Abstract
Background
Parkinson’s disease (PD) induces progressive deficits in motor and cognitive functions as well as impaired dual-task performance requiring both motor and cognitive functions. This systematic review and meta-analysis evaluated the effects of non-invasive brain stimulation (NIBS) on dual-task performance in patients with PD.
Methods
11 studies met the following inclusion criteria: (a) patients with PD, (b) NIBS intervention, (c) comparison with the sham stimulation group, (d) motor and cognitive performance outcomes during dual tasks, and (e) randomized controlled trials with parallel or crossover designs. Individual effect size (i.e., comparison) was quantified by comparing motor and cognitive performances changes during dual tasks between active NIBS and sham stimulation conditions. Thus, higher values of the overall effect size indicate more improvements in either motor or cognitive performances after NIBS. Moreover, moderator variable analyses determined whether NIBS effects on dual-task performances differed depending on targeted brain regions. Finally, meta-regression analyses determined whether NIBS effects on dual-task performances were associated with demographic characteristics.
Results
The random-effects model meta-analysis revealed that NIBS significantly improved motor (73 comparisons from 11 studies) and cognitive (12 comparisons from four studies) performances during dual tasks in patients with PD. Specifically, anodal transcranial direct current stimulation protocols on the dorsolateral prefrontal cortex were effective. Moreover, greater improvements in motor performance during dual tasks significantly correlated with decreased age and increased proportion of females, respectively.
Conclusion
This meta-analysis suggests that excitatory stimulation on the dorsolateral prefrontal cortex may be effective for improving dual-task performance in patients with PD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01505-8.
Keywords: Parkinson’s disease, Dual task, Non-invasive brain stimulation, Transcranial direct current stimulation, Dorsolateral prefrontal cortex
Introduction
Parkinson’s disease (PD), the second-most common neurodegenerative disorder, normally induces progressive deficits in motor and cognitive functions, as indicated by slower gait speed and impaired executive functions in patients with PD [1–5]. Moreover, patients with PD presented more impairments when performing dual tasks that simultaneously require motor and cognitive task goals than healthy older adults [6–8]. For example, patients with PD exhibited 18% reduction of step length from single-task walking to dual-task walking, whereas healthy older adults showed only 2% decrease in step length [9]. Given that many activities of daily living frequently require dual tasks such as reading text messages on a smartphone while walking, patients with PD may be challenging for increasing independent life without specific rehabilitation protocols that effectively address dual-task impairments [10, 11].
Non-invasive brain stimulation (NIBS) techniques including transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (rTMS) have been explored as intervention protocols for improving dual-task performance due to their potential effects on modulating cortical excitability and facilitating neuroplasticity [12–15]. According to the central capacity sharing model [16], cognitive resources may be divided when concurrently processing multiple stimuli due to a limited capacity. Thus, dual tasks such as using a smartphone while walking can lead to cognitive-motor interference presumably impairing the performance of either one or both tasks [17]. Importantly, patients with PD typically showed impaired gait automaticity so that they may use more cognitive resources for successful locomotion while increasing cognitive-motor interference patterns [18, 19]. For example, although patients with PD revealed greater excitability in the dorsolateral prefrontal cortex (DLPFC) for executive functions, their gait patterns during dual tasks were slower and more variable than those of age-matched healthy older adults [20]. These findings indicated that patients with PD may need more neural resources in the brain (e.g., DLPFC excitability) to compensate for their motor and executive deficits. Potentially, the primary motor cortex (M1) may be an additional key area to preserve motor functions during dual tasks because M1 excitability may advance the cortico-basal ganglia connections affected by striatal dopamine depletion [21–23]. Thus, NIBS protocols targeting these cortical regions may improve dual-task performance by attenuating cognitive-motor interferences. A systematic review and meta-analysis investigating tDCS effects on dual-task performances in older adults reported that anodal tDCS on the DLPFC significantly reduced dual-task cost of gait speed [24]. Taken together, quantifying potential overall effects of NIBS on dual-task performances in patients with PD may provide meaningful information on identifying optimal rehabilitation protocols contributing to increasing their independent life.
A recent systematic review study performed by Lin and colleagues [25] revealed potential positive effects of NIBS protocols on gait speed and timed up and go (TUG) during dual tasks in patients with PD from three studies [14, 26, 27]. Although this study suggested a possibility of improvements in dual-task performances after NIBS protocols, these findings were still insufficient because of the limited number of included studies and no quantitative evidence by conducting data synthesis procedures. Thus, we investigated effects of NIBS on dual-task performances in patients with PD by conducting a systematic review and meta-analysis. Moreover, NIBS effects can vary with different targeted brain areas [28, 29]. Based on these findings, we addressed two leading questions: (1) Do NIBS techniques improve motor and cognitive performances during dual tasks in patients with PD? and (2) Do the effects of NIBS on motor and cognitive performances in patients with PD differ depending on the targeted brain regions?
