Abstract
Background
Mentha consumption may associated with blood pressure improvement in humans, but the recent evidence from randomized controlled trials (RCTs) showed inconsistent results. The present study provides a systematic review and meta-analysis of RCTs to investigate the effect of Mentha on blood pressure.
Methods
To cover all relevant literature, a complete search was conducted across PubMed, ISI Web of Science, and SCOPUS databases before March 2024 using PRISMA guidelines. In addition, Google Scholar, SID databases, the reference lists of the related reviews, and meta-analyses were searched for this purpose. Also, a “snowball search” was applied to include other relevant trials that may have been missed. A random-effects model was used for quantitative data synthesis, with weight mean difference (WMD) and 95% confidence intervals (CI). Standard methodologies were utilized to assess kappa statistics between the authors, GRADE evidence profiles, heterogeneity, meta-regression, sensitivity analysis, and publication bias.
Results
Out of 476 publications identified, seven RCTs were eligible and included in this systematic review and meta-analysis. There was perfect agreement in study selection between the reviewers (К statistic, 0.86; p < 0.001). Meta-analysis showed a 1.227 mmHg reduction in systolic blood pressure (SBP) (95% CI: -6.61,4.16, p = 0.655), 2.997 mmHg reduction in long-term SBP (95% CI: -8.00,2.00, p = 0.241), 1.830 mmHg reduction in diastolic blood pressure (DBP) (95% CI: -5.06,1.40, p = 0.268), and 2.857 mmHg reduction in long-term DBP (95% CI: -6.01, 0.30, p = 0.076) after Mentha consumption in intervention group compared to control. In sub-group analysis, a statistically and clinically significant reduction in SBP and DBP was observed in the participants with ages above 30 years and in the participants with SBP > 130 mmHg or DBP > 80 mmHg.
Conclusions
Our findings showed that Mentha consumption might not have a statistically significant effect on lowering SBP, DBP, long-term SBP, and long-term DBP. However, it can lead to a clinically significant reduction in both long-term SBP and long-term DBP. Besides, Mentha may have potential benefits for patients with pre-hypertension and hypertension. Nevertheless, further well-designed RCTs are needed to confirm our results.
PROSPERO Registration No: CRD42023459490
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04701-0.
Keywords: Mentha, Blood pressure, Hypertension, Systematic review, Meta-analysis
Introduction
Hypertension is a widespread chronic disease that impacts more than 30% of adults aged over 25 years, worldwide [1]. This health problem is a significant risk factor for cardiovascular disease (CVD). Hypertension raises the risk of coronary artery disease, cardiac failure, stroke, and peripheral arterial disease. Hypertension is also related to other diseases, such as diabetes mellitus and renal failure.
Hypertension results from increased cardiac output and/or an increase in peripheral resistance [2]. Hypertension is defined as a systolic blood pressure (SBP) of 140 mmHg or higher and/or a diastolic blood pressure (DBP) of 90 mmHg or higher. It is often called the “silent killer” because it has no symptoms until serious complications develop. Therefore, its prevention is better than cure [1]. However, various treatments have been offered for the management of hypertension, including pharmacotherapy. Pharmacotherapy, which involves using medications like diuretics and beta-blockers, is commonly applied to control hypertension; nevertheless, these medications can have adverse side effects. It is important to note that hypertension is a warning sign suggesting the need for lifestyle changes, such as increasing physical activity and following a healthy diet, in addition to pharmacotherapy [1, 2]. In this regard, some herbal medicines with minimal side effects can be used to manage hypertension [3].
Herbal medicines have been used for years to control hypertension [3]. Some evidence showed that phytotherapy with some herbs such as garlic [4], cinnamon [5], celery [6], nigella sativa [7], and Mentha [8] may help lower blood pressure. Among these herbs, the genus Mentha (from the Lamiaceae family), commonly known as mint, is popular in many world regions. In addition to its traditional use as food flavorings, Mentha is also known for its therapeutic properties, particularly in treating colds, fever, gastric, and CVDs. These pharmacological benefits of Mentha can be attributed to its bioactive phytochemicals like terpenoids, alcohols, rosmarinic acid, and antioxidant phenolics. Mentha mainly comprises volatile bioactive components, including menthol, menthone, menthofuran, piperitenone, pulegone, and linalool [9].
Mentha has been recognized for various biological activities, including antioxidant, antimicrobial, anticancer, antiviral, antiallergic, anti-inflammatory, and antihypertensive properties [9]. Mentha’s possible antihypertensive effect has been attributed to a mixture of vasodilator and cardiac depressant components. The vasodilatory effect was mediated through a combination of calcium channel blockade and endothelium-dependent pathways linked to vascular muscarinic receptors [9]. In this respect, literature has reported the significant role of the antioxidant properties of phenolics and total flavonoid contents [10, 11]. Some studies suggest that menthol, a compound found in Mentha, activates transient receptor potential melastatin subtype 8 (TRPM8), a cold-sensing cation channel in blood vessels. Activation of TRPM8 improves vascular function and blood pressure by inhibiting calcium signaling-mediated RhoA/Rho kinase stimulation in the vasculature. Therefore, nutritional menthol consumption may be beneficial in managing hypertension [12]. However, available published randomized controlled trials (RCTs) show inconsistent results regarding the effect of Mentha on blood pressure. Some trials have shown a significant reduction in SBP [8, 12–14] or DBP [12–14] when using Mentha compared to placebo, while some did not show such an effect [15–17]. Given that RCTs about this effect had a limited sample size with a diverse population and duration of intervention, the present systematic review and meta-analysis was performed to investigate the overall effects of Mentha on blood pressure.
Methods
Search Strategy
This systematic review and meta-analysis study was designed following the guidelines of the PRISMA statement [18]. We searched three databases (PubMed, ISI Web of Sciences, and SCOPUS) for RCTs published before March 2024 that examined Mentha or its products (whole or Mentha extract, menthol supplement, or Mentha oil) and their influence on blood pressure. In the following, we represent the MeSH terms and relevant keywords that we have identified regarding the topic: (“Mentha”[MeSH Terms] OR “Mentha”[Title/Abstract] OR “Mentha piperita”[Title/Abstract] OR “mint”[Title/Abstract] OR “Spearmint”[Title/Abstract] OR “Mentha longifolia”[Title/Abstract] OR “Mentha spicata”[Title/Abstract] OR “Mentha aquatica”[Title/Abstract] OR “Mentha rotundifolia”[Title/Abstract] OR “Mentha citrata”[Title/Abstract] OR “lemon mint”[Title/Abstract] OR “Mentha arvensis”[Title/Abstract] OR “Bergamot mint”[Title/Abstract] OR “Mentha viridis”[Title/Abstract] OR “Mentha suaveolens”[Title/Abstract] OR “Mentha gattefossei”[Title/Abstract] OR “corn mint”[Title/Abstract] OR “Mentha canadensis”[Title/Abstract] OR “Water mint”[Title/Abstract] OR “field mint”[Title/Abstract] OR “ginger mint”[Title/Abstract] OR “wild mint”[Title/Abstract] OR “Japanese mint”[Title/Abstract] OR “horsemint”[Title/Abstract] OR “Peppermint”[Title/Abstract] OR “eau de cologne mint”[Title/Abstract] OR “orange mint”[Title/Abstract] OR “Mosquito plant”[Title/Abstract] OR “pennyroyal mint”[Title/Abstract] OR “pennyrile”[Title/Abstract] OR “squaw mint”[Title/Abstract] OR “common mint”[Title/Abstract] OR “Apple mint”[Title/Abstract] OR “pineapple mint”[Title/Abstract] OR “Grapefruit mint”[Title/Abstract]) AND (“Hypertension”[MeSH Terms] OR “Blood Pressure”[MeSH Terms] OR “systolic blood pressure”[Text Word] OR “diastolic blood pressure”[Text Word] OR “hypertens”[Title/Abstract] OR “SBP”[Text Word] OR “DBP”[Text Word] OR “Hypertension”[Title/Abstract] OR " “Blood Pressure”[Title/Abstract]).
A full description of the search strategy used in three databases (PubMed, ISI Web of Science, and SCOPUS) can be found in Appendix A1 of this article’s Supplemental Information. In addition, we searched the Google Scholar and SID databases and the references of associated reviews and meta-analyses. Also, a “snowball search” was applied to include other relevant trials that may have been missed. To identify new articles that may have been published after our search, we activated PubMed’s ‘My NCBI’ (National Centre for Biotechnology Information) email alert service. The study protocol was recorded in the PROSPERO database, which is the worldwide prospective registry for systematic reviews. (http://www.crd.york.ac.uk/PROSPERO; registration No: CRD42023459490).
Study selection
The eligibility of studies for the present systematic review and meta-analysis was determined by reviewing titles and abstracts of articles by F.N and G.K. Then, F.N and Ab.M reviewed the full text of selected articles. We resolved the discrepancies by discussing with N.S. We calculated the kappa statistic to determine the level of agreement between reviewers for study selection using SPSS software (ver. 26). To this end, the following interpretation of kappa was used: chance agreement (≤ 0), slight agreement (0.01–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80), almost perfect agreement (0.81–0.99) [19].
