantly lower than AL-alone (p = 0.015) but higher than MM-alone (16.6%, p = 0.026). This translocation juxtaposes the IGH gene on chromosome 14 next to the proto-oncogene CCND1 on chromosome 11, resulting in overexpression of cyclin D1 and is predictive of response to therapy. Several studies have reported that patients with t(11;14) are less likely to have a favorable hematologic response and survival with PIs (particularly bortezomib) [9, 12, 13] and IMiDs [9] but tend to respond more favorably to alkylator therapy (including ASCT) [14, 15] and Daratumumab [16]. However, patients with the coexistence of AL amyloidosis and MM were excluded from these studies, and the prognostic implication of CA in MM-AL patients was not addressed. In our cohort, most of the MM-AL patients included were treated with PIs/IMiDs-based therapies. We found a relatively unfavorable prognosis of t(11;14) in the MM-AL group but no significant difference. The median PFS and OS of MM-AL patients with t(11;14)-positivity/ negativity was 5.8/15.8 months (p = 0.422), and 8.2/31.5 months (p = 0.229), respectively. One possible explanation is that there was a tendency for t(11;14)-negative patients to have MM-AL, and MM-AL itself was associated with a significantly poorer prognosis. Thus, the impact of t(11;14) appeared to be masked by the dominance of MM-AL. This is in line with the discovery of Muchtar E [9] that the adverse effect of t(11;14) on survival only persisted in AL patients with favorable prognostic features; patients with unfavorable features did poorly regardless of their t(11;14) status.
Unlike MM, the prevalence of HRCA is low in AL amyloidosis, as the genetic abnormality t(11;14) suppresses subclones. In AL amyloidosis, t(4;14) comprises about 2% of CA detected by the iFISH method [28]. Del17p and t(14;16) occur at a rate of less than 5% [10, 28] and 3% [28, 29], respectively. HRCA predicts worse outcomes in MM patients, but their role in the AL population, notably in those with MM-AL, is not fully defined, given their rarity. Chesi M [30] reported no adverse prognosis impact was found in t(4;14) in AL patients receiving bortezomib-based. In a multicenter study [18] of 44 AL patients with del17p, patients with del17p in more than 50% of plasma cells had a trend toward inferior survival. However, the sample sizes in these studies are small, and large-scale studies are needed to confirm these findings. In this study, we integrated MM-AL patients with del17p, t(4;14), and t(14;16) into the HRCA-positive group (N = 17: del17p-positive, n = 4; t(4;14)-positive, n = 9; del17p + t(4;14)-positive, n = 2; t(14;16)-positive, n = 2). We found that the PFS and OS of MM-AL patients with HRCA-positivity were inferior to those with HRCA-negativity (median PFS, 5.6 vs. 13.8 months, p = 0.671; median OS, 16.4 vs. 25.5 months, p = 0.391), although the difference didn’t reach statistical significance, partly due to the limitation of patient numbers. What’s more, among 106 MM-AL patients, 11.7% (2/17) of HRCA-positive and 27.0% (24/89) of HRCA-negative patients had coexistent t(11;14)-positivity, respectively. As t(11;14)-positivity was considered as an inferior prognostic factor, the impact of HRCA on the survival of MM-AL patients might be distracted. Nevertheless, high-risk cytogenetic abnormalities in MM hold certain prognostic value for patients with MM-AL, and we suggest that it is necessary to conduct the FISH test at baseline.
The prognostic impact of increased BMPC infiltration in AL amyloidosis was observed by the Mayo group [20] that > 10% BMPC translated into shorter OS, which was similar to outcomes of AL patients who presented CRAB symptoms. Tovar N [31] emphasized that the worse prognosis of AL patients with > 10% BMPC and without myeloma features might be due to a higher cardiac involvement and was not related to the clonal plasma cell expansion. In this study, among AL-alone patients without CRAB-SLiM criteria (n = 151), 71 of the patients had > 10% BMPC, and 78 had ≤ 10% BMPC; BMPC data of the rest 2 patients were missing. To investigate the prognostic effect of BMPC infiltration, we further defined them as smoldering multiple myeloma-AL (sMM-AL) and monoclonal gammopathy of undetermined significance (MGUS-AL) group, respectively. The EFS and OS of MGUS-AL and sMM-AL groups were both significantly superior to MM-AL patients (median EFS, 27.6 vs. 31.3 vs. 8.2 months, p = 0.011; median OS, not reached vs. not reached vs. 25.2 months, p < 0.001) (Supplementary Fig. 4). However, no statistical difference was found in EFS (p = 0.964) and OS (p = 0.726) between MGUS-AL and sMM-AL patients. The early mortality rate within 3 and 6 months of diagnosis of MGUS-AL and sMM-AL groups were close as well (6.4% vs. 9.9%, p = 0.440; 7.7% vs. 12.7%, p = 0.313). In terms of treatment response, MGUS-AL and sMM-AL patients shared a similar hematologic response rate (ORR, 83.6% vs. 82.7%, p = 0.897; ≥VGPR, 77.0% vs. 69.2%, p = 0.348; CR, 37.7% vs. 46.2%, p = 0.364). MRD negativity rates of MGUS-AL and sMM-AL patients were 37% (17/46) and 47.5% (19/40), respectively (p = 0.583). To sum up, it seems that in our patients, myeloma-defining events (CRAB-SLiM) play a more dominant role than BMPC infiltration in the overall clinical outcomes of patients with AL amyloidosis.