Methods
Literature search and study inclusion criteria
We conducted the systematic review and meta-analysis consistent with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [30]. To formulate convincing eligibility criteria [31], we used Population, Intervention, Comparison, Outcomes, and Study design (PICOS) framework. Specifically, five inclusion criteria included: (1) Population: patients with PD; (2) Intervention: NIBS protocols; (3) Comparison: controls who received sham stimulation; (4) Outcome: motor and cognitive performance during dual tasks; and (5) Study design: studies that included randomized controlled trials (RCT) with either a parallel or crossover design. Moreover, we excluded case studies, animal studies, review articles, and studies that reported insufficient data for calculating effect sizes. Using two databases including PubMed and Web of Science, the literature search was performed from July 12, 2023, to August 14, 2023. For both search engines, we used the following keywords: (PD OR Parkinson’s disease OR Parkinson) AND (NIBS OR non-invasive brain stimulation OR tDCS OR transcranial direct current stimulation OR tACS OR transcranial alternating current stimulation OR TMS OR transcranial magnetic stimulation OR rTMS OR repetitive transcranial magnetic stimulation OR tPCS OR transcranial pulsed current stimulation OR tRNS OR transcranial random noise stimulation) AND (dual-task OR dual task OR concurrent OR walk OR gait OR locomotion OR cognition OR interference).
Data synthesis for meta-analysis
Meta-analysis procedures were conducted using the Comprehensive Meta-Analysis software version 4.0 (Englewood, NJ, USA). Individual effect size (i.e., comparison) and overall effect size (i.e., effect size after data synthesis) were quantified by calculating standardized mean difference (SMD) with a 95% confidence interval (CI). We included multiple comparisons from one study when each comparison could be calculated based on different types of dual tasks, outcome variables, and NIBS protocols (e.g., targeted regions and timing). Higher SMD values indicate more improvements in dual-task performances after applying active NIBS protocols than those for sham stimulation condition. For RCT with a parallel design, individual effect sizes were calculated by comparing mean and standard deviation values of motor and cognitive performances between active and sham stimulation groups. For RCT with a crossover design, we calculated individual effect sizes using a paired analysis that applied the sample size and mean difference values with standard error [32–34]. To synthesize individual effect sizes, we used the random-effects model meta-analysis based on the traditional assumptions that inherent heterogeneity may exist among individual studies because of different experimental characteristics (e.g., participants, study protocols, and outcome measures). This approach may minimize the potential variability of effect sizes by reducing the influence of these methodological differences across individual studies [35].
To estimate the heterogeneity levels across individual effect sizes, we used Higgins and Green’s I-squared (I2) indicating relationship between the distribution of true effects and observed effects [36]. Typically, the 25%, 50%, and 75% values of I2 denote low, moderate, and high heterogeneity levels, respectively [37]. Moreover, the Egger’s regression test was performed to determine whether significant levels of publication bias exist across individual effect sizes. A P-value for the intercept (β0) of less than 0.05 indicates significant levels of publication bias [38].
Moderator variable analysis
Additional moderator variable analyses were performed to specify effects of NIBS protocols on motor and cognitive performances during dual tasks in patients with PD. First, we investigated how NIBS influenced dual-task performances based on different types of motor functions (i.e., gait speed, cadence, double support time, stride time, stride length, step length, step width, stride time variability, stride length variability, TUG, time to return, and writing amplitude) and cognitive functions (i.e., number of correct generating words and counting). Second moderator analysis specified NIBS effects on dual-task performances according to different targeted brain areas (i.e., DLPFC, supplementary motor area; SMA, M1, and cerebellum). Finally, we conducted additional meta-regression analyses to determine whether the effects of NIBS on motor and cognitive performances were associated with different demographic characteristics (i.e., mean age, proportion of the females, and duration since PD diagnosis).
Methodological quality assessment
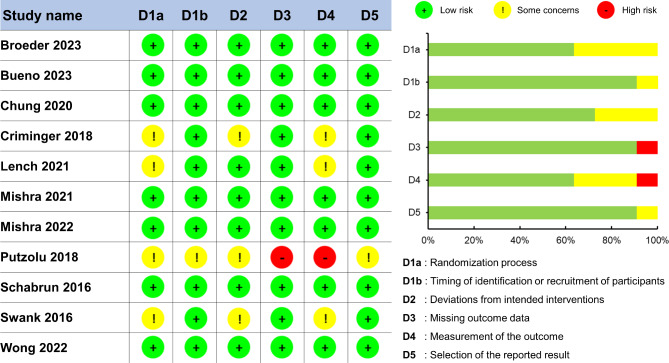
Using the Cochrane risk of bias assessment tool version 2 [39], two researchers (H.L. and B.J.C.) independently evaluated potential methodological issues based on six specific domains: (1) randomization process (2), timing of identification or recruitment of participants (3), deviations from intended intervention (4), missing outcome data (5), measurement of the outcome, and (6) selection of the reported result [40]. Based on the criteria for algorithms in the Cochrane risk of bias assessment tool [41], we judged the risk of bias for each domain by assigning one of three levels: (1) low risk of bias (2), some concern, and (3) high risk of bias. In the case of any discrepancy between the researchers, one leading researcher (N.K.) made a final decision.
