Abstract
Background
Most Canadians receive their care in community hospitals, yet most clinical research is conducted in academic hospitals. This study aims to compare patients with community acquired pneumonia (CAP) treated in academic and community hospitals with respect to their demographics, clinical characteristics, treatments and outcomes.
Methods
This nested observational cohort substudy of the Community Acquired Pneumonia: Toward InnoVAtive Treatment (CAPTIVATE) trial included 1,329 hospitalized adults with CAP recruited between March 1st, 2018 and September 31st, 2023 from 15 Canadian hospitals. Unadjusted and adjusted analyses for age, sex and co-morbidities using logistic, Cox and censored quantile regressions were conducted.
Results
Patients in community hospitals were older (mean [SD] 75.0 [15.7] years vs. 68.3 [16.2] years; p < 0.001), were more likely to be female (49.7% vs. 41.0%, p = 0.002), and had more comorbidities (75.9% vs. 64.8%, p < 0.001). More patients in community hospitals received corticosteroids (49.2% vs. 37.4%, p < 0.001). Community hospital patients had a higher likelihood of developing acute respiratory distress syndrome (OR 3.13, 95% CI: 1.87, 5.24, p = < 0.001), and acute cardiac injury (OR 2.53, 95% CI: 1.33, 4.83, p = 0.005). In unadjusted and adjusted analyses, 28-day mortality difference did not meet statistical significance (OR 1.43, 95% CI: 0.98, 20.7, p = 0.062 and OR 1.23, 95% CI: 0.81, 1.87, p = 0.332, respective).
Conclusion
Patients with CAP in Canadian community and academic hospitals differed with respect to their age, clinical characteristics, treatments and outcomes, emphasizing the importance of including more community hospitals in clinical research studies to ensure the generalizability of results.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-024-00143-x.
Keywords: Community-acquired pneumonia, Mortality, Corticosteroids, Community hospital
Background
Community hospitals represent over 90% of hospitals in Canada [1]. Although they provide the majority of inpatient clinical care, they do not frequently participate in clinical research studies [2]. Relative to academic hospitals, community hospitals are more likely to be located in suburban and rural communities [3, 4] and are more likely to serve populations with higher proportions of recent immigrants [5] lower socioeconomic status [6–8] and reduced access to subspecialized care [9, 10]. In addition, patients in community hospital tend to be older, with more comorbidities, increased frailty and a higher risk of in-hospital mortality [8]. Thus, research conducted exclusively in academic hospitals may not accurately reflect the patient population in community hospitals.
Community-acquired pneumonia (CAP) affects 330,000 Canadians per year, causing 6,000 deaths and disproportionately affecting older individuals and those with comorbidities [11, 12]. Given the differences in baseline populations between academic and community hospitals, we hypothesized that there are clinically relevant differences in patient baseline characteristics, treatments, and outcomes of patients with CAP in community and academic hospitals.
Methods
This is a retrospective observational study nested within the Community Acquired Pneumonia: Toward InnoVAtive Treatment (CAPTIVATE) Research program – a multi-centre, pan-Canadian cohort study. Inclusion criteria were hospitalized patients > 18 years of age with an admitting diagnosis of acute CAP defined by having one of fever, chills, leukocytosis, leukopenia; one of cough, sputum, dyspnea; and new infiltrates on chest x-ray consistent with CAP [13–16]. Exclusion criteria were Emergency Department visits without hospital admission, readmissions, and admissions for other reasons. In this nested observational study, we included patients enrolled between March 1, 2018 and September 31, 2023 in 15 Canadian hospitals. We calculated the SMART-COP (systolic blood pressure, multilobar infiltrates, albumin, respiratory rate, tachycardia, confusion, oxygen, and pH) CAP severity score in all patients to understand severity of CAP between community and academic hospitals [17].
The primary outcome was 28-day mortality; patients discharged before day 28 and lost to follow up were assumed 28-day survivors [18]. Secondary outcomes were hospital mortality, Intensive Care Unit (ICU) admission rates, organ dysfunction, and ICU and hospital length of stay. Organ dysfunction was scored first, as frequency of invasive ventilation, vasopressors and Renal Replacement Therapy (RRT) and second, as days alive and free (DAF) of these therapies within the first 14 days [19] determined by subtracting numbers of days on ventilation, vasopressors or RRT from 14. Deaths within 14 days were assigned 0 DAF.
Hospital sites were included by invitation and based on agreement to participate in the study. Hospital status was determined according to the Canadian Institute for Health Information (CIHI) classification, which differentiates hospitals by teaching status [1]. For the purpose of this study, we defined CIHI “teaching” hospitals as “academic” and CIHI “non-teaching” hospitals as “community”. Study outcomes included patient demographics, clinical characteristics, treatments, and clinical outcomes (organ dysfunction, length of stay and mortality).