The studies included in the analysis were selected based on the PICOS criteria (participants, interventions, comparisons, outcomes, and study design), shown in Table 1. The subsequent inclusion criteria considered for this purpose are 1) The experiment followed a placebo-controlled approach, using either a parallel or crossover design; 2) It is possible to extract information regarding the impact of Mentha on blood pressure from the study. It is noteworthy that sufficient data on blood pressure, including standard deviations (SDs), standard errors (SEs), or 95% confidence intervals (CIs), should be provided at baseline and the final of the intervention; 3) The study had a suitably controlled design where the only change between the control and intervention clusters was Mentha consumption; 4) having a short-term or long-term intervention duration. Trials that lasted for more than 1 day as interventions were considered long-term and those with an intervention duration of less than 1 day were deemed short-term. Any articles that fulfilled the following conditions were excluded:1) we could not extract the effect of Mentha or its product consumption on blood pressure from the article; 2) non-RCTs or animal studies; 3) unavailability of blood pressure values at the baseline or end of the intervention.
Table 1.
PICOS criteria used to define the research question
| Participants | Interventions | Comparisons | Outcomes | Study design |
|---|---|---|---|---|
| All humans | Mentha consumption | Blood pressure | SBP and DBP | PR and CR |
Abbreviations: PR parallel, CR cross over, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure
Data collection
The required data were collected according to the guidelines of the PRISMA statement [18]. Screening forms were used to identify eligible articles for this research having the inclusion criteria. The data of selected articles were independently reviewed by two authors (Ab.M. and F.N.). The continuance data collection process included extracting the following data from each study using Microsoft Office Excel 2016 MSO (16.0.4266.1001) software spreadsheet: first author’s name, publication year, country, the sample size in total and each group, type and dose of intervention and placebo, study design (crossover or parallel), duration of the intervention, participant’s status health, and other information including mean age, gender, baseline Systolic Blood Pressure (bSBP), and baseline Diastolic Blood Pressure (bDBP) of the participants. We extracted the mean values and standard deviations for the outcomes at baseline, post-intervention, and the changes between them. If data were collected at several time points, just the last measurement values were utilized. Both authors (Ab.M. and F.N.) separately summarized the data from the included studies and resolved any discrepancies by consulting with N.S. Finally, К statistic was calculated to determine the agreement level between reviewers for data extraction using SPSS software (ver. 26) [19].
Assessment of quality of studies
F.N. and Ab.M. systematically assessed the risk of bias using the Cochrane quality assessment tool for including studies [20]. There are seven criteria in this tool for determining the quality of studies: 1) random sequence generation (selection bias), 2) allocation sequence concealment (selection bias), 3) blinding of participants and personnel (performance bias), 4) blinding of outcome valuation (detection bias), 5) inadequate outcome data (attrition bias), 6) selective outcome reporting (reporting bias), and 7) other causes of bias. The study’s risk of bias was classified as either low, high, or uncertain. Also, the К statistic was calculated to determine the level of agreement between reviewers for assessing the quality of included studies using SPSS software (ver. 26) [19].
Additionally, GRADE evidence profiles were applied to evaluate the overall evidence quality regarding blood pressure [21].
Data synthesis and statistical analysis
We evaluated the effect of consuming Mentha on SBP (mmHg) and DBP (mmHg). The effect sizes were expressed as weighted mean differences (WMDs) along with 95% confidence intervals. We computed the net changes in blood pressure by extracting the mean (± SD) of pre- and post-intervention periods for both the Mentha and control groups: the value change between the end of the study and the beginning of the study is to subtract the value at baseline from the value at the end. The mean difference was calculated using the following method: (value at the end of follow-up in the treatment group—value at baseline in the treatment group) minus (value at the end of follow-up in the control group—value at baseline in the control group). When there was no informed standard deviation of the mean difference, the result was determined through a mathematical calculation using the following technique: SD = square root [(SD pre-treatment)2 + (SD post-treatment)2—(2 R × SD pre-treatment × SD post-treatment)], assuming a correlation coefficient of 0.5, as a conservative estimate for R which ranges between 0 and 1 [22]. In the case of medians and ranges or 95% CIs, mean and SD values were calculated utilizing the method developed by Hozo et al. [23]. Heterogeneity was tested using Cochran’s Q-test (with significance set at p < 0.1) and the I2 test to estimate the percentage of heterogeneity (I2 value ≥ 50% representing significant heterogeneity). When heterogeneity existed, a random effects model was applied; otherwise, a fixed-effects model was applied. Also, a 95% prediction interval (PI) was calculated. Furthermore, a leave-one-out sensitivity analysis was performed to evaluate each study’s effect on the total effect size [23]. Subgroup analysis was conducted to assess the effect of baseline blood pressure, dose, consumption duration, gender, and mean age on the results. The correlation between effect size and moderator variables (e.g., such as mean age, baseline values, and sample size of the studies) was examined by performing a meta-regression. The Hartung-Knapp adjustment was used for the meta-regression analysis. The potential publication bias was identified using the funnel plot, Begg’s rank correlation, and Egger’s weighted regression tests. Also, the analysis of the effects of publication bias was adjusted using the Duval & Tweedie “trim and fill” and “failsafe N” methods [24]. In this research, the meta-analysis was performed using the Comprehensive Meta-Analysis (CMA) software (ver. 3; Biostat, NJ) [25]. A p-value less than 0.05 was considered statistically significant.
Results
Results of the search and trial flow
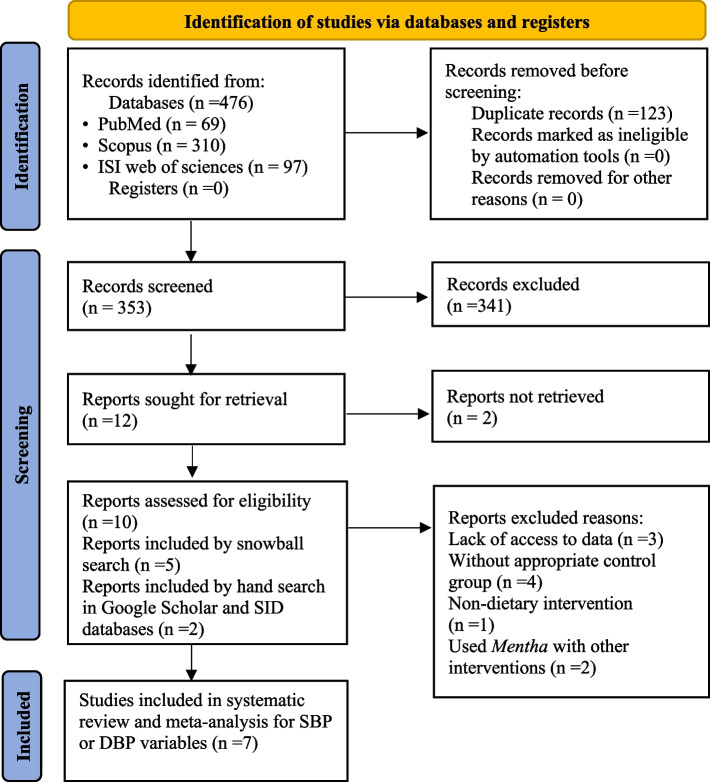
Figure 1 illustrates the step-by-step process used to select the appropriate studies for the research [18]. Initially, 476 articles were identified and 353 articles remained after eliminating the duplicate entries (n = 123). The remaining articles underwent screening based on their titles and abstracts. After carefully reviewing the titles and abstracts, 341 out of 353 articles were excluded for not being RCTs or irrelevant to the present study. A total of 12 articles were recognized as potentially related and were chosen for intensive assessment and thorough checks of their content. In this stage, there was perfect agreement in study selection between the reviewers (К statistic, 0.86; p < 0.001).
Fig. 1.
Flow diagram of the study selection procedure showing the number of eligible studies for the meta-analysis of the effect of Mentha on blood pressure
After careful valuation and excluding studies without clear and sufficient data needed for this study [26–28], seven eligible RCTs met the inclusion criteria. Thus, they were included in the systematic review and meta-analysis for SBP or DBP variables. All of these seven studies have reported the effect of Mentha on both SBP and DBP [8, 12–17].
Study characteristics
A summary of the demographic characteristics of the study participants included in the meta-analysis is provided in Table 2. This research combined data from 7 studies that met the eligibility criteria. These studies included 297 randomly assigned subjects, with 148 participants in the intervention group and 149 in the control group. The number of participants in these studies varied from 8 [15] to 106 [17] individuals. The mean age of the participants ranged between 16.7 [16] and 56.9 [12] years. Only one trial [13] was conducted exclusively on men, two trials were conducted on women [15, 16], and the remaining trials were conducted on both sexes [8, 12, 14, 17]. The included trials were published between 2004 [15] and 2023 [14], and performed in Lebanon [8], China [12], Iran [13], Turkey [15], Pakistan [16], USA [17], and UK [14]. Four trials had an intervention with capsules [12, 15–17], one with extract [8], and two with the essential oil [13, 14] of Mentha. The duration of Mentha consumption varied from 1 h [13] to 112 days [8]. Four trials selected subjects with healthy status [13–15, 17]; one of the three remaining trials was focused on patients who had pre-hypertension [12], one included patients with primary dysmenorrhea [16], and another study was conducted on patients diagnosed with mild hypertension [8]. Six studies [8, 12–14, 16, 17] used a parallel design, and one used a crossover design [15]. The baseline values of SBP and DBP of the participants in the intervention groups varied from 112 [16] to 137.6 [8] and 72.8 [17] to 87.4 [8] mmHg, respectively. The baseline values of SBP and DBP of the participants in the control groups varied from 111 [16] to 137.4 [8] and 72.6 [17] to 86.9 [8] mmHg, respectively.