Results
Study identification procedure
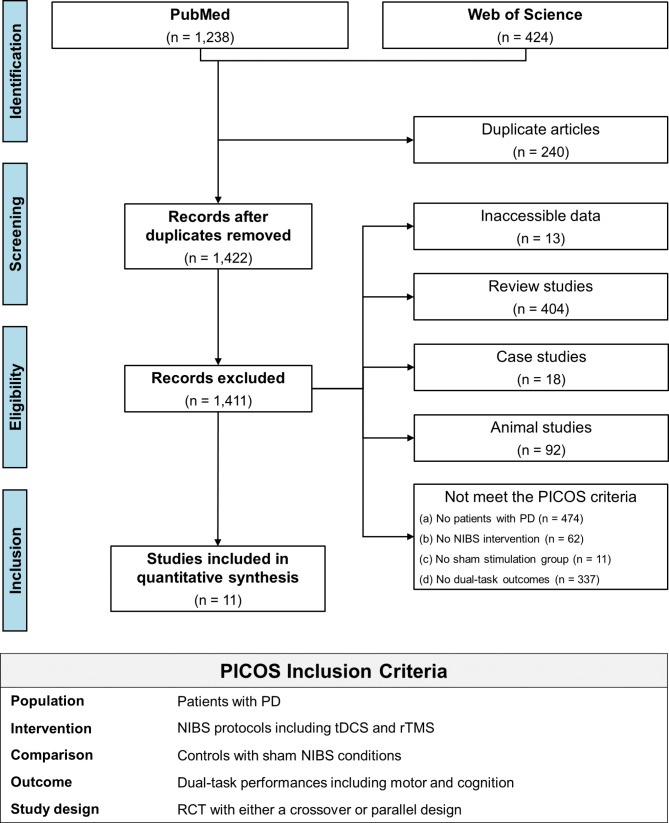
Initially, a systematic literature search identified 1,662 studies including 1,238 from the PubMed and 424 from the Web of Science, and then we removed 240 duplicated studies. The title and abstract of 1,422 studies were firstly screened, and 527 studies were excluded because of the following: (1) 404 review articles (2), 92 animal studies (3), 18 case studies, and (4) 13 studies that reported inaccessible contents. Full texts of the remaining 895 studies were carefully reviewed based on our inclusion and exclusion criteria, and 884 studies were further excluded: (1) 474 studies that did not focus on patients with PD (2), 62 studies that did not use NIBS intervention (3), 11 studies that did not involve sham stimulation group, and (4) 337 studies that did not estimate dual-task performances. Finally, 11 studies qualified for this meta-analysis [14, 15, 26, 27, 42–48]. Our study identification procedures are described in Fig. 1.
Fig. 1.
PRISMA flowchart for the study identification procedure. PD = Parkinson’s disease; NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; rTMS = repetitive transcranial magnetic stimulation; RCT = randomized control trials
Demographic information on patients with PD
From the 11 qualified studies, 284 patients with PD participated in experiments (a range of mean age = 50.1–72.8 years and a range of mean duration after PD diagnosis = 3.5–9.3 years). Motor impairments in patients with PD at baseline was mild to moderate [49–51]: (1) a range of mean Hoehn and Yahr scale (H&Y) = 1.7–2.3 and (2) a range of mean unified Parkinson’s disease rating scale part III (UPDRS-III) = 15.8–47.7. Cognitive function of the participants was relatively normal [52, 53]: (1) a range of mean mini-mental state examination (MMSE) = 25.5–29.7 and (2) Montreal cognitive assessment (MoCA) = 26.1–28.0. The included studies reported that all patients with PD were on medication state. Table 1 shows specific details on demographic information of patients with PD.
Table 1.