Statistical analysis
Baseline and clinical characteristics were compared using Chi-square test, Fisher’s exact test, Analysis of Variance (ANOVA) or Kruskal–Wallis test as appropriate. Unadjusted and adjusted regression analyses (adjusting for pre-defined adjustment factors: age, sex, co-morbidities and CAP severity as measured by modified SMART-COP), logistic, Cox and censored quantile regression were used to compare binary outcomes, survival time and length of stay, respectively [20]. For length of stay analysis, in-hospital deaths were considered as never discharged and censored at the longest observed length of stay [21]. The observed days alive and free (DAF) of ventilation, vasopressors, and renal replacement therapy over the first 14 days post hospital admission data exhibited a U-shape distribution, with most data concentrated at 0 and 14. We thus used 0–1 inflated beta regression to model this data [14]. Results were expressed as odds ratio (OR), hazard ratio (HR), difference in median length of stay and mean difference in DAF with 95% confidence interval (CI).
Approximately 5% of patients had missing data and were therefore excluded from the adjusted regression analyses. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and R 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant.
Ethical considerations
This study was approved by Providence Health Care and the University of British Columbia (UBC) Human Research Committee and by each of the participating sites. Collection of anonymized clinical data and discarded plasma from clinical blood tests were deemed low risk and the requirement for informed consent was waived by all the participating REBs.
Results
Hospital site characteristics
The CAPTIVATE Research Program included 15 hospital sites across Canada of which 10 (66.7%) were academic hospitals and 5 (33.3%) were community hospitals. Amongst 1,329 patients, 744 (56.0%) were admitted to academic hospitals and 585 (44.0%) to community hospitals, translating to 88 and 142 patients per 1,000 hospital beds respectively. Site characteristics are found in Additional File 1. Median enrollment was numerically higher per site in community sites compared to academic sites (99 [Range: 22–195] vs. 20 [Range: 1-221], p = 0.27).
Patient demographics
Patients enrolled in community hospitals were older (mean [SD] 75.0 [15.7] years vs. 68.3 [16.2] years; p < 0.001), more likely to be female (49.7% vs. 41.0%, p = 0.002), and were more likely to have comorbidities including chronic cardiac disease, chronic kidney disease, hypertension and diabetes (75.9% vs. 64.8%, p < 0.001) (Table 1) than patients enrolled in academic hospitals. Specifically, community hospital patients had higher proportions of chronic kidney disease (23.1% vs. 15.3%, p < 0.001), hypertension (60.8% vs. 48.4%, p < 0.001), chronic neurological disorders (16.1% vs. 9.2%, p < 0.001), rheumatologic disorders (19.1% vs. 12.5%, p < 0.001) and dementia (14.0% vs. 8.0%, p < 0.001) (Table 1). Conversely, academic hospital patients had a higher proportion of acquired immune deficiency syndrome and human immunodeficiency virus infection (AIDS/HIV) (1.5% vs. 0.2%, p = 0.011) (Table 1).
Table 1.
Patient demographics and clinical characteristics
| Variable | Community (n = 585) | Academic (n = 744) | P value |
|---|---|---|---|
| Province, n/total (%) | < 0.001 | ||
| AB | 0/585 (0.0) | 1/744 (0.1) | |
| BC | 109/585 (18.6) | 188/744 (25.3) | |
| NF | 0/585 (0.0) | 6/744 (0.8) | |
| ON | 281/585 (48.0) | 259/744 (34.8) | |
| QC | 195/585 (33.3) | 290/744 (39.0) | |
| Sex, n/total (%) | 0.002 | ||
| Unknown | 1/585 (0.1) | 3/744 (0.4) | |