Table 2.
Demographic characteristics of the included studies
| First Author | Year | Country | Study population | Design | Sample size (n) | Female | Male | Mean age (years) |
Duration | In-bSBP (mmHg) |
Pl-bSBP (mmHg) |
In-bDBP (mmHg) |
Pl-bDBP (mmHg) |
Mentha | Placebo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jing Sun [12] | 2014 | China | Pre-hypertensive | PR | 36 | 20 | 16 | 56.9 | 56 days | 129 | 129 | 81 | 78 | 144 mg/day menthol capsule | Placebo capsule |
| Ayse Gelal [15] | 2004 | Turkey | Healthy | CR | 8 | 8 | 0 | 26.2 | 8 h | NR | NR | NR | NR | 10 mg felodipine tablet + 200 mg menthol capsule | Felodipine tablet + glucose capsule |
| Abbas Meamarbashi [13] | 2014 | Iran | Healthy | PR | 30 | 0 | 30 | 24.8 | 1 h | 129.9 | 133 | 74.6 | 81.4 | 50 μl of Mentha essential oil | Mineral water |
| Ali A. Samaha [8] | 2019 | Lebanon | Mild hypertension | PR | 29 | 10 | 19 | 53.5 | 112 days | 137.64 | 137.41 | 87.41 | 86.91 | 300 mL/day of Mentha extract mixed with distilled water | Distilled water with food colorant |
| Sana SULTAN [16] | 2021 | Pakistan | Primary dysmenorrhea | PR | 60 | 60 | 0 | 16.7 | 5 days | 112 | 111 | 77 | 76 | 750 mg /day of powdered dried Mentha capsules | Lactose capsule |
| Jonathan Sinclair [14] | 2023 | UK | Healthy | PR | 35 | 12 | 23 | 28.5 | 20 days | 118.44 | 118.53 | 76.72 | 75.29 | 50 μl of peppermint oil with 100 mL of water twice daily |
Peppermint flavored placebo, diluted in 100 mL of water |
| Paul H. Falcone [17] | 2018 | USA | Healthy | PR | 142 | 98 | 44 | 27.5 | 90 days | 114.02 | 114.72 | 72.84 | 72.65 | two capsules of spearmint (providing 900 mg spearmint extract) /day | Placebo capsule |
Values are expressed as mean
Abbreviations: PR parallel, CR crossover, bSBP baseline Systolic Blood Pressure, bDBP baseline Diastolic Blood Pressure, In Intervention group, Pl placebo group, NR not reported
There was substantial agreement in data extraction between the reviewers (К statistic, 0.72; p = 0.047).
Assessment of the quality of the studies
Two authors (Ab.M. and F.N.) independently assessed the quality of selected studies using the Cochrane Risk-of-Bias (RoB2) tool [29]. This tool was applied to assess the possible bias from randomization, timing of identification or recruitment of participants, deviation from intended intervention, measurement of outcome, missing outcome data, and the selection of reported results. The overall quality of each study was considered low risk of bias, some concerns of bias, or high risk of bias.
The quality assessment results are presented in Table 3 and Fig. 2. Among these seven RCTs, five had a high risk of bias, and two had a low risk of bias (Fig. 2) [8, 12–17]. All studies reported randomization. However, two of these trials did not explain the randomization procedure [12, 16]. There was substantial agreement between the reviewers regarding the quality of the studies (К statistic, 0.69; p = 0.053). A third researcher (N.S.) judged any disagreement. The general quality of evidence related to blood pressure was assessed utilizing the GRADE evidence profiles (Table 4).
Table 3.
Quality assessment of the studies included according to Cochrane guidelines investigating the effect of Mentha on blood pressure
| Studies | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete Outcome data | Selective outcome reporting | Other potential threats to validity |
|---|---|---|---|---|---|---|---|
| Abbas Meamarbashi [13] | U | H | U | U | L | L | U |
| Ali A. Samaha et al. [8] | L | L | H | U | L | L | U |
| Ayse Gelal et al. [15] | L | L | L | U | H | L | L |
| Jing Sun et al. [12] | H | U | U | U | L | L | L |
| Jonathan Sinclair et al. [14] | L | L | L | L | L | L | U |
| Paul H. Falcone et al. [17] | L | L | L | L | L | L | U |
| Sana SULTAN et al. [16] | H | U | U | U | L | L | L |
Abbreviations: H high risk of bias, L low risk of bias, U unclear risk of bias
Fig. 2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Table 4.
GRADE profile regarding the effect of Mentha on blood pressure
| Quality assessment | Summary of findings | Quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Number of intervention/control | WMD,95% CI | |
| SBP | Serious limitation | Very serious limitation | No serious limitation | Serious limitation | Serious limitation | 148/149 |
-1.22 mmHg, -6.61,4.16 |
Low ⊕ ⊕ 〇〇 |
| DBP | Serious limitation | Very serious limitation | No serious limitation | Serious limitation | No serious limitation | 148/149 |
-1.83 mmHg, -5.06,1.40 |
Low ⊕ ⊕ 〇〇 |
Abbreviations: SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, WMD Weight Mean Difference
Effect of Mentha on blood pressure
The forest plots in Fig. 3 (panels A—D) represent the meta-analysis of trials regarding the impact of Mentha on SBP and DBP. The results for SBP and DBP in 7 comparisons and for long-term SBP and DBP in 5 comparisons were reported separately using the random-effects model because of the existing significant heterogeneities. Pooled results indicated that Mentha intake lowered SBP in the intervention group compared to the control. The reduction observed was not significant from a statistical point of view (WMD: -1.227 mmHg, 95% CI: -6.61,4.16, p = 0.655), and the studies showed significant differences among each other (I2 = 90.400%, p = 0.000, Mean PI = -1.227, 95% PI: -18.84,16.39) (Fig. 3 panel A).
Fig. 3.
Forest plot presenting the weighted mean difference and 95% confidence intervals for the effect of Mentha consumption on systolic and diastolic blood pressure
Results revealed that long-term SBP was reduced in the intervention group compared to the control after the Mentha intake. However, this reduction was not statistically significant (WMD: -2.997 mmHg, 95% CI: -8.00,2.00, p = 0.241), and there was significant heterogeneity between the studies (I2 = 90.11%, p = 0.000, Mean PI = -1.227, 95% PI: -21.60,15.61) (Fig. 3 panel B).
According to the combined effects from the included studies, the consumption of Mentha resulted in a decrease in DBP in the intervention group compared with the control. But, the decrease observed was not statistically significant (WMD: -1.830 mmHg, 95% CI: -5.06,1.40, p = 0.268), and there was significant heterogeneity between the studies (I2 = 79.939%, p = 0.000, Mean PI = -1.830, 95% PI: -11.90,8.24) (Fig. 3 panel C).
The combined results obtained from the articles suggest that the consumption of Mentha led to a reduction in long-term DBP in the intervention group compared with the control. However, this reduction was not statistically significant (WMD: -2.857 mmHg, 95% CI: -6.01, 0.30, p = 0.076), and there was significant heterogeneity between the studies (I2 = 81.140%, p = 0.000, Mean PI = -2.857, 95% PI: -14.07,8.36) (Fig. 3 panel D).