Participants characteristics
| Study | Study Design | Total (N) | Age (yrs) | Gender | Disease Duration (yrs) | LEDD (mg) | Motor Function | Cognitive Function | ||
|---|---|---|---|---|---|---|---|---|---|---|
| H&Y | UPDRS-3 | MMSE | MoCA | |||||||
| Broeder 2023 | Parallel | Trt: 20 | 62.9 ± 8.3 | 3 F, 17 M | 3.5 (2.8, 8.0) | 588.4 ± 379.0 | 2.0 (2.0, 3.0) | 23.4 ± 12.1 | NA | 28.0 (25.3, 29.0) |
| Con: 19 | 63.5 ± 8.5 | 5 F, 14 M | 6.0 (4.0, 9.0) | 748.8 ± 381.8 | 2.0 (2.0, 3.0) | 29.1 ± 12.1 | 28.0 (25.0, 29.0) | |||
| Bueno 2023 | Parallel | Trt 1: 12 | 63.9 ± 11.9 | 5 F, 7 M | 5.1 ± 4.1 | 786.6 ± 707.1 | 2.2 ± 0.7 | 36.7 ± 14.7 | 25.5 ± 3.9 | NA |
| Trt 2: 12 | 60.8 ± 10.6 | 3 F, 9 M | 5.2 ± 4.1 | 654.2 ± 350.3 | 2.0 ± 0.3 | 29.5 ± 18.6 | 28.3 ± 2.0 | |||
| Con: 13 | 69.6 ± 6.2 | 5 F, 8 M | 6.5 ± 5.3 | 651.2 ± 460.6 | 1.9 ± 0.3 | 28.5 ± 15.7 | 27.2 ± 2.5 | |||
| Chung 2020 | Parallel | Trt 1: 17 | 62.1 ± 5.7 | 8 F, 9 M | 7.5 ± 4.9 | 512.5 ± 359.9 | 2.2 ± 0.4 | 27.1 ± 9.6 | NA | NA |
| Trt 2: 17 | 62.7 ± 6.8 | 7 F, 10 M | 5.2 ± 3.4 | 484.2 ± 336.4 | 2.2 ± 0.3 | 27.9 ± 10.5 | ||||
| Con: 16 | 62.1 ± 5.7 | 9 F, 7 M | 6.9 ± 3.3 | 493.3 ± 523.9 | 2.3 ± 0.3 | 29.7 ± 10.6 | ||||
| Criminger 2018 | Crossover | Total: 16 | 68.1 ± 9.8 | 4 F, 12 M | 8.7 ± 9.8 | ‘ON’ state | NA | 23.4 ± 9.7 | NA | NA |
| Lench 2021 | Parallel | Trt: 12 | 66.6 ± 7.5 | 5 F, 7 M | 8.7 ± 7.1 | 1074.4 ± 493.9 | 2.3 ± 0.4 | 16.8 ± 4.1 | 29.1 ± 1.2 | NA |
| Con: 8 | 64.5 ± 8.9 | 1 F, 7 M | 8.0 ± 5.6 | 1304.4 ± 757.3 | 2.3 ± 0.3 | 15.8 ± 6.0 | 28.4 ± 1.7 | |||
| Mishra 2021 | Crossover | Total: 20 | 67.8 ± 8.3 | 6 F, 14 M | 4.8 ± 3.8 | ‘ON’ state | 1.9 ± 0.9 | NA | NA | 26.1 ± 2.2 |
| Mishra 2022 | Crossover | Total: 20 | 67.8 ± 8.3 | 6 F, 14 M | 4.8 ± 3.8 | ‘ON’ state | 1.9 ± 0.9 | NA | NA | 26.1 ± 2.2 |
| Putzolu 2018 | Crossover | FOG: 10 | 70.1 ± 3.8 | 4 F, 6 M | 9.3 ± 5.5 | ‘ON’ state | NA | 20.1 ± 8.4 | 29.0 ± 1.9 | NA |
| Non-FOG: 10 | 72.8 ± 6.9 | 5 F, 5 M | 7.2 ± 5.2 | NA | 22.9 ± 8.1 | 29.1 ± 0.9 | ||||
| Schabrun 2016 | Parallel | Trt: 8 | 72.0 ± 4.9 | 0 F, 8 M | 6.9 ± 4.4 | 730.0 ± 341.0 | NA | 47.7 ± 7.5 | 29.0 ± 0.8 | NA |
| Con: 8 | 63.0 ± 11.0 | 2 F, 6 M | 4.6 ± 3.9 | 523.0 ± 398.0 | NA | 37.7 ± 9.8 | 29.7 ± 0.5 | |||
| Swank 2016 | Crossover | Total: 10 | 68.7 ± 10.2 | 2 F, 8 M | 7.9 ± 7.1 | ‘ON’ state | NA | 37.0 ± 12.9 | NA | NA |
| Wong 2022 | Parallel | Trt 1: 9 | 54.2 ± 4.1 | 1 F, 8 M | 7.8 ± 5.7 | 592.1 ± 208.2 | 1.9 ± 0.6 | 33.2 ± 13.1 | 28.1 ± 1.8 | NA |
| Trt 2: 9 | 50.1 ± 2.4 | 3 F, 6 M | 6.2 ± 3.3 | 603.9 ± 357.3 | 1.7 ± 0.5 | 25.6 ± 17.0 | 28.9 ± 1.8 | |||
| Trt 3: 9 | 61.3 ± 7.9 | 7 F, 2 M | 4.1 ± 3.3 | 468.2 ± 212.1 | 2.1 ± 0.6 | 24.2 ± 9.9 | 27.3 ± 2.2 | |||
| Con: 9 | 58.3 ± 8.0 | 6 F, 3 M | 8.3 ± 12.3 | 426.1 ± 243.7 | 1.8 ± 0.7 | 23.4 ± 14.7 | 28.9 ± 2 | |||
Data is mean ± standard deviations or median (1st quartile, 3rd quartile)
Abbreviation. LEDD = levodopa equivalent daily dose; H&Y = Hoehn and Yahr scale; UPDRS-3 = unified Parkinson’s disease rating scale; MMSE = mini-mental state examination; MoCA = Montreal cognitive assessment; Trt = treatment; Con = control; FOG = freezing of gait; F = female; M = male
NIBS protocols for dual tasks