| Male | 294/585 (50.3) | 437/744 (59.0) | |
| Female | 290/585 (49.7) | 304/744 (41.0) | |
| Age, years | < 0.001 | ||
| Mean (SD) | 75.0 (15.7) | 68.3 (16.2) | |
| Median (IQR) | 77.0 (66.0, 87.0) | 70.0 (60.0, 80.0) | |
| Range | (20.0, 103.0) | (20.0, 103.0) | |
| Co-morbidities, n/total (%) | |||
| Any of the foura | 443/584 (75.9) | 481/742 (64.8) | < 0.001 |
| Chronic cardiac disease | 239/585 (40.9) | 275/742 (37.1) | 0.159 |
| Chronic kidney disease | 135/584 (23.1) | 113/740 (15.3) | < 0.001 |
| Hypertension | 355/584 (60.8) | 359/741 (48.4) | < 0.001 |
| Diabetes | 156/585 (26.7) | 183/741 (24.7) | 0.414 |
| Chronic pulmonary disease (not asthma) | 172/585 (29.4) | 243/740 (32.8) | 0.181 |
| Asthmab | 56/585 (9.6) | 78/741 (10.5) | 0.567 |
| Liver disease | 26/585 (4.4) | 32/739 (4.3) | 0.920 |
| Chronic neurological disorder | 94/584 (16.1) | 68/739 (9.2) | < 0.001 |
| Malignant neoplasm | 125/585 (21.4) | 135/740 (18.2) | 0.155 |
| Chronic hematologic disease | 30/585 (5.1) | 48/740 (6.5) | 0.297 |
| AIDS / HIV | 1/585 (0.2) | 11/732 (1.5) | 0.011 |
| Obesity (as defined by clinical staff) | 33/585 (5.6) | 44/734 (6.0) | 0.786 |
| Rheumatologic disorder | 112/585 (19.1) | 92/738 (12.5) | < 0.001 |
| Dementia | 82/585 (14.0) | 59/738 (8.0) | < 0.001 |
| Malnutrition | 10/582 (1.7) | 8/727 (1.1) | 0.340 |
| Positive culturec, n/total (%) | |||
| Streptococcus pneumoniae | 131/574 (22.8) | 152/728 (20.9) | 0.399 |
| Staphylococcus aureus | 28/573 (4.9) | 43/723 (5.9) | 0.405 |
| Haemophilus influenza | 15/572 (2.6) | 25/725 (3.4) | 0.393 |
| Klebsiella/Enterobacter | 6/573 (1.0) | 12/724 (1.7) | 0.351 |
| Other | 8/573 (1.4) | 8/726 (1.1) | 0.633 |
| Influenza, n/total (%) | 80/574 (13.9) | 87/727 (12.0) | 0.291 |
| Admitted to ICU on hospital admission day, n/total (%) | 41/584 (7.0) | 63/743 (8.5) | 0.326 |
| Organ support on admission day, n/total (%) | |||
| Oxygen therapy | 287/583 (49.2) | 324/732 (44.3) | 0.073 |
| Invasive mechanical ventilation | 30/585 (5.1) | 45/744 (6.0) | 0.471 |
| Renal replacement therapy | 4/583 (0.7) | 4/742 (0.5) | 0.737 |
| Vasopressors | 35/585 (6.0) | 45/744 (6.0) | 0.960 |
| Modified SMART-COP Scored | 0.233 | ||
| Unknown | 3 | 46 | |
| 0 | 352 (60.5) | 465 (66.6) | |
| 1 | 129 (22.2) | 124 (17.8) | |
| 2 | 67 (11.5) | 73 (10.5) | |
| 3 | 21 (3.6) | 22 (3.2) | |
| ≥ 4 | 13 (2.2) | 14 (2.0) | |
aChronic cardiac disease, chronic kidney disease, hypertension or diabetes
bDiagnosed by a physician
cBlood or sputum, within 48 h before or after hospital admission
dModified SMART-COP = Systolic blood pressure, respiratory rate, tachycardia, oxygen. Chest x-ray, albumin, Glasgow Coma Score, PaO2/FiO2 and arterial pH were not included as the score component as they were not consistently captured in the database
Clinical characteristics
Rates of laboratory-confirmed bacterial and influenza CAP were comparable between patients enrolled in community and academic hospitals (Table 1). The frequency of organ support and need for oxygen therapy at admission were also similar, as was the rate of ICU admission. There was no difference in Modified SMART-COP scores [17].
Hospital interventions and treatments
Almost all patients received antibiotics in both settings (99.7% vs. 97.8%, p = 0.005). The proportions of patients receiving any corticosteroids (49.2% vs. 37.4%, p < 0.001) were higher in community hospitals relative to academic hospitals (Table 2), however the times to initiation were similar (Table 2). The proportion of patients receiving organ support was similar between community and academic hospitals.
Table 2.