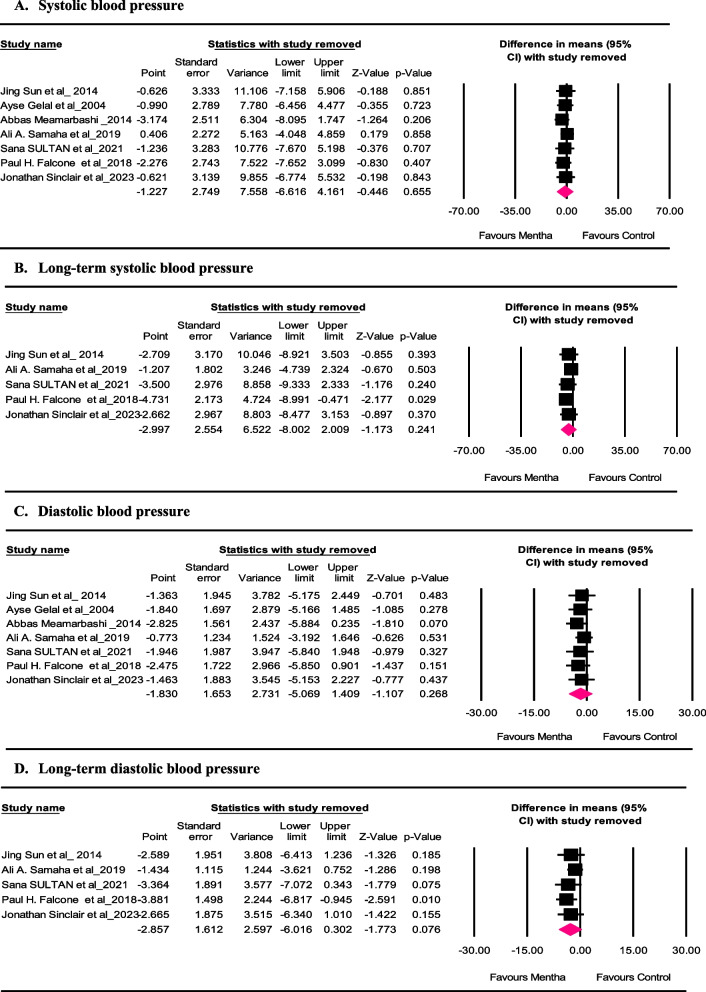
Sensitivity analysis
We conducted a leave-one-out sensitivity analysis to detect each trial’s effect on the pooled effect size. It was determined that omitting each trial would not significantly alter the meta-analysis regarding the effect size of Mentha on SBP (Fig. 4 panel A). However, the effect size for the influence of Mentha on long-term SBP was sensitive to the study of “Paul H. Falcone et al. “ [17] (Fig. 4 panel B). It was determined that omitting each trial would not significantly change the meta-analysis regarding the effect size of Mentha on DBP (Fig. 4 panel C). However, the effect size for the influence of Mentha on long-term DBP was sensitive to the study of “Paul H. Falcone et al. “ [17] (Fig. 4 panel D).
Fig. 4.
Leave-one-out sensitivity analysis of the effect of Mentha consumption on systolic and diastolic blood pressure, as well as long-term systolic and diastolic blood pressure
Sub-group analysis
Based on certain variables, we conducted subgroup analyses as follows: mean age and gender of the participants, sample size, type and duration of intervention, dose of Mentha supplements, baseline SBP and DBP, as well as long-term blood pressure.
The results of the subgroup analysis for the effect of Mentha on SBP and DBP are presented in Table 5. Based on a stratified analysis by mean age of the participants, higher reductions in SBP were observed in the ages above 30 years (WMD: -6.799, 95% CI: -10.89, -2.70, P = 0.001). Furthermore, SBP declined in studies with a sample size < 29 (WMD: -8.363, 95% CI: -9.14, -7.58, P < 0.001) compared to the studies with a sample size > 29 (WMD: 0.597, 95%CI: -3.94,5.14, P = 0.797). The results showed a decline in SBP after the Mentha intake when the baseline values of the intervention group were more significant than 130 mmHg (WMD: 0.597, 95%CI: -3.94, 5.14, P = 0.797). No statistically significant differences in SBP were observed when the analysis was stratified based on the dose of Mentha (Table 4). Furthermore, Mentha increased SBP in the studies conducted on male participants (WMD: 11.200, 95% CI: 3.34, 19.05, P = 0.005). Also, SBP had a reduction in the study conducted using the Mentha extract (WMD: -8.360, 95% CI: -9.14, -7.57, P < 0.001), compared to the trials using Mentha in the form of capsule or Mentha essential oil.
Table 5.
Results of subgroup analysis of the included trials regarding the effect of Mentha on blood pressure
| Variables | Mean age (years) | Sample Size (n) | Intervention Group Baseline value (mmHg) | Dose of Mentha supplements (mg/day) |
Gender | Type of Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | < 30 | > 30 | < 29 | > 29 | < 130 | > 130 | < 150 | > 150 | Both | Female | Male | Capsule | Essential Oil | Extract |
| Number of studies | 5 | 2 | 2 | 5 | 5 | 1 | 1 | 3 | 4 | 2 | 1 | 4 | 2 | 1 |
|
WMD (95% CI) |
1.632 (-3.56,6.83) |
-6.799 (-10.89,-2.70) |
-8.363 (-9.14,-7.58) |
0.597 (-3.94,5.14) |
0.597 (-3.94,5.14) |
-8.363 (-9.14,-7.57) |
-4.000 (-8.61,0.61) |
0.918 (-3.19,5.03) |
-3.500 (-9.33,-2.33) |
-1.271 (-4.96,2.71) |
11.200 (3.34, 19.05) |
-0.628 (-4.52, 3.26) |
3.194 (-12.20, 18.59) |
-8.360 (-9.14,-7.57) |
| P-value | 0.539 | 0.001 | < 0.001 | 0.797 | 0.797 | < 0.001 | 0.090 | 0.662 | 0.240 | 0.565 | 0.005 | 0.752 | 0.684 | < 0.001 |
| I2(%) | 66.811 | 69.941 | 0.000 | 73.428 | 73.428 | 0.000 | 0.000 | 31.193 | 89.536 | 0.000 | 0.000 | 48.667 | 88.858 | 0.000 |
| P-heterogeneity | 0.017 | 0.068 | 0.690 | 0.005 | 0.005 | 1.000 | 1.000 | 0.234 | 0.000 | 0.466 | 1.000 | 0.119 | 0.003 | 1.000 |
| 95% PI | (-14.93,18.20) | _ | _ | (-15.13,16.32) | (-15.13,16.32) | _ | _ | (-36.42,38.26) | (-30.38,23.38) | _ | _ | (-14.85,13.59) | _ | _ |
| DBP (mmHg) | < 30 | > 30 | < 29 | > 29 | < 80 | > 80 | < 150 | > 150 | Both | Female | Male | Capsule | Essential Oil | Extract |
| Number of studies | 5 | 2 | 2 | 5 | 4 | 2 | 1 | 3 | 4 | 2 | 1 | 4 | 2 | 1 |
|
WMD (95% CI) |
-0.056 (-2.50,2.39) |
-5.951 (-7.32,-4.57) |
-6.148 (-7.59,-4.69) |
-0.764 (-3.38,1.85) |
-0.004 (-2.77,2.76) |
-5.951 (-7.32,-4.57) |
-4.00 (-8.33,0.33) |
-0.201 (-2.33, 1.93) |
-3.364 (-7.07,0.34) |
-0.995 (-3.79,1.80) |
5.300 (-0.97, 11.57) |
-0.958 (-2.93, 1.02) |
0.579 (-8.36, 9.52) |
-6.170 (-7.62,-4.71) |
| P value | 0.964 | < 0.001 | < 0.001 | 0.567 | 0.998 | < 0.001 | 0.070 | 0.853 | 0.075 | 0.485 | 0.098 | 0.343 | 0.899 | < 0.001 |
| I2(%) | 26.945 | 0.000 | 0.000 | 50.082 | 45.187 | 0.000 | 0.000 | 0.000 | 80.147 | 0.000 | 0.000 | 3.849 | 78.829 | 0.000 |
| P-heterogeneity | 0.242 | 0.352 | 0.637 | 0.091 | 0.140 | 0.352 | 1.000 | 0.689 | 0.002 | 0.979 | 1.000 | 0.373 | 0.030 | 1.000 |
| 95% PI | (-6.10,5.99) | _ | _ | (-8.60,7.07) | (-10.08,10.07) | _ | _ | (-14.04,13.63) | (-14.09,14.24) | _ | _ | (-5.67,3.75) | _ | _ |
Abbreviations: SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, WMD Weight Mean Difference
When stratified by the mean age of the participants, a higher decline in DBP was observed in trials with a mean age above 30 years (WMD: -5.951, 95% CI: -7.32, -4.57, P < 0.001) compared to the mean age < 30 years. DBP was decreased in the studies with a sample size of less than 29 participants (WMD: -6.148, 95% CI: -7.59, -4.69, P < 0.001) compared to those with a sample size greater than 29. After the intake of Mentha, DBP declined more in trials with the baseline values of the intervention group above 80 mmHg (WMD: -5.951, 95% CI: -7.32, -4.57, P < 0.001). There was no significant difference in DBP when stratified by dose of Mentha. Also, there was no significant difference in DBP when stratified by gender of the participants (Table 4). It was observed that in the study using Mentha extract (WMD: -6.170, 95% CI: -7.62, -4.71, P < 0.001), a higher reduction in DBP was found compared to the studies that applied Mentha in the form of capsule or Mentha essential oil.
After subgroup analysis based on participants’ gender, sample size, baseline SBP of the intervention group, and type of intervention, the RCTs’ heterogeneity for SBP was reduced to less than 50%. Similarly, after analyzing sub-groups based on participants’ gender, sample size, and intervention type, the heterogeneity of RCTs for DBP was reduced to less than 50%.
Table 6 provides the results of the subgroup analysis of the effect of Mentha on long-term blood pressure. When stratified based on the mean age, trials with a mean age of participants greater than 30 years showed a significant reduction in long-term SBP (WMD: -6.799, 95% CI: -10.89, -2.70, P = 0.001) compared to trials with a mean age of less than 30 years. Furthermore, the long-term SBP declined in the studies with a sample size of less than 29 participants (WMD: -8.360, 95% CI: -9.14, -7.57, P < 0.001). Long-term SBP was decreased significantly in the intervention group, with baseline values in the intervention group above 130 mmHg (WMD: -8.363, 95% CI: -9.14, -7.57, P < 0.001). When stratified based on the study duration, there was no significant difference in long-term SBP in the trials with a more or less than 50 days duration. Also, when stratified based on the participants’ gender, there was no significant difference in long-term SBP in the trials with both genders compared to the study conducted on female participants. Long-term SBP had a significant reduction in the study conducted using the Mentha extract (WMD: -8.360, 95% CI: -9.14, -7.57, P < 0.001) compared to those conducted using Mentha in the form of capsule or Mentha essential oil.