It has been well documented that achievement of MRD negativity was associated with improved survival outcomes in MM across different regimens and lines of therapy [32]. Whereas for AL amyloidosis, plasma cells are known to be indolent with a low proliferative index, and the clinical picture is dominated by organ dysfunction resulting from amyloid deposition rather than from clonal progression. As is the case for MM, recent studies have shown that MRD eradication is related to improved hematologic and organ response (particularly cardiac response) and better survival in AL amyloidosis [33–35], which suggests that even low levels of light chains produced by residual plasma cells can be cardiotoxic. Thus, for patients with MM-AL who experience a dual risk of clonal progression and amyloid deposition, MRD testing seems to be indispensable in the assessment of treatment response. We found that patients with MM-AL who achieved MRD negativity in our cohort had significantly longer EFS, longer OS, and a higher likelihood of cardiac response. Hence, we suggest testing MRD in MM-AL patients, especially if not accompanied by cardiac response.
In the current study, MM-AL patients who received over six cycles of first-line induction therapy achieved significantly higher hematologic response rates and longer EFS on landmark analysis. When we address the impact of maintenance therapy on MM-AL patients, those who died within three months from diagnosis did not achieve hematologic response (including stable disease and progressive disease) or had no available evaluation of treatment response were excluded. There was no significant discrepancy in baseline characteristics and pre-maintenance hematologic and organ response rate between the two groups (Supplementary Table 4), except patients who received maintenance therapy had higher levels of baseline BMPC (median, 20% vs. 10%; p = 0.030) and more cycles of induction therapy (median, 9 vs. 5.5; <0.001). A total of 17 patients receiving maintenance therapy and 15 patients without maintenance were analyzed. The maintenance therapy included bortezomib (n = 1), lenalidomide (n = 8), thalidomide (n = 4), ixazomib (n = 2), lenalidomide + bortezomib (n = 1) and bortezomib + cyclophosphamide (n = 1). The EFS of patients with maintenance therapy with the best hematologic response during first-line induction therapy ≥ PR was significantly superior to those without maintenance (median, not reached vs. 41.5 months; p = 0.026; Supplementary Fig. 3A). Among patients with hematologic response ≥ VGPR, those with maintenance also tended to have longer EFS (median, not reached vs. 41.5 months; p = 0.076; Supplementary Fig. 3C), while for patients who had achieved CR, maintenance did not bring benefits to EFS (median, not reached vs. not reached; p = 0.106; Supplementary Fig. 3E), indicating that for MM-AL patients who did not achieve deeper hematologic response during first-line treatment, maintenance therapy may exert a positive impact on EFS. Nevertheless, no significant difference in OS between subgroups was found. So far, several clinical guidelines have been mentioned about the management of MM-AL patients. As recently suggested by the Mayo Clinic group [1], employment of myeloma treatment schemes is recommended in AL patients with symptomatic myeloma or HRCA, that is, induction therapy for 6 to 12 months with consideration of maintenance therapy. The use of maintenance therapy in AL patients with myeloma phenotype was also suggested by Palladini G [36] and Gertz Morie A [37]. However, with the reservation of data paucity, evidence for these recommendations is not supported by randomized trials. Despite the limited sample size, our findings confirm the necessity of extended duration of induction therapy in this population and support the employment of maintenance therapy, particularly in those who have not achieved deep hematologic response after first-line treatment.
Limitations of our study include its retrospective nature and the conventional therapeutic regimens. The number of patients with MM-AL treated with novel agents was limited. Daratumumab, which has demonstrated promising efficacy in treating patients with MM [38] and AL [39], was received by only 3 patients with MM-AL in our cohort. No MM-AL patients received the oral Bcl-2 inhibitor venetoclax, which was reported to correlate to deep response in patients with t(11;14)-positive PCDs [40, 41], as t(11;14) is associated with a higher dependency of the plasma cell on the anti-apoptotic protein Bcl-2. We will monitor the clinical outcomes of MM-AL patients receiving novel agents in future medical practice.