Hospital interventions
| Intervention | Community (n = 585) | Academic (n = 744) | P value |
|---|---|---|---|
| Co-intervention while hospitalized, n/total (%) | |||
| Antiviral agent | 66/583 (11.3) | 85/741 (11.5) | 0.932 |
| Remdesivir | 7/583 (1.2) | 16/741 (2.2) | 0.185 |
| Antibiotic | 583/585 (99.7) | 728/744 (97.8) | 0.005 |
| Corticosteroid | 288/585 (49.2) | 278/744 (37.4) | < 0.001 |
| Dexamethasone | 46/585 (7.9) | 32/744 (4.3) | 0.006 |
| Antifungal agent | 24/583 (4.1) | 29/744 (3.9) | 0.840 |
| Time to initiation of corticosteroid, days, n/total (%) | 0.434 | ||
| Unknown | 21/288 (7.3) | 8/278 (2.9) | |
| 0 | 138/288 (47.9) | 154/278 (55.4) | |
| 1 | 65/288 (22.6) | 61/278 (21.9) | |
| > 1 | 64/288 (22.2) | 55/278 (19.8) | |
| Organ support while hospitalized, n/total (%) | |||
| Invasive mechanical ventilation | 57/585 (9.7) | 74/744 (9.9) | 0.902 |
| Renal replacement therapy | 16/583 (2.7) | 15/742 (2.0) | 0.388 |
| Vasopressors | 64/585 (10.9) | 77/744 (10.3) | 0.729 |
Clinical outcomes
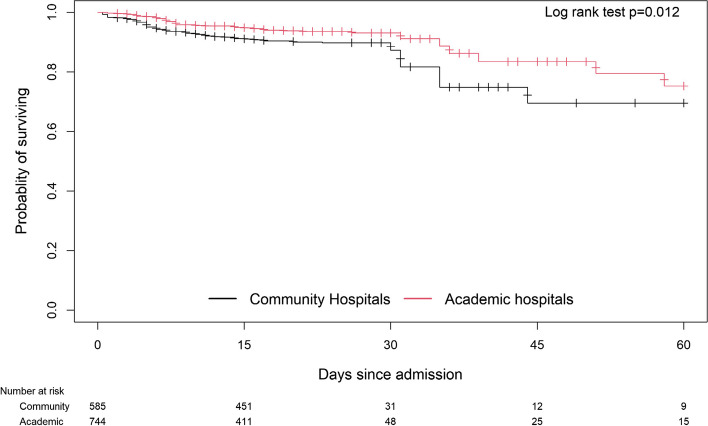
In unadjusted analyses, community hospital patients had higher in-hospital mortality (OR 1.91, 95% CI: 1.28, 2.85, p = 0.001) than academic hospital patients (Tables 3 and 4). Kaplan-Meier survival curves also showed significantly better survival for academic hospital patients (Log Rank p = 0.012, Fig. 1). However, when analyses were adjusted for age, sex, co-morbidities and CAP severity, the difference in survival was no longer significant (Table 4).
Table 3.
Clinical outcomes for community hospital patients vs academic hospital patients
| Variable | Community (n=585) | Academic (n=744) | P value |
|---|---|---|---|
| Mortality, n/total (%) | |||
| Primary: 28-day | 63/585 (10.8) | 58/744 (7.8) | 0.061 |
| In-hospital | 63/585 (10.8) | 44/744 (5.9) | 0.001 |
| Admitted to ICU, n/total (%) | 90/585 (15.4) | 121/744 (16.3) | 0.663 |
| During the first 14 days, DAFa of, mean (SD) | |||
| Invasive mechanical ventilation | 12.4 (4.2) | 13.0 (3.3) | 0.037 |
| Renal replacement therapy | 12.7 (4.0) | 13.3 (2.9) | <0.001 |
| Vasopressors | 12.5 (4.0) | 13.3 (3.1) | 0.018 |
| Hospital length of stay – deceased (time to death) | 0.084 | ||
| n/total (%) | 63/585 (10.8) | 44/744 (6.0) | |
| Median, days (IQR) | 6.0 (4.0, 14.0) | 8.0 (6.0, 18.0) | |
| Hospital length of stay – survivors | 0.092 | ||
| n/total (%) | 522/585 (89.2) | 700/744 (94) | |
| Median, days (IQR) | 6.5 (4.0, 11.0) | 6.0 (4.0, 11.0) | |
| ICU length of stay – deceasedb | 0.208 | ||
| n/total (%) | 18/585 (3.1) | 17/744 (2.3) | |
| Median, days (IQR) | 11.5 (4.0, 21.0) | 7.0 (4.0, 8.0) | |
| ICU length of stay – survivorsb | 0.012 | ||
| n/total (%) | 72/585 (12.3) | 93/744 (12.5) | |
| Median, days (IQR) | 7.5 (4.0, 12.0) | 5.0 (3.0, 10.0) | |
| Septic shock, n/total (%) | 41/581 (7.1) | 48/734 (6.5) | 0.711 |
| Acute respiratory distress syndrome, n/total (%) | 53/584 (9.1) | 25/741 (3.4) | <0.001 |
| Acute kidney injury, n/total (%) | 118/579 (20.4) | 105/733 (14.3) | 0.004 |
| Acute cardiac injury, n/total (%) | 29/585 (5.0) | 19/711 (2.7) | 0.030 |
aDAF = Days Alive and Free
bAmong those who were admitted to ICU
Table 4.