Table 6.
Results of subgroup analysis of the RCTs regarding the effect of Mentha on long-term blood pressure
| Variables | Mean age (years) | Sample Size (n) | Intervention Group Baseline value (mmHg) | Duration (day) |
Gender | Type of Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | < 30 | > 30 | < 29 | > 29 | < 130 | > 130 | < 50 | > 50 | Both | Female | Male | Capsule | Essential Oil | Extract |
| Number of studies | 3 | 2 | 1 | 4 | 4 | 1 | 2 | 3 | 4 | 1 | 0 | 3 | 1 | 1 |
|
WMD (95% CI) |
-0.231 (-4.41,3.95) |
-6.799 (-10.89,-2.70) |
-8.360 (-9.14,-7.57) |
-1.207 (-4.73,2.32) |
-1.207 (-4.73,2.32) |
-8.360 (-9.14,-7.57) |
-1.887 (-5.22,1.44) |
-3.169 (-10.41,4.07) |
-3.500 (-9.33,2.33) |
-1.000 (-4.85, 2.85) |
_ |
-0.493 (-4.58,3.59) |
-4.520 (-11.160, 2.120) |
-8.360 (-9.14, -7.57) |
| P value | 0.914 | 0.001 | < 0.001 | 0.503 | 0.503 | < 0.001 | 0.267 | 0.391 | 0.240 | 0.611 | _ | 0.813 | 0.182 | < 0.001 |
| I2(%) | 54.144 | 69.941 | 0.000 | 53.754 | 53.754 | 0.000 | 0.000 | 92.774 | 89.536 | 0.000 | _ | 62.120 | 0.000 | 0.000 |
| P-heterogeneity | 0.113 | 0.068 | 1.000 | 0.090 | 0.090 | 1.000 | 0.369 | 0.000 | 0.000 | 1.000 | _ | 0.071 | 1.000 | 1.000 |
| 95% PI | (-44.07,43.61) | _ | _ | (-14.89,12.48) | (-14.89,12.48) | _ | _ | (-50.11,43.77) | (-30.38,23.38) | _ | _ | (-45.35, 44.37) | _ | _ |
| DBP (mmHg) | < 30 | > 30 | < 29 | > 29 | < 80 | > 80 | < 50 | > 50 | Both | Female | Male | Capsule | Essential Oil | Extract |
| Number of studies | 3 | 2 | 1 | 4 | 3 | 2 | 2 | 3 | 4 | 1 | 0 | 3 | 1 | 1 |
|
WMD (95% CI) |
-0.737 (-2.90,1.42) |
-5.951 (-7.32,-4.57) |
-6.170 (-7.62,-4.71) |
-1.434 (-3.62,0.75) |
-0.737 (-2.90,1.42) |
-5.951 (-7.32,-4.57) |
-1.617 (-4.10,0.87) |
-3.212 (-7.84,1.42) |
-3.364 (-7.07,0.34) |
-1.000 (-3.81, 1.81) |
_ |
-1.056 (-3.51,1.40) |
-3.830 (-9.16, 1.50) |
-6.170 (-7.62,-4.71) |
| P value | 0.504 | < 0.001 | < 0.001 | 0.198 | 0.504 | < 0.001 | 0.203 | 0.174 | 0.075 | 0.487 | _ | 0.400 | 0.159 | < 0.001 |
| I2(%) | 12.181 | 0.000 | 0.000 | 27.090 | 12.181 | 0.000 | 0.000 | 86.626 | 80.147 | 0.000 | _ | 35.890 | 0.000 | 0.000 |
| P-heterogeneity | 0.320 | 0.352 | 1.000 | 0.249 | 0.320 | 0.352 | 0.358 | 0.001 | 0.002 | 1.000 | _ | 0.210 | 1.000 | 1.000 |
| 95% PI | (-17.26,15.79) | _ | _ | (-8.37,5.50) | (-17.26,15.79) | _ | _ | (-59.74,53.32) | (-14.09,14.24) | _ | _ | (-24.07, 21.95) | _ | _ |
Abbreviations: SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, WMD Weight Mean Difference
The sub-group analysis regarding the mean age of participants revealed that in the mean age above 30 years, a significant reduction occurred in long-term DBP after Mentha consumption (WMD: -5.951, 95% CI: -7.32, -4.57, P < 0.001). Long-term DBP was declined in the studies with a sample size of less than 29 participants (WMD: -6.170, 95% CI: -7.62, -4.71, P < 0.001). Also, long-term DBP was decreased in the intervention group with baseline values of more than 80 mmHg (WMD: -5.951, 95% CI: -7.32, -4.57, P < 0.001). When stratified based on the study duration, there was no significant difference in long-term DBP in the trials with more or less than 50 days of duration. Furthermore, when stratified based on the participants’ gender, long-term DBP had no significant difference. It was discovered that long-term DBP had a significant reduction in the study that used the Mentha extract (WMD: -6.170, 95% CI: -7.62, -4.71, P < 0.001) compared to studies using Mentha in the form of capsule or Mentha essential oil.
By conducting sub-group analysis based on participants’ gender, sample size, study duration, baseline SBP of the intervention group, and types of intervention, the RCTs’ heterogeneity for long-term SBP was reduced to less than 50%. Similarly, after analyzing sub-groups based on participants’ gender and study duration, the heterogeneity of RCTs for long-term DBP was reduced to less than 50%.
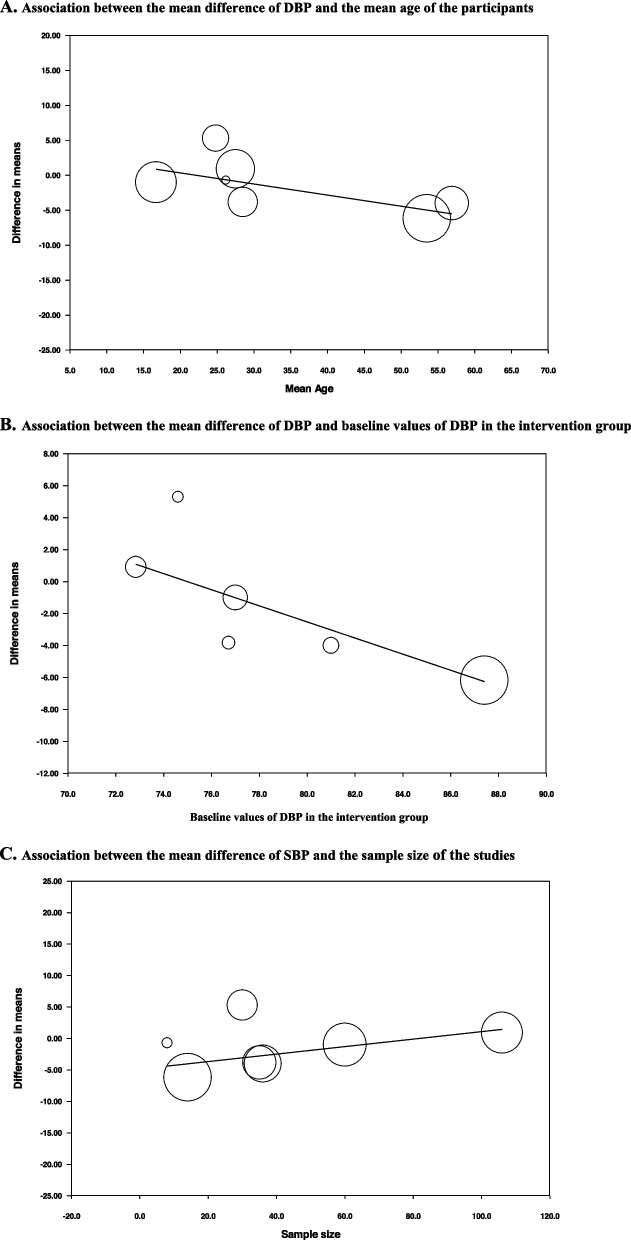
Results of meta-regression
The results of meta-regression analysis showed no significant associations between the change in SBP and the mean ages of participants (slope: -0.23; 95%CI: -0.61 to 0.14; p = 0.178); between SBP and the baseline values in the intervention group (slope: -0.18; 95%CI: -1.04 to 0.67; p = 0.588); and between SBP and the sample size (slope: 0.09; 95%CI: -0.12 to 0.30; p = 0.322). (Fig. 5, panels A-C).
Fig. 5.