For 11 qualified studies, nine studies applied tDCS and two studies used rTMS. For specific brain region of NIBS stimulation, tDCS protocols targeted: (1) DLPFC from five studies (2), M1 from three studies, and (3) three different targeted regions (i.e., DLPFC, M1, and cerebellum) from one study. Two studies that used rTMS protocols stimulated M1 and SMA, respectively. For the stimulation protocol type, nine tDCS studies applied anodal stimulation. For rTMS protocols, one study applied inhibitory stimulation (≤ 1 Hz), whereas another study used both inhibitory and excitatory stimulation (> 5 Hz). For the number of sessions, eight tDCS studies applied a single session and one study administered multiple sessions. Two rTMS studies applied multiple stimulation sessions. For the timing of NIBS protocols, seven tDCS studies used off-stimulation (i.e., tDCS before dual tasks) and two studies administered on-stimulation (i.e., tDCS during dual tasks) and off-stimulation, respectively. Two rTMS studies applied off-stimulation. Specifically, six out of the studies that used off-stimulation provided additional training while administering NIBS protocols: (1) writing figure-8 (2), physical therapy from one study (3), treadmill walking from one study (4), stationary bicycle and golf video game from one study, and (5) dual-task walking from two studies. In Table 2, we describe the specific parameters of NIBS techniques.
Table 2.
Specific parameters of NIBS protocols
| Study | Group | NIBS | Stimulation Site | Intensity | Session | Timing | Duration | Surface | Additional Training |
|---|---|---|---|---|---|---|---|---|---|
| Broeder 2023 | Trt | tDCS | M1 | 1 mA | 1 | Off | 20 min | 35 cm2 | Writing figure-8 |
| Bueno 2023 | Trt 1 | tDCS | M1Cz | 2 mA | 1 | Off | 20 min | NA | Physical therapy |
| Trt 2 | M1C3 − Cz−C4 | ||||||||
| Chung 2020 | Trt 1 | rTMS | M1 | 1 Hz, 80% of RMT | 12 | Off | 600 pulses | NA | Treadmill walking |
| Trt 2 | 25 Hz, 80% of RMT | ||||||||
| Criminger 2018 | Trt 1 | tDCS | DLPFC | 2 mA | 1 | Off | 20 min | 15 cm2 | Resting |
| Trt 2 | Stationary bicycle | ||||||||
| Trt 3 | Golf video game | ||||||||
| Lench 2021 | Trt | rTMS | SMA | 1 Hz, 110% of RMT | 10 | Off | 1200 pulses | NA | Dual-task walking |
| Mishra 2021 | Trt | tDCS | DLPFC | 2 mA | 1 | On | 30 min | 35 cm2 | Resting |
| Off | |||||||||
| Mishra 2022 | Trt | tDCS | DLPFC | 2 mA | 1 | On | 30 min | 35 cm2 | Resting |
| Off | |||||||||
| Putzolu 2018 | Trt | tDCS | DLPFC | 1.5 mA | 1 | Off | 20 min | 25 cm2 | Resting |
| Schabrun 2016 | Trt | tDCS | M1 | 2 mA | 9 | Off | 20 min | 35 cm2 | Dual-task walking |
| Swank 2016 | Trt | tDCS | DLPFC | 2 mA | 1 | Off | 20 min | NA | Resting |
| Wong 2022 | Trt 1 | tDCS | DLPFC | 2 mA | 1 | Off | 20 min | 35 cm2 | Resting |
| Trt 2 | M1 | ||||||||
| Trt 3 | Cerebellum |
Abbreviation. NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; rTMS = repetitive transcranial magnetic stimulation; Trt = treatment; M1 = primary motor cortex; DLPFC = dorsolateral prefrontal cortex; SMA = supplementary motor area; RMT = resting motor threshold; min = minute
Motor and cognitive performances during dual tasks
Nine out of 11 included studies tested following motor performance during dual tasks: (1) gait (e.g., speed and cadence) from four studies (2), TUG from four studies, and (3) time to turn from one study. The remaining one study assessed both gait and TUG, and the other study evaluated writing amplitude. Moreover, three out of four studies estimated following cognitive performance during dual tasks: (1) the number of correct generating words from two studies and (2) the number of correct counting from one study. The remaining one study assessed both the number of correct generating words and counting. Table 3 shows the specific motor and cognitive performances during dual tasks.