Comparison of outcomes for community hospital patients vs academic hospital patients by regression analysis
| Unadjusted analysis | Adjusted analysis | |||
| Outcome | Odds/hazard ratio (95% CI) | P value | Odds/hazard ratio (95% CI) | P value |
| Primary outcome: 28-day mortality | 1.43 (0.98, 2.07) | 0.062 | 1.23 (0.81, 1.87) | 0.332 |
| In-hospital death | 1.91 (1.28, 2.85) | 0.001 | 1.38 (0.90, 2.11) | 0.142 |
| Time to death | 1.63 (1.11, 2.40) | 0.013 | 1.20 (0.79, 1.82) | 0.389 |
| Admitted to ICUa | 0.94 (0.70, 1.26) | 0.669 | 1.17 (0.83, 1.64) | 0.367 |
| Organ support while hospitalized | ||||
| Invasive mechanical ventilation | 0.98 (0.68, 1.41) | 0.909 | 1.28 (0.86, 1.92) | 0.226 |
| RRT | 1.36 (0.68, 2.75) | 0.386 | 1.68 (0.76, 3.71) | 0.197 |
| Vasopressors | 1.07 (0.75, 1.51) | 0.723 | 1.19 (0.81, 1.75) | 0.387 |
| Organ support during first 14 days | ||||
| Invasive mechanical ventilation | 1.01 (0.70, 1.45) | 0.978 | 1.33 (0.88, 2.00) | 0.176 |
| RRT | 1.38 (0.65, 2.92) | 0.402 | 1.77 (0.76, 4.10) | 0.182 |
| Vasopressors | 1.06 (0.74, 1.51) | 0.752 | 1.18 (0.80, 1.75) | 0.398 |
| Septic shock | 1.09 (0.71, 1.67) | 0.704 | 1.25 (0.78, 2.01) | 0.346 |
| Acute respiratory distress syndrome | 2.83 (1.74, 4.59) | <0.001 | 3.13 (1.87, 5.24) | <0.001 |
| Acute kidney injury | 1.53 (1.15, 2.04) | 0.004 | 1.23 (0.89, 1.70) | 0.199 |
| Acute cardiac injury | 1.88 (1.05, 3.37) | 0.034 | 2.53 (1.33, 4.83) | 0.005 |
| Unadjusted analysis | Adjusted analysis | |||
| Outcome | Difference in median/mean (95% CI) | P value | Difference in median/mean (95% CI) | P value |
| Hospital length of stay | 1.0 (0.2, 1.9) | 0.021 | 0.5 (-0.2, 1.3) | 0.173 |
| ICU length of stay | 3.0 (0.3, 5.7) | 0.031 | 1.7 (-1.9, 5.3) | 0.363 |
| DAFa first 14 days | ||||
| Invasive mechanical ventilation | -0.6 (-1.0, -0.2) | 0.003 | -0.5 (-1.0, -0.1) | 0.020 |
| RRTa or dialysis | -0.7 (-1.1, -0.3) | <0.001 | -b | |
| Vasopressors | -0.6 (-1.0, -0.2) | 0.003 | -0.4 (-0.8, 0.0) | 0.056 |
aDAF = days alive and free, ICU = intensive care unit, RRT = renal replacement therapy
bAdjusted regression analysis was not feasible numerically as few patients received renal replacement therapy during the first 14 days
Fig. 1.
Kaplan-Meier survival estimates of hospitalized CAP patients in community versus academic hospitals
Community hospital patients had greater frequencies of acute respiratory distress syndrome (ARDS) (9.1% vs. 3.4% p < 0.001), acute kidney injury (AKI) (20.4% vs. 14.3%, p = 0.004), and acute cardiac injury (ACI) (5.0% vs. 2.7%, p = 0.030). The differences for ARDS and ACI remained statistically significant after regression adjustment (Table 4).
Community hospital patients had fewer Days Alive and Free (DAF) [19] of invasive mechanical ventilation, vasopressors and renal replacement therapy during the first 14 days of hospitalization in unadjusted analysis. While overall hospital length of stay was similar between community and academic hospital patients, ICU survivors had a greater length of ICU stay in community hospitals (7.5 vs. 5.0 days, p = 0.012).
Discussion
In our multicenter cohort study, there were important baseline differences between patients from community versus academic hospitals. We observed that patients admitted to Canadian community hospitals with CAP were older, more often female, and had more co-morbidities than their academic hospital counterparts. They also had higher severity of illness and a higher proportion of patients developed ARDS, AKI and ACI. In unadjusted analyses, in-hospital mortality was higher in community hospital patients. However, logistic regression analyses revealed that differences at baseline accounted for the higher mortality. With respect to treatments, almost all patients in both settings received antibiotics, while community hospital patients were more likely to receive corticosteroids. However, they were equally likely to receive organ support with mechanical ventilation, vasopressors, and renal replacement therapy. Notably, nearly 95% of patients in both settings had a Modified SMART-COP score of 0–2, reflecting the similar rate of ICU admissions, vasopressor support and mortality (after adjusted analyses) between both groups. ICU stay was longer for community ICU survivors than for academic ICU survivors.