Meta-regression of the associations between the mean differences of SBP and mean age, baseline values and the sample size of the studies
Also, the analysis revealed no significant relationship between changes in DBP and the mean age of the participants (slope: -0.15; 95%CI: -0.33 to 0.01; p = 0.069) and between DBP and the sample size (slope: 0.05; 95%CI: -0.04 to 0.16; p = 0.210). However, the results of the meta-regression analysis showed significant associations between DBP and the baseline values in the intervention group (slope: -0.50; 95%CI: -0.77 to -0.22; p = 0.007) (Fig. 6, panels A-C).
Fig. 6.
Meta-regression of the associations between the mean differences of DBP and mean age, baseline values and sample size of the studies
Publication bias
The funnel plots of the effect of Mentha on SBP and DBP are illustrated in Fig. 7. After applying the “trim and fill” method, some studies were added to account for missing data in the meta-analysis of SBP or DBP for adjusting publication bias (Fig. 7 panels A and B). Table 7 summarizes the results of Begg’s rank correlation, Egger’s liner regression, “fail-safe N” tests, and correlated effect size. The result of Egger's linear regression test revealed publication bias for the effect of Mentha on SBP (Table 7).
Fig. 7.
Funnel plots of the effect of Mentha on blood pressure
Table 7.
Assessing publication bias regarding the effect of Mentha consumption on blood pressure
| Corrected effect size | Begg’s rank correlation test | Egger’s liner regression test | Fail-safe N test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WMD | 95% CI | Kendall’s Tau | z-value | p-value | Intercept | 95% CI | t-value | df | p-value | n | |
| SBP | -1.22 | -6.61,4.16 | 0.00 | 0.00 | 1.00 | 2.92 | 0.02,5.82 | 2.58 | 5.00 | 0.048 | 108 |
| DBP | -1.83 | -5.06,1.40 | 0.19 | 0.60 | 0.54 | 2.31 | -0.98,5.61 | 1.80 | 5.00 | 0.13 | 20 |
Abbreviations: SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, WMD Weight Mean Difference
Discussion
To the best of our knowledge, the present study is the first systematic review and meta-analysis to assess the effects of Mentha on blood pressure, including seven RCTs. The results of the meta-analysis indicated that Mentha consumption lowered SBP and DBP. However, this reduction was not statistically significant in SBP, DBP, long-term SBP, and long-term DBP. Notably, this reduction was clinically significant in long-term SBP and long-term DBP. Overall, reductions in SBP and DBP of ≥ 2 mm Hg can significantly reduce CVD incidence in both hypertensive and normotensive individuals. Therefore, small reductions in this value are considered clinically meaningful [30, 31].
In sub-group analysis, a statistically and clinically significant reduction in SBP, DBP, long-term DBP, and long-term SBP was found in the ages above 30 years and the participants with a baseline SBP > 130 mmHg or DBP > 80 mmHg.
The antihypertensive effect of Mentha has been observed in some in-vitro studies [12]. These studies showed that dietary menthol, i.e., the effective compound in Mentha, can lower blood pressure in hypertensive rats by inhibiting RhoA/Rho kinase expression and activity [12].
The overall effect of Mentha on blood pressure in the present meta-analysis had been confirmed by a trial with a long-term duration of intervention [16]. However, our results differ from those of other experimental [12, 13] and clinical trials [32]. In this respect, the findings of animal studies [12, 33, 34] regarding the effect of Mentha on blood pressure are different from those reported in certain human studies [15, 16]. This variance may be due to the varied blood pressure or physiological differences between humans and animals, particularly in the transient receptor potential melastatin subtype 8 (TRPM8) channel. It is of note that this channel is activated by menthol [12].
In the current study, the significant heterogeneity among the included studies makes it challenging to interpret our findings accurately. This apparent heterogeneity indicates that Mentha consumption may have varying effects on SBP and DBP across different populations. The included trials involved adult participants of both genders, including healthy individuals, as well as patients with pre-hypertension, mild hypertension, or primary dysmenorrhea.
Generally, there have been inconsistent findings on the effect of Mentha on SBP and DBP. Similar to our findings, some studies reported a slight reduction or no significant reduction in SBP, DBP, long-term SBP or long-term DBP [15, 16], while some trials reported significant effects [12, 13, 35].
The exact mechanisms by which Mentha probably affects blood pressure are not entirely comprehended. However, previous studies have suggested various mechanisms for Mentha’s mild blood pressure-lowering effect. Activation of TRPM8 by menthol has been found to inhibit calcium signaling-mediated RhoA/Rho kinase activation in the vasculature, leading to endothelium-dependent hypotensive and vasorelaxant effects [12, 34, 36, 37]. The nearly high levels of potassium found in Mentha can increase cellular Ca2 + levels by allowing Ca2 + influx through receptor-operated and voltage-dependent Ca2 + channels (VDCs) [38]. Furthermore, Mentha consumption seems to enhance glutathione peroxidase activity [39]. Additionally, Mentha has been hypothesized to inhibit the arachidonic acid metabolite thromboxane B2 [39] and angiotensin I-converting enzyme (ACE) [40]. These mechanisms are some of the findings of studies conducted on the effect of Mentha on blood pressure [9, 11, 41]. Among these mechanisms, the role of the TRPM8 channel seems more critical. The TRPM8 cation channel can be activated by menthol, a compound with a naturally cold sense in mint [12]. This nonselective cation channel controls Ca2+ homeostasis [42, 43]. This channel is present in various organs and tissues, regulating vital processes such as cell proliferation, migration, apoptosis, inflammatory reactions, immunomodulation, pain, and vascular muscle tension [42].
The mechanisms mentioned for the probably antihypertensive action of Mentha are due to its compounds. Mentha species contain menthol, menthone, isomenthone, menthyl acetate, linalool, lippione, pulegone, carvone, piperitenone oxide, and cis-piperine epoxide as their principal components [44–48]. Mentha species are known for their high polyphenol content [49]. They also contain caffeic acid, caftaric acid, cinnamic acid, ferulic acid, and oleanolic acid [48, 50–52]. Moreover, flavonoids such as apigenin, acacetin, diosmin, salvigenin, and thymonin have been found in Mentha species. These compounds account for about 10 to 70 compounds out of the total phenolics. Additionally, flavanols like catechin and epicatechin, as well as coumarins including esculetin and scopoletin, have been detected in Mentha [48, 53–56].
In the subgroup analysis of the current study, a significant decrease in SBP and DBP was detected only in trials that used Mentha extract [8]. This finding may be due to the presence of nearly all Mentha compounds in the extract or higher menthol concentration in Mentha extract than in other formations. Alternatively, it may be due to better absorption of extract. Moreover, in subgroup analysis, the effect of Mentha on long-term SBP and DBP was not significant in the trials with a duration of more or less than 50 days. This result is inconsistent with animal studies showing the effects of Mentha on blood pressure [12]. Based on the findings of the present meta-analysis, it seems that the effect of Mentha on long-term blood pressure does not depend on the duration of intervention. However, because the heterogeneity in the trials with an intervention duration of more than 50 days was significant, the obtained result should be interpreted cautiously. In the present study, subgroup analyses also indicated that the consumption of Mentha led to a statistically and clinically significant reduction in blood pressure when the baseline values for the SBP were more than 130 mmHg and the baseline values for the DBP were more than 80 mmHg. Therefore, it seems that Mentha consumption may have a beneficial effect on blood pressure in patients with pre-hypertension and hypertension than in healthy individuals. This effect is probably due to the activation of the TRPM8 channel, which may not be completely active in patients with pre-hypertension and hypertension. It is of note that patients with hypertension are usually older adults aged over 25 years [1]. This age difference may explain why sub-group analyses in our study showed that the intake of Mentha significantly decreased both SBP and DBP in ages above 30 years compared to the lower ages. This reduction was statistically and clinically significant.
In the present meta-analysis, only one study showed an increase in SBP, unlike the other studies included [13]. The participants of this RCT were only men. The result observed in this RCT may be attributed to the short duration of the intervention, which was only 1 h. Besides, the young age and low baseline blood pressure of the participants in this study may have contributed to the results. It is also possible that the exercise implemented in the trial affected the outcome. Notably, this study was one of the two RCTs that had an intervention with the essential oil of Mentha [13]. Therefore, this finding should be interpreted with caution.
Although Mentha species are generally safe and have some beneficial impacts, they are not free from side effects for all individuals. Some of these side effects can be severe for some persons. Mentha consumption can trigger allergic reactions in sensitive individuals. There is limited reporting on the toxicity of Mentha species and the authors of this research found no chronic toxicity study in humans [48]. Past studies reported no side effects by consuming 0.24 mL of pure Mentha daily for three consecutive weeks [57, 58] or 144 mg/day of menthol for eight weeks [8]. The World Health Organization (WHO) has evaluated the safe consumption of menthol as being up to 4.0 mg/kg daily [15].
Mentha has properties that distinguish it from other herbs that help lower blood pressure. In addition to high levels of potassium in Mentha, which aids in lowering blood pressure, it contains high levels of menthol [38]. Menthol is a monoterpene alcohol that can activate the TRPM8 channel, which has a vasodilator effect on the vasculature and creates a cooling sensation. This activation of the TRPM8 channel can help patients with hypertension and stress to feel better [12]. There is accumulating evidence that several of menthol’s cellular targets are involved in cardiovascular diseases, such as hypertension. Therefore, menthol can play important preventive and therapeutic roles, which requires further investigation [59]. Besides, Mentha consumption seems to enhance glutathione peroxidase activity [39]. Many studies show that consuming foods and beverages with antioxidant potential is related to protection against cardiovascular diseases. This result is attributed to their impact on nitric oxide metabolism, which enhances vascular tone control and helps to manage hypertension [60]. Furthermore, Mentha is generally safe and can be easily added to daily foods and various products [12]. These properties of Mentha distinguish it from other herbs that aid in lowering blood pressure.