Conclusions
This comparative study presented an apparent discrepancy in the distribution and prognostic implication of CA in different PCDs. Compared with patients with MM or AL alone, patients with AL amyloidosis and concomitant symptomatic myeloma had the worst overall outcomes, requiring extended cycles of induction therapy and the use of maintenance therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Natural Science Foundation of Shanghai (22ZR1411400). The authors thank the patients who participated in the study.
Abbreviations
- MM
Multiple myeloma
- AL
Light chain amyloidosis
- MM-AL
Light chain amyloidosis with concomitant symptomatic multiple myeloma
- PCD
Plasma cell dyscrasia
- IMWG
International Myeloma Working Group
- CA
Cytogenetic abnormality
- HRCA
High-risk cytogenetic abnormalities
- MRD
Minimal resident disease
- OS
Overall survival
- PFS
Progression-free survival
- EFS
Event-free survival
- BMPC
Bone marrow plasma cell
- PI
Proteasome inhibitor
- IMiD
Immunomodulatory agent
- ASCT
Autologous stem cell transplantation
- IFE
Immunofixation electrophoresis
- FLC
Free light-chain
- ORR
Overall response rate
- PR
Partial response
- VGPR
Very good partial response
- CR
Complete response
- SD
Stable disease
- PD
Progressive disease
Author contributions
CQY, JL, and THX contributed equally to this study. PL, JL, and THX conceived the idea; CQY, THX, WJW, YY, PW, and CZ conducted the data curation; CQY led the formal analysis and wrote the original draft of the manuscript; PL and JL edited and revised the paper; all authors read and approved the final version of the manuscript.
Funding
This study was supported by Natural Science Foundation of Shanghai (22ZR1411400).
Data availability
The data that support the findings of this study are available from the corresponding author (Peng Liu) upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Zhongshan Hospital Fudan University (approval number: B2023-185R), Shanghai, China. Informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenqi Yu MD, Jing Li MD and Tianhong Xu MD contributed equally to this work.
References
- 1.Muchtar E, Dispenzieri A, Gertz MA, Kumar SK, Buadi FK, Leung N et al. Treatment of AL Amyloidosis: Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Statement 2020 Update. Mayo Clin Proc. 2021;96(6):1546-77. [DOI] [PubMed]
- 2.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. [DOI] [PubMed] [Google Scholar]
- 3.Siragusa S, Morice W, Gertz MA, Kyle RA, Greipp PR, Lust JA, et al. Asymptomatic immunoglobulin light chain amyloidosis (AL) at the time of diagnostic bone marrow biopsy in newly diagnosed patients with multiple myeloma and smoldering myeloma. A series of 144 cases and a review of the literature. Ann Hematol. 2011;90(1):101–6. [DOI] [PubMed] [Google Scholar]
- 4.Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1). [DOI] [PubMed]
- 5.Madan S, Dispenzieri A, Lacy MQ, Buadi F, Hayman SR, Zeldenrust SR et al. Clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma. Mayo Clin Proc. 2010;85(3):232-8. [DOI] [PMC free article] [PubMed]
- 6.Ramadas P, Tambe A, Lee M. Amyloidosis and plasma cell dyscrasias: a single Institution experience. Blood. 2019;134(Supplement1):5463. [Google Scholar]
- 7.Pardanani A, Witzig TE, Schroeder G, McElroy EA, Fonseca R, Dispenzieri A, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101(3):827–30. [DOI] [PubMed] [Google Scholar]
- 8.Dinner S, Witteles W, Witteles R, Lam A, Arai S, Lafayette R, et al. The prognostic value of diagnosing concurrent multiple myeloma in immunoglobulin light chain amyloidosis. Br J Haematol. 2013;161(3):367–72. [DOI] [PubMed] [Google Scholar]
- 9.Muchtar E, Dispenzieri A, Kumar SK, Ketterling RP, Dingli D, Lacy MQ, et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia. 2017;31(7):1562–9. [DOI] [PubMed] [Google Scholar]
- 10.Bochtler T, Merz M, Hielscher T, Granzow M, Hoffmann K, Krämer A, et al. Cytogenetic intraclonal heterogeneity of plasma cell dyscrasia in AL amyloidosis as compared with multiple myeloma. Blood Adv. 2018;2(20):2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for multiple myeloma: a Report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33(12):1371–8. [DOI] [PubMed] [Google Scholar]
- 13.Dumas B, Yameen H, Sarosiek S, Sloan JM, Sanchorawala V. Presence of t(11;14) in AL amyloidosis as a marker of response when treated with a bortezomib-based regimen. Amyloid. 2020;27(4):244–9. [DOI] [PubMed] [Google Scholar]