Community hospital patients with CAP often have higher severity of illness compared to their academic hospital counterparts [8, 22, 23]. In unadjusted analyses, in-hospital mortality was higher amongst community hospital patients but after adjustment for age, sex, comorbidities and CAP severity, this gap was no longer detected; suggesting that differences in baseline patient characteristics and severity of illness account for much of the observed mortality difference. Data on social determinants of health, including income, education, and race, were not collected in CAPTIVATE but may also have contributed to poorer outcomes among community hospital patients [24–26]. With respect to medical treatments, community hospital patients were more likely to receive corticosteroids than their academic hospital counterparts which may reflect differences in baseline patient characteristics and also practice patterns that could impact outcomes. Overall, these findings demonstrate that the patient populations in community and academic hospitals differ with respect to their baseline and clinical characteristics, suggesting the need to include more community hospital patients in clinical research to ensure the generalizability of results to the wider population.
Although community hospitals represent more than 90% of Canadian hospitals, they represented only 33% of the hospitals included in this study. The underrepresentation of community hospitals is commonly observed in research studies, including clinical trials [2]. Yet, research results generated in academic hospitals are routinely used to guide care in community hospitals. Our results show that in patients with CAP, baseline characteristics, the provision of treatment and clinical outcomes differ between community and academic hospitals. Considering these differences, it is vital to increase access to research for patients in community hospitals in order to generate clinical evidence that is more applicable to their care. Insufficient research infrastructure, inadequate funding, a lack of research experience and limited organizational commitment to research are known barriers to community hospital research participation [27–32]. However, a recent study demonstrated that community hospitals participating in a randomized control trial had similar consent rates, enrolment rates and protocol adherence to academic hospitals [8]. Moreover, in this study, we observed less missing data in community hospitals compared to academic hospitals. Thus, community hospitals have the ability to participate in clinical trials with similar trial metrics as well as strong potential for study recruitment.
The strengths of this study include the large sample size and the substantial representation of community hospital patients. Additionally, the waived consent model reduced the likelihood of bias in patient recruitment. Limitations included the post-hoc retrospective study design, the relatively small number of community hospitals that participated and that our data represents Ontario, BC and Quebec with very small representation from Alberta and Newfoundland. Although community hospital site participation was low, community hospital patients represented almost half of the patients in the study which suggests that these observed differences may be generalizable among CAP patients in community versus academic hospitals. However, it should be noted that the community hospitals included in this study may not be entirely representative of the characteristics (i.e., size, participation in research) of all Canadian community hospitals. While the focus of the current study was to observe rather than explain differences in patient characteristics, an additional limitation is that data on social determinants of health and race/ethnicity, which may have impacted patient outcomes, were not collected. Furthermore, the definition of “teaching” and “non-teaching” hospital may not be precise, noting that some community hospitals have trainees.
Conclusions
In conclusion, community hospital patients with CAP enrolled in the CAPTIVATE trial differed from academic hospital patients with respect to their baseline and clinical characteristics, treatments and outcomes. After adjusted analyses, in-hospital mortality was the same between community and academic hospital patients however, community hospital patients were older and presented with more comorbidities. These results emphasize the need to increase community hospital participation in studies focused on the causes and treatment of pneumonia. Moreover, these findings call into question the generalizability of clinical research results that are generated from studies conducted exclusively in academic hospitals, highlighting the need to increase community hospital patient representation in clinical research. Increasing community hospital participation in health research has the potential to improve study recruitment, accelerate study completion, and improve the generalizability of study results for more Canadians.
Supplementary Information
Acknowledgements
The authors would like to thank the patients and families who participated in the CAPTIVATE trial as well as the clinical staff at participating sites for their support.
Abbreviations
- CAP
Community Acquired Pneumonia
- ICU
Intensive Care Unit
- CAPTIVATE
Community Acquired Pneumonia: Toward InnoVAtive Treatment
- SMART-COP
Systolic blood pressure, Multilobar infiltrates, Albumin, Respiratory rate, Tachycardia, Confusion, oxygen, and PH
- DAF
Days Alive and Free
- ANOVA
Analysis of Variance
- OR
Odds Ratio
- HR
Hazard Ratio
- CI
Confidence Interval
- AIDS/HIV
Acquired Immune Deficiency Syndrome and Human Immunodeficiency Virus
- ARDS
Acute Respiratory Distress Syndrome
- AKI
Acute Kidney Injury
- ACI
Acute Cardiac Injury
Authors’ contributions
J.A.R. and J.L.Y.T. contributed to the conception and design of the study. J.A.R., J.L.Y.T., K.R., A.B. and T.L. drafted the manuscript and interpreted the data. J.A.R. and T.L. acquired the data and led data analysis. All authors revised the manuscript critically for important intellectual context, gave final approval of the version to be published, and agreed to be accountable for all aspects of this work.
Funding
Support for CAPTIVATE was obtained from grants to Dr. James A. Russell from the Canadian Institutes of Health Research (grant number: 439993) and St. Paul’s Hospital Foundation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval was received from the University of British Columbia Providence Health Care Research Ethics Board (REB Number: H20-00600) and by each of the participating sites.
Consent for publication
Not applicable.