There were some strong points in our study. To date, the present study is the first systematic review and meta-analysis to evaluate the effect of Mentha on blood pressure. This study considers Mentha’s long-term and short-term effects on SBP and DBP. To cover all relevant literature, a complete search was conducted across five databases (PubMed, ISI Web of Science, SCOPUS, Google Scholar, and SID) using PRISMA guidelines. In addition, the reference lists of the related reviews were searched. Also, this study uses a snowball search technique to include other relevant trials that may have been missed. Standard methodologies were utilized to assess kappa statistics between the authors, heterogeneity, meta-regression, sensitivity analysis, and publication bias. There was perfect agreement in study selection between the reviewers. Also, the reviewers had substantial agreement regarding data extraction and quality assessment. In addition, the GRADE evidence profiles were applied to assess the total quality of evidence related to the blood pressure-lowering effect of Mentha. However, the current study has some limitations that need to be considered. First, the included RCTs were limited and had a modest sample size, which resulted in insufficient statistical power to recognize an effect in the present meta-analysis. Second, according to the GRADE evidence profiles, the quality of evidence regarding the effect of Mentha on SBP and DBP was low. Therefore, our confidence in the estimate of these effects is limited.
It is worth noting that most of the included RCTs did not provide enough data on the participants’ weight, height, and body mass index (BMI). As a result, it was not possible to conduct a sub-group analysis based on these variables, even though blood pressure can be influenced by the weight and BMI of the participants [61, 62]. Moreover, due to the limited available data in the included trials, we could not analyze the correlation between Mentha consumption and sodium and potassium serum levels that seem to be related to blood pressure [63].
The sensitivity analysis in the present study showed that omitting each trial would not significantly alter the meta-analysis regarding the effect size of Mentha on DBP or SBP; however, the effect size for the influence of Mentha on long-term SBP or long-term DBP was sensitive to the study performed by Paul H. Falcone et al. [17]. In other words, eliminating this study from the analysis significantly changes the effect of Mentha on long-term blood pressure; hence, it is advisable to take caution while interpreting our findings.
Furthermore, the included trials were heterogeneous in terms of the mean age and gender of participants, sample size, duration of intervention, and baseline blood pressure. It seems that potential differences in the type of Mentha supplements and the duration of intervention may have a confounding impact on the changes in blood pressure.
It is suggested to conduct more RCTs with larger sample sizes and longer durations of intervention regarding the effect of Mentha on blood pressure in the future. Specifically, these trials can focus on the effect of Mentha on blood pressure in patients with pre-hypertension and hypertension with different races in different countries. Also, these trials can focus on the effect of menthol supplementation on blood pressure. This focus is especially important considering the effect of Mentha or menthol supplementation on the participants’ sodium and potassium serum levels. Moreover, because of the relationship between blood pressure and heart rate, it is recommended to conduct a systematic review and meta-analysis of RCTs on the effect of Mentha on heart rate. Furthermore, it is suggested that more studies be conducted on the mechanisms regarding the effect of Mentha on blood pressure in the future.
Conclusion
Our findings showed that Mentha consumption might not have a statistically significant impact on reducing SBP, DBP, long-term SBP, and long-term DBP. However, it can lead to a clinically significant reduction in both long-term SBP and long-term DBP. Additionally, Mentha may have potential benefits for patients with pre-hypertension and hypertension. It is important to note that further well-designed RCTs are needed to confirm our results.
Supplementary Information
Acknowledgements
We sincerely thank the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences and the Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran.
Abbreviations
- RCTs
Randomized Controlled Trials
- WMD
Weighted Mean Difference
- CI
Confidence Intervals
- PI
Prediction Interval
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- CVD
Cardiovascular Diseases
- TRPM8
Transient Receptor Potential Melastatin Subtype 8
- NCBI
National Centre for Biotechnology Information
- WHO
World Health Organization
- VDCs
Voltage-Dependent Ca + + Channels
- ACE
Angiotensin I-Converting Enzyme
- CMA
Comprehensive Meta-Analysis
- P-Value
Probability Value
- PR
Parallel
- CR
Crossover
- bSBP
Baseline Systolic Blood Pressure
- bDBP
Baseline Diastolic Blood Pressure
- In
Intervention Group
- Pl
Placebo Group
- NR
Not Reported
- BMI
Body mass index
Authors’ contributions
F.N. conceived the study. F.N. and N.S. wrote the proposal. F.N. was responsible for conducting the literature search. F.N. and G.K. carried out the literature screening. F.N. and Ab.M. were responsible for extracting and reviewing data independently. F.N. and Ab.M. performed a quality evaluation. F.N. conducted data analysis and interpretation. F.N. and N.S. wrote the manuscript. N.S. and Al.M. critically revised the manuscript. All authors involved in this study have thoroughly studied the manuscript and agreed on its contents.
Funding
The present study was funded by the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences, Kashan, Iran (Grant Number: 402143).
Data availability
The data sets generated or analyzed during the present study are not publicly accessible due to the regulations of the Research Center for Biochemistry and Nutrition in Metabolic Diseases at Kashan University of Medical Science. However, they can be accessible from the corresponding author upon logical request.
Declarations
Ethics approval and consent to participate
This systematic review and meta-analysis study was designed following the guidelines of the PRISMA statement. The study was approved by the Ethics Committee of Kashan University of Medical Sciences, Kashan, Iran (IR.KAUMS.NUHEPM.REC.1402.063).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nasrin Sharifi, Email: sharifi-na@kaums.ac.ir.
Alireza Milajerdi, Email: amkhv@yahoo.com.
References
- 1.Haldar R. Global brief on hypertension: silent killer, global public health crisis. Indian J Phys Med Rehabil. 2013;24(1):2. [Google Scholar]
- 2.Saxena T, Ali AO, Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther. 2018;16(12):879–87. [DOI] [PubMed] [Google Scholar]
- 3.Verma T, Sinha M, Bansal N, Yadav SR, Shah K, Chauhan NS. Plants Used as Antihypertensive. Natural Products and Bioprospecting. 2021;11(2):155–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr. 2016;146(2):389S–S396. [DOI] [PubMed] [Google Scholar]
- 5.Ghavami A, Haghighian HK, Roshanravan N, Ziaei R, Ghaedi E, Moravejolahkami AR, Askari G. What is the impact of cinnamon supplementation on blood pressure? A systematic review and meta-analysis. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2021;21(5):956–65. [DOI] [PubMed]
- 6.Shayani Rad M, Moohebati M, Mohajeri SA. Effect of celery (Apium graveolens) seed extract on hypertension: A randomized, triple-blind, placebo-controlled, crossover, clinical trial. Phytother Res. 2022;36(7):2889–907. [DOI] [PubMed] [Google Scholar]
- 7.Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22(4):447–52. [DOI] [PubMed] [Google Scholar]
- 8.Samaha AA, Fawaz M, Salami A, Baydoun S, Eid AH. Antihypertensive indigenous lebanese plants: ethnopharmacology and a clinical trial. Biomolecules. 2019;9(7):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AJ, Bukhari IA, Gilani AH. Mentha longifolia lowers blood pressure in anesthetized rats through multiple pathways. ||| Bangladesh Journal of Pharmacology. 2016;11(4):784–92.
- 10.Patonay K, Korozs M, Muranyi Z, Konya EP. Polyphenols in northern Hungarian Mentha longifolia (L.) L. treatedwith ultrasonic extraction for potential oenological uses. Turkish Journal of Agriculture and Forestry. 2017;41(3):208–17.
- 11.Bahadori MB, Zengin G, Bahadori S, Dinparast L, Movahhedin N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). International Journal of Food Properties. 2018;21(1):183–93.
- 12.Sun J, Yang T, Wang P, Ma S, Zhu Z, Pu Y, et al. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension. 2014;63(6):1354–63. [DOI] [PubMed] [Google Scholar]
- 13.Meamarbashi A. Instant effects of peppermint essential oil on the physiological parameters and exercise performance. Avicenna journal of phytomedicine. 2014;4(1):72. [PMC free article] [PubMed] [Google Scholar]
- 14.Sinclair J, Murray H, Smith V, Tom N, Cruz TC, Taylor PJ, et al. Effects of peppermint oil (Mentha piperita L.) on cardiometabolic and other health-related outcomes: a parallel placebo randomized controlled trial. Sport Sciences for Health. 2023;19(4):1329–38.