- 14.Bal S, Estrada-Merly N, Costa LJ, Qazilbash MH, Kumar S, D’Souza A. Outcomes of t(11;14) light chain (AL) amyloidosis after autologous stem cell transplantation: benchmark for new therapies. Blood Cancer J. 2023;13(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochtler T, Hegenbart U, Kunz C, Benner A, Kimmich C, Seckinger A, et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood. 2016;128(4):594–602. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Dispenzieri A, Bhutani D, Gertz M, Wechalekar A, Palladini G et al. Impact of cytogenetic abnormalities on treatment outcomes in patients with amyloid light-chain amyloidosis: subanalyses from the ANDROMEDA study. Amyloid. 2023;30(3):268–278. [DOI] [PubMed]
- 17.Bochtler T, Hegenbart U, Kunz C, Benner A, Seckinger A, Dietrich S et al. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid. 2014;21(1). [DOI] [PubMed]
- 18.Wong SW, Hegenbart U, Palladini G, Shah GL, Landau HJ, Warner M, et al. Outcome of patients with newly diagnosed systemic light-chain amyloidosis Associated with deletion of 17p. Clin Lymphoma Myeloma Leuk. 2018;18(11):e493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozga M, Zhao Q, Benson D, Elder P, Williams N, Bumma N, et al. AL amyloidosis: the effect of fluorescent in situ hybridization abnormalities on organ involvement and survival. Cancer Med. 2021;10(3):965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol. 2013;31(34):4319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H, Lu J, Qiang W, Liu J, Liang A, Du J. The Landscape of Cytogenetic aberrations in Light-Chain Amyloidosis with or without Coexistent multiple myeloma. J Clin Med. 2023;12(4). [DOI] [PMC free article] [PubMed]
- 22.Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88(4):360 – 76. [DOI] [PubMed]
- 23.Kumar SK, Callander NS, Adekola K, Anderson LD, Baljevic M, Campagnaro E, et al. Systemic light Chain Amyloidosis, Version 2.2023, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(1):67–81. [DOI] [PubMed] [Google Scholar]
- 24.Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612–5. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46. [DOI] [PubMed] [Google Scholar]
- 27.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9. [DOI] [PubMed] [Google Scholar]
- 28.Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015;5(5):e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryce AH, Ketterling RP, Gertz MA, Lacy M, Knudson RA, Zeldenrust S, et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica. 2009;94(3):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesi M, Bergsagel PL. Molecular pathogenesis of multiple myeloma: basic and clinical updates. Int J Hematol. 2013;97(3):313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tovar N, Rodríguez-Lobato LG, Cibeira MT, Magnano L, Isola I, Rosiñol L, et al. Bone marrow plasma cell infiltration in light chain amyloidosis: impact on organ involvement and outcome. Amyloid. 2018;25(2):79–85. [DOI] [PubMed] [Google Scholar]
- 32.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease with Superior Survival Outcomes in patients with multiple myeloma: a Meta-analysis. JAMA Oncol. 2017;3(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palladini G, Paiva B, Wechalekar A, Massa M, Milani P, Lasa M, et al. Minimal residual disease negativity by next-generation flow cytometry is associated with improved organ response in AL amyloidosis. Blood Cancer J. 2021;11(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidana S, Muchtar E, Sidiqi MH, Jevremovic D, Dispenzieri A, Gonsalves W, et al. Impact of minimal residual negativity using next generation flow cytometry on outcomes in light chain amyloidosis. Am J Hematol. 2020;95(5):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staron A, Burks EJ, Lee JC, Sarosiek S, Sloan JM, Sanchorawala V. Assessment of minimal residual disease using multiparametric flow cytometry in patients with AL amyloidosis. Blood Adv. 2020;4(5):880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palladini G, Merlini G. How I treat AL amyloidosis. Blood. 2022;139(19):2918–30. [DOI] [PubMed] [Google Scholar]
- 37.Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818–29. [DOI] [PubMed] [Google Scholar]
- 38.Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol. 2022;40(25):2901–12. [DOI] [PubMed] [Google Scholar]
- 39.Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-based treatment for Immunoglobulin Light-Chain Amyloidosis. N Engl J Med. 2021;385(1):46–58. [DOI] [PubMed] [Google Scholar]
- 40.Premkumar VJ, Lentzsch S, Pan S, Bhutani D, Richter J, Jagannath S, et al. Venetoclax induces deep hematologic remissions in t(11;14) relapsed/refractory AL amyloidosis. Blood Cancer J. 2021;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman JL, Gasparetto C, Schjesvold FH, Moreau P, Touzeau C, Facon T, et al. Targeting BCL-2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am J Hematol. 2021;96(4):418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Peng Liu) upon reasonable request.