Table 3.
Specific motor and cognitive performances during dual tasks
| Study | Motor Performance | Cognitive Performance | Dual Task |
|---|---|---|---|
| Broeder 2023 | DTC-writing amplitude | NA | Writing figure-8 patterns while counting low and high tones |
| Bueno 2023 | Speed, Cadence, Step length, Step width | NA | Walking while counting backwards by three |
| Chung 2020 | TUG | NA | TUG test while counting backwards by three |
| Criminger 2018 | TUG | NA |
TUG test while carrying a full cup of water TUG test while counting backwards by three |
| Lench 2021 | Time to turn | NA | TUG test while counting backwards by seven |
| Mishra 2021 | Speed, DTC-speed | Number of correct generated words, DTC-number of correct generated words | Walking while generating words starting from a given alphabet |
| Mishra 2022 | TUG, DTC-TUG | Number of correct generated words | TUG test while generating words starting from a given alphabet |
| Putzolu 2018 | DTC-speed, DTC-step length, DTC-double support time, DTC-stance time | NA | Walking while counting backwards by seven |
| Schabrun 2016 | Speed, Cadence, Stride length, Double support time, TUG | Number of correct generated words, Number of correct backward counts |
Walking while counting backwards by three Walking while generating words starting from a particular letter Walking while conversation TUG test while counting backwards by three TUG test while generating words starting from a particular letter |
| Swank 2016 | TUG, DTC-TUG | Number of correct backward counts, DTC-number of correct backward counts |
TUG test while carrying a full cup of water TUG test while counting backwards by three |
| Wong 2022 | Speed, Cadence, Stride length, Stride time, Stride length variability, Stride time variability, DTC-speed | NA | Walking while counting backwards by three |
Abbreviation. TUG = timed up and go; DTC = dual-task cost
Methodological quality assessment results
The Cochrane risk of bias assessment for the 11 qualified studies showed a relatively low risk of methodological biases across three domains: (1) timing of identification or recruitment of participants (2), missing outcome data, and (3) selection of the reported result. However, a relatively moderate risk of methodological biases was confirmed in following three domains: (1) randomization process (2), deviation from intended interventions, and (3) measurements of the outcome. Specifically, four studies failed to mention a specific randomization process and did not report information about the blinding of people who implemented the interventions or assessed performances. The methodological quality assessment for the included studies are described in Fig. 2.
Fig. 2.
Methodological quality assessment using Cochrane risk-of-bias tool
Meta-analytic findings
NIBS effects on motor performances during dual tasks in PD
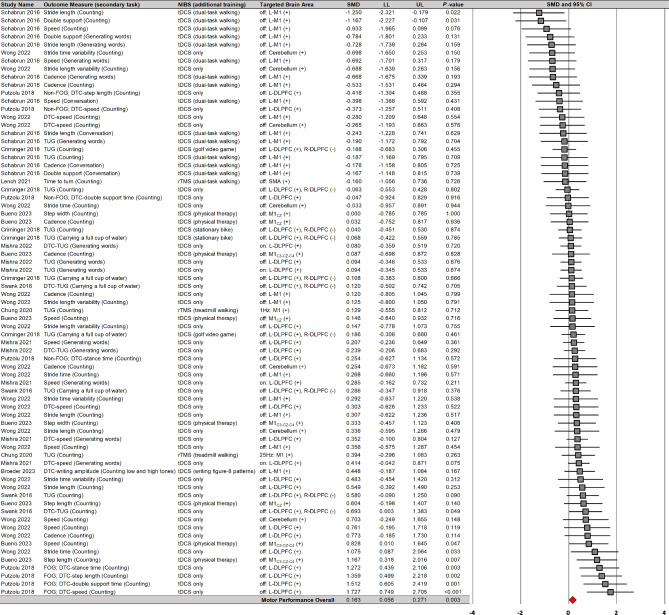
A random-effects model meta-analysis confirmed that NIBS significantly improved motor performances during dual tasks on 73 comparisons from 11 studies (Fig. 3): SMD = 0.163; SE = 0.055; 95% CI = 0.056–0.271; Z = 2.975; P = 0.003. Heterogeneity tests indicated that overall variability of individual effect sizes was relatively moderate (I2 = 33.7%). Publication bias assessment indicated relatively symmetrical distribution of individual effect size (Egger’s β0 = − 0.402 with P = 0.395). These findings indicated that NIBS protocols may slightly improve motor performances during dual tasks with small heterogeneity.