- 15.Gelal A, Balkan D, Ozzeybek D, Kaplan YC, Gurler S, Guven H, Benowitz NL. Effect of menthol on the pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur J Clin Pharmacol. 2005;60(11):785–90. [DOI] [PubMed] [Google Scholar]
- 16.Sultan S, Ahmed Z, Afreen A, Rashid F, Majeed F, Khalid N. Analgesic effect of ginger and peppermint on adolescent girls with primary dysmenorrhea. Food Science and Technology. 2020;41:833–9. [Google Scholar]
- 17.Falcone PH, Tribby AC, Vogel RM, Joy JM, Moon JR, Slayton CA, et al. Efficacy of a nootropic spearmint extract on reactive agility: a randomized, double-blind, placebo-controlled, parallel trial. J Int Soc Sports Nutr. 2018;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906. [DOI] [PubMed] [Google Scholar]
- 19.Cantor AB. Sample-size calculations for Cohen’s kappa. Psychol Methods. 1996;1(2):150. [Google Scholar]
- 20. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. London, UK. 2011.
- 21.Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave. 2021;21(02). [DOI] [PubMed]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis Englewood. NJ; 2005.
- 26.Shepherd K, Peart DJ. Aerobic capacity is not improved following 10-day supplementation with peppermint essential oil. Appl Physiol Nutr Metab. 2017;42(5):558–61. [DOI] [PubMed] [Google Scholar]
- 27.Herrlinger KA, Nieman KM, Sanoshy KD, Fonseca BA, Lasrado JA, Schild AL, et al. Spearmint extract improves working memory in men and women with age-associated memory impairment. The Journal of Alternative and Complementary Medicine. 2018;24(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao T-Y, Huang C-F, Liu D-Y, Chen C-T, Yang C-C. Effects of Mentha Piperita Essential Oil Uptake or Inhalation on Heart Rate Variability and Cardiopulmonary Regulation during Exercise. Montenegrin Journal of Sports Science & Medicine. 2021;10(2).
- 29.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366. [DOI] [PubMed]
- 30.Turnbull F, Neal B, Algert C, Chalmers J, Woodward M, MacMahon S, et al. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–35. [DOI] [PubMed] [Google Scholar]
- 31.Wong GW, Wright JM. Blood pressure lowering efficacy of nonselective beta‐blockers for primary hypertension. Cochrane Database of Systematic Reviews. 2014(2). [DOI] [PMC free article] [PubMed]
- 32.Mohammadtaheri F, Gheysari R, Akhlaghdoost M. The effectiveness of oral peppermint extract on migraine. Anesthesiology and Pain. 2016;7(1):1–12. [Google Scholar]
- 33.Ajebli M, Eddouks M. Vasorelaxant and antihypertensive effects of Mentha pulegium L. in rats: an in vitro and in vivo approach. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2021;21(7):1289–99. [DOI] [PubMed]
- 34.Lahlou S, Carneiro-Leão RFL, Leal-Cardoso J. Cardiovascular effects of the essential oil of Mentha x villosa in DOCA-salt-hypertensive rats. Phytomedicine. 2002;9(8):715–20. [DOI] [PubMed] [Google Scholar]
- 35.Anwar F, Abbas A, Mehmood T, Gilani AH, Rehman Nu. Mentha: A genus rich in vital nutra‐pharmaceuticals—A review. Phytotherapy Research. 2019;33(10):2548–70. [DOI] [PubMed]
- 36.González-Muñiz R, Bonache MA, Martín-Escura C, Gómez-Monterrey I. Recent progress in TRPM8 modulation: an update. Int J Mol Sci. 2019;20(11):2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guedes DN, Silva D, Barbosa-Filho J, De Medeiros IA. Endothelium-dependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine. 2004;11(6):490–7. [DOI] [PubMed] [Google Scholar]
- 38.Turkoglu S. Assessment of wild mint from Tunceli as source of bioactive compounds, and its antioxidant Activity. Cell Mol Biol (Noisy-le-grand). 2015;61(8):63–8. [PubMed] [Google Scholar]
- 39.Gul S, Gul H, Nawaz R. Possible mechanism of action of Mentha arvensis in cardiovascular diseases. International Journal of Endorsing Health Science Research. 2014;2(1).
- 40.Alu’datt MH, Rababah T, Alhamad MN, Ereifej K, Al‐Mahasneh M, Brewer S, Rawshdeh M. Optimization Extraction Conditions for Phenolic Compounds, Antioxidant and Inhibitory Activities of A ngiotensin I‐C onverting E nzyme (ACE), α‐Glucosidase and α‐Amylase from M entha Spicata L. Journal of Food Biochemistry. 2016;40(3):335–44.
- 41.Cam M, Basyigit B, Alasalvar H, Yilmaztekin M, Ahhmed A, Sagdic O, et al. Bioactive properties of powdered peppermint and spearmint extracts: Inhibition of key enzymes linked to hypertension and type 2 diabetes. Food Biosci. 2020;35: 100577. [Google Scholar]
- 42.Liu Y, Mikrani R, He Y, Baig MMFA, Abbas M, Naveed M, et al. TRPM8 channels: A review of distribution and clinical role. Eur J Pharmacol. 2020;882: 173312. [DOI] [PubMed] [Google Scholar]
- 43.Hawthorn M, Ferrante J, Luchowski E, Rutledge A, Wei X, Triggle D. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther. 1988;2(2):101–18. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence B. Mint: The Genus Mentha. CRC: Press. Taylor & Francis Group, Florida, USA; 2006. [Google Scholar]
- 45.Maffei M, Codignola A. Photosynthesis, photorespiration and herbicide effect on terpene production in peppermint (Mentha piperita L.). Journal of Essential Oil Research. 1990;2(6):275–86.
- 46.Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, Van Griensven LJ. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14(1):238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moghaddam M, Pourbaige M, Tabar HK, Farhadi N, Hosseini SMA. Composition and antifungal activity of peppermint (Mentha piperita) essential oil from Iran. Journal of Essential Oil Bearing Plants. 2013;16(4):506–12. [Google Scholar]
- 48.Tafrihi M, Imran M, Tufail T, Gondal TA, Caruso G, Sharma S, et al. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules. 2021;26(4):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Pereira O, M Cardoso S. Overview on Mentha and Thymus polyphenols. Current Analytical Chemistry. 2013;9(3):382–96.
- 50.Benedec D, Vlase L, Oniga I, Mot AC, Silaghi-Dumitrescu R, Hanganu D, et al. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note I Farmacia. 2013;61(2):262–7. [Google Scholar]
- 51.Taamalli A, Arráez-Román D, Abaza L, Iswaldi I, Fernández-Gutiérrez A, Zarrouk M, Segura-Carretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem Anal. 2015;26(5):320–30. [DOI] [PubMed] [Google Scholar]
- 52.Wu Z, Tan B, Liu Y, Dunn J, Martorell Guerola P, Tortajada M, et al. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules. 2019;24(15):2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koşar M, Dorman HD, Can Başer KH, Hiltunen R. Screening of free radical scavenging compounds in water extracts of Mentha samples using a postcolumn derivatization method. J Agric Food Chem. 2004;52(16):5004–10. [DOI] [PubMed] [Google Scholar]
- 54.Bimakr M, Rahman RA, Taip FS, Ganjloo A, Salleh LM, Selamat J, et al. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food and bioproducts processing. 2011;89(1):67–72.
- 55.Salin O, Tormakangas L, Leinonen M, Saario E, Hagstrom M, Ketola RA, et al. Corn mint (Mentha arvensis) extract diminishes acute Chlamydia pneumoniae infection in vitro and in vivo. J Agric Food Chem. 2011;59(24):12836–42. [DOI] [PubMed] [Google Scholar]
- 56.Fatiha B, Didier H, Naima G, Khodir M, Martin K, Léocadie K, et al. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Industrial crops and products. 2015;74:722–30.
- 57.Amui Roknabad M, Sarafraz N. Comparison between the effect of supermint and ibuprofen on primary dysmenorrheal: a randomized clinical trial. Qom University of Medical Sciences Journal. 2011;5(3):37–41. [Google Scholar]
- 58.Nasiri A, Pakmehr M, Shahdadi H, Balouchi A, Sepehri Z, Ghalenov A. A comparative study of dimethicone and supermint anti-flatulence effects on reducing flatulence in patients with irritable bowel syndrome. Pharm Lett. 2016;8(1):97–101. [Google Scholar]
- 59.Silva H. Current knowledge on the vascular effects of menthol. Front Physiol. 2020;11: 523469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schini-Kerth VB, Auger C, Kim J-H, Étienne-Selloum N, Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflügers Archiv-European Journal of Physiology. 2010;459:853–62. [DOI] [PubMed] [Google Scholar]
- 61.Linderman GC, Lu J, Lu Y, Sun X, Xu W, Nasir K, et al. Association of body mass index with blood pressure among 1.7 million Chinese adults. JAMA network open. 2018;1(4):e181271-e. [DOI] [PMC free article] [PubMed]
- 62.Sundström J, Lind L, Lampa E, Angerås O, Bachus E, Bergström G, et al. Weight gain and blood pressure. J Hypertens. 2020;38(3):387–94. [DOI] [PubMed] [Google Scholar]
- 63.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356(19):1966–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated or analyzed during the present study are not publicly accessible due to the regulations of the Research Center for Biochemistry and Nutrition in Metabolic Diseases at Kashan University of Medical Science. However, they can be accessible from the corresponding author upon logical request.