Competing interests
Support for CAPTIVATE was obtained from grants to J.A.R. from the Canadian Institutes of Health Research (grant number: 439993) and St. Paul’s Hospital Foundation. J.B. is a recipient of a Providence Health Care Research Scholarship. K.W. is supported by Canadian Institutes of Health Research (CIHR) Foundation Grant (FDN 154311). A.F.T. is the chairholder of the Canada Research Chair in Critical care neurology and trauma. D.C.V. is supported by the Fonds de recherche du Québec – Santé (FRQS) clinician-scientist Senior scholar award. D.C.V. has received funding support from the Jeffrey Modell Foundation, FRQS, and Canadian Institutes of Health Research. D.C.V. has served on advisory boards for: Astra Zeneca; CSL Behring; Novartis Canada; Moderna; Takeda. D.C.V. has received speaker honoraria from: CSL Behring; Merck Canada. DCV has a patent application pending (Electronic Filing System ID: 40101099) unrelated to this work. M.C. reports grants from the Canadian Institutes of Health Research during the conduct of the study and is supported by the Fonds de Recherche du Québec – Santé. M.C. reports personal fees from GEn1E Lifesciences and from nomic bio as a member of the scientific advisory board, as well as honoraria from AstraZeneca, Takeda, Merck, and Pfizer. M.C. reports research support from Cidara therapeutics, from Scynexis, Inc., and from Amplyx Pharmaceutics during the conduct of the study but outside the submitted work. M.C. is the co-founder of Kanvas Biosciences, Inc. and owns equity in the company. M.C. has pending patents, including: i) Methods for detecting tissue damage, graft versus host disease, and infections using cell-free DNA profiling, ii) Methods for assessing the severity and progression of SARS-CoV-2 infections using cell-free DNA pending.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
James A. Russell, Email: Jim.Russell@hli.ubc.ca
For CAPTIVATE Investigators:
Jennifer L.Y. Tsang, Terry Lee, Anne Mccarthy, Juthaporn Cowan, Patrick Archambault, Francois Lellouche, Alexis F. Turgeon, Jennifer Yoon, Francois Lamontagne, Allison Mcgeer, Josh Douglas, Peter Daley, Robert Fowler, David M. Maslove, Brent W. Winston, Todd C. Lee, Karen C. Tran, Matthew P. Cheng, Donald C. Vinh, John H. Boyd, Keith R. Walley, Joel Singer, John C. Marshall, Gregory Haljan, Fagun Jain, and James A. Russell
References
- 1.Canadian Institute for Health Information. Hospital beds staffed and in operation 2020–2021. 2022. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjUsJC03qKBAxUTAjQIHQ-iCWIQFnoECB0QAQ&url=https%3A%2F%2Fwww.cihi.ca%2Fsites%2Fdefault%2Ffiles%2Fdocument%2Fbeds-staffed-and-in-operation-2020-2021-en.xlsx&usg=AOvVaw1MA_XDmKP5eZLf_jSkRX6V&opi=89978449.
- 2.DiDiodato G, DiDiodato JA, McKee AS. The research activities of Ontario’s large community acute care hospitals: a scoping review. BMC Health Serv Res. 2017;17(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CP, Senft Everson N, Shelburne N, Norton WE. Demographic and health behavior factors associated with clinical trial invitation and participation in the United States. JAMA Netw Open. 2021;4(9):e2127792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmen. Visible minorities now the majority in 5 B.C. cities. CBCNews; 2017. Available from: https://www.cbc.ca/news/canada/british-columbia/visible-minorities-now-the-majority-in-5-b-c-cities-1.4375858.