Fig. 3.
NIBS effects on motor performances during dual tasks. NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; rTMS = repetitive transcranial magnetic stimulation; DLPFC = dorsolateral prefrontal cortex; M1 = primary motor cortex; SMA = supplementary motor area; L = left; R = right; DTC = dual-task cost; FOG = freezing of gait; TUG = timed up and go
Moreover, a moderator variables analysis showed that NIBS on the DLPFC significantly improved motor performances during dual tasks on 33 comparisons from six studies (Fig. 4): SMD = 0.298; SE = 0.069; 95% CI = 0.163–0.433; Z = 4.317; P < 0.001; I2 = 35.5%; Egger’s β0 = 2.048 with P = 0.003. Importantly, the six studies that reported overall positive effects used tDCS protocols. However, NIBS on the M1 failed to report significant effects on motor performances during dual tasks on 32 comparisons from five studies (Additional file 1): SMD = − 0.014; SE = 0.094; 95% CI = − 0.199–0.171; Z = − 0.145; P = 0.885; I2 = 27.8%; Egger’s β0 = − 5.525 with P < 0.001. These findings suggested that tDCS protocols targeted the DLPFC may produce slight enhancements in motor performances during dual tasks with small heterogeneity.
Fig. 4.
NIBS effects on motor performances during dual tasks based on stimulation site. NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; DLPFC = dorsolateral prefrontal cortex; L = left; R = right; DTC = dual-task cost; FOG = freezing of gait; TUG = timed up and go
NIBS effects on cognitive performances during dual tasks in PD
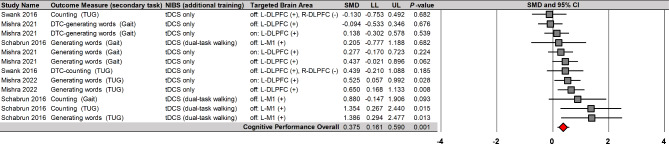
A random-effects model meta-analysis found that NIBS significantly enhanced cognitive performances during dual tasks on 12 comparisons from four studies (Fig. 5): SMD = 0.375; SE = 0.110; 95% CI = 0.161–0.590; Z = 3.427; P = 0.001. Importantly, the four studies that reported overall positive effects used tDCS protocols. Heterogeneity tests showed that overall variability of individual effect sizes was relatively moderate (I2 = 36.3%). Publication bias assessment showed a no significant publication bias across individual effect sizes (Egger’s β0 = 2.047 with P = 0.070). These findings implied that tDCS protocols may moderately improve cognitive performances during dual tasks with small heterogeneity.
Fig. 5.
NIBS effects on cognitive performances during dual tasks. NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; DLPFC = dorsolateral prefrontal cortex; M1 = primary motor cortex; L = left; R = right; DTC = dual-task cost; TUG = timed up and go
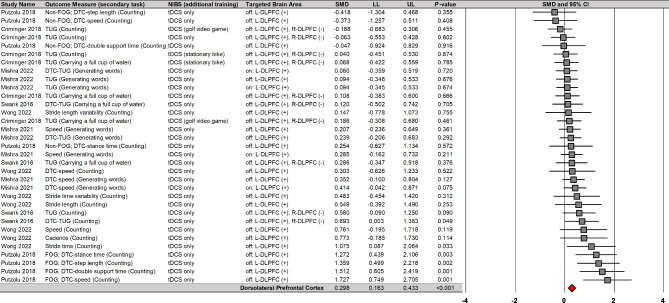
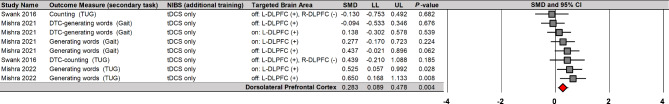
Moreover, moderator variables analysis reported that NIBS on the DLPFC significantly improved cognitive performances during dual tasks on eight comparisons from three studies (Fig. 6): SMD = 0.283; SE = 0.099; 95% CI = 0.089–0.478; Z = 2.860; P = 0.004; I2 = 20.9%; Egger’s β0 = − 0.061 with P = 0.985. Importantly, the three studies that reported overall positive effects used tDCS protocols. These findings showed tDCS protocols on the DLPFC may lead to small enhancements in cognitive performances during dual tasks with minimal heterogeneity.
Fig. 6.