- 6.Fleet RMD, Audette LDMD, Marcoux J, Villa J, Archambault PMD, Poitras JMD. Comparison of access to services in rural emergency departments in Quebec and British Columbia. CJEM. 2014;16(6):437–48. [DOI] [PubMed] [Google Scholar]
- 7.Fleet R, Pelletier C, Marcoux J, Maltais-Giguère J, Archambault P, Audette LD, et al. Differences in access to services in rural emergency departments of Quebec and Ontario. PLoS One. 2015;10(4): e0123746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsang JLY, Binnie A, Duan EH, Johnstone J, Heels-Ansdell D, Reeve B, et al. Academic and community ICUs participating in a critical care randomized trial: a comparison of patient characteristics and trial metrics. Crit Care Explor. 2022;4(11):e0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapral MK, Hall R, Gozdyra P, Yu AYX, Jin AY, Martin C, et al. Geographic access to stroke care services in rural communities in Ontario, Canada. Can J Neurol Sci. 2020;47(3):301–8. [DOI] [PubMed] [Google Scholar]
- 10.Walker MJ, Wang J, Mazuryk J, Skinner SM, Meggetto O, Ashu E, et al. Delivery of cancer care in Ontario, Canada, during the first year of the COVID-19 pandemic. JAMA Netw Open. 2022;5(4):e228855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.File TM, Marrie TJ. Burden of community-acquired pneumonia in north American adults. Postgrad Med. 2010;122(2):130–41. [DOI] [PubMed] [Google Scholar]
- 12.Grajales Beltrán AG, Lytle D, Vojicic J, Grover P, Latifovic L, Golden S, et al. Burden of acute-care hospitalization for community-acquired pneumonia in Canadian adults aged 50 years or older: focusing on most responsible diagnosis tells only part of the story. Vaccines. 2023;11(4):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T, Walley KR, Boyd JH, Cawcutt KA, Kalil A, Russell JA. Impact of the COVID-19 pandemic on non-COVID-19 community-acquired pneumonia: a retrospective cohort study. BMJ Open Resp Res. 2023;10(1):e001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T, Cheng MP, Vinh DC, Lee TC, Tran KC, Winston BW, et al. Outcomes and characteristics of patients hospitalized for COVID-19 in British Columbia, Ontario and Quebec during the Omicron wave. Cmajo. 2023;11(4):E672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocheleau GLY, Lee T, Mohammed Y, Goodlett D, Burns K, Cheng MP, et al. Renin-angiotensin system pathway therapeutics associated with improved outcomes in males hospitalized with COVID-19. Crit Care Med. 2022;50(9):1306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best JR, Wang M, Lee T, Russell JA, DeMarco ML, ARBs CORONA I Investigators. Early increases in anti-SARS-CoV-2 antibody isotypes associated with organ dysfunction and mortality in patients hospitalized with COVID-19. Intensive Care Med. 2022;48(5):616–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memon RA, Rashid MA, Avva S, Anirudh Chunchu V, Ijaz H, Ahmad Ganaie Z, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022. Available from: https://www.cureus.com/articles/105033-the-use-of-the-smart-cop-score-in-predicting-severity-outcomes-among-patients-with-community-acquired-pneumonia-a-meta-analysis. Cited 2024 Jan 4. [DOI] [PMC free article] [PubMed]
- 18.Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87. [DOI] [PubMed] [Google Scholar]
- 19.Russell JA, Lee T, Singer J, De Backer D, Annane D. Days alive and free as an alternative to a mortality outcome in pivotal vasopressor and septic shock trials. J Crit Care. 2018;47:333–7. [DOI] [PubMed] [Google Scholar]
- 20.Peng L, Huang Y. Survival analysis with quantile regression models. J Am Stat Assoc. 2008;103(482):637–49. [Google Scholar]
- 21.Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol. 2011;11(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke LG, Frakt AB, Khullar D, Orav EJ, Jha AK. Association between teaching status and mortality in US hospitals. JAMA. 2017;317(20):2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czarnecki A, Qiu F, Koh M, Cheskes S, Dorian P, Scales DC, et al. Association between hospital teaching status and outcomes after out-of-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2019;12(12):e005349. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy DA, Soo A, Niven DJ, Jolley RJ, Posadas-Calleja J, Stelfox HT, et al. Socio-demographic characteristics associated with hospitalization for sepsis among adults in Canada: a census-linked cohort study. Can J Anesth/J Can Anesth. 2020;67(4):408–20. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram ME, Calzavara A, Mishra S, Kustra R, Chan AK, Hamilton MA, et al. Individual and social determinants of SARS-CoV-2 testing and positivity in Ontario, Canada: a population-wide study. CMAJ. 2021;193(20):E723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Ma H, Moloney G, Velásquez García HA, Sirski M, Janjua NZ, et al. Geographic concentration of SARS-CoV-2 cases by social determinants of health in metropolitan areas in Canada: a cross-sectional study. CMAJ. 2022;194(6):E195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gehrke P, Binnie A, Chan SPT, Cook DJ, Burns KEA, Rewa OG, et al. Fostering community hospital research. CMAJ. 2019;191(35):E962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamontagne F, Rowan KM, Guyatt G. Integrating research into clinical practice: challenges and solutions for Canada. CMAJ. 2021;193(4):E127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snihur A, Mullin A, Haller A, Wiley R, Clifford P, Roposa K, et al. Fostering clinical research in the community hospital: opportunities and best practices. Healthc Q. 2020;23(2):30–6. [DOI] [PubMed] [Google Scholar]
- 30.Tsang JLY, Fowler R, Cook DJ, Ma H, Binnie A. How can we increase participation in pandemic research in Canada? Can J Anaesth. 2021;69(3):293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang JLY, Fowler R, Cook DJ, Burns KEA, Hunter K, Forcina V, et al. Motivating factors, barriers and facilitators of participation in COVID-19 clinical research: a cross-sectional survey of Canadian community intensive care units. PLoS One. 2022;17(4):e0266770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Dolovich L, Holbrook A, Jack SM. Factors that influence community hospital involvement in clinical trials: a qualitative descriptive study. Evaluation Clin Pract. 2022;28(1):79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.