NIBS effects on cognitive performances during dual tasks based on stimulation site
NIBS = non-invasive brain stimulation; tDCS = transcranial direct current stimulation; DLPFC = dorsolateral prefrontal cortex; L = left; R = right; DTC = dual-task cost; TUG = timed up and go
Meta-regression analyses
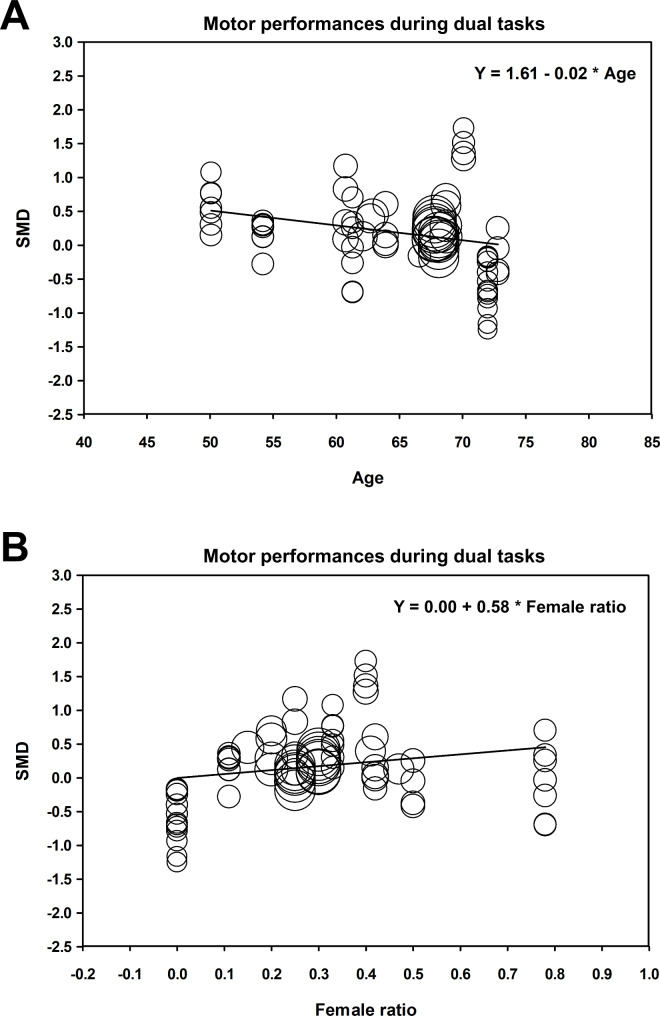
The random-effects meta-regression analyses revealed that greater improvements in motor performances during dual tasks after NIBS were significantly associated with decreased age (Y = 1.61 − 0.02X; P = 0.004; Fig. 7A) and increased proportion of females in total patients with PD (Y = 0.00 + 0.58X; P = 0.026; Fig. 7B), respectively. However, the amount of duration since PD diagnosis was not significantly associated with improvements in motor performances during dual tasks after NIBS (Y = 0.10 + 0.01X; P = 0.727). The meta-regression analyses found no significant relationships between improvements in cognitive performances during dual tasks after NIBS and following demographic characteristics: (1) age (Y = − 9.13 + 0.14X; P = 0.052) (2), proportion of females in total patients with PD (Y = 0.80 − 1.77X; P = 0.075), and (3) duration since PD diagnosis (Y = 0.17 + 0.04X; P = 0.686).
Fig. 7.
NIBS effects on motor performances during dual tasks versus age (A) and female ratio (B). SMD = standardized mean difference
Discussion
This meta-analysis investigated effects of NIBS techniques including tDCS and rTMS on motor and cognitive performances during dual tasks in patients with PD. The findings revealed that NIBS protocols significantly enhanced motor and cognitive performances during dual tasks. Specifically, these improvements were observed when tDCS stimulated the DLPFC regions. Moreover, the meta-regression analyses revealed that greater improvements in motor performances during dual tasks after NIBS protocols were significantly associated with decreased age and increased proportion of females in patients with PD, respectively.
The meta-analytic findings demonstrated that tDCS protocols increasing DLPFC excitability improved motor performances during dual tasks in patients with PD. Successful walking in well-functioning people is typically based on the automaticity allowing the motor system to quickly and efficiently coordinate movements with minimal cognitive involvement [54]. However, individuals with impaired automaticity such as older adults and patients with stroke may require more cognitive resources (e.g., executive control involving conscious processing of information for organizing, managing, and controlling movements) for executing gait performances [55, 56]. Several studies reported impaired automaticity during gait performances in patients with PD due to dopamine depletion in the posterior putamen, a sensorimotor region of the striatum that potentially acquires, stores, and facilitates automated motor skills [18, 57–59]. However, patients with PD who exhibited greater DLPFC excitability showed no significant impairments in normal gait performances as compared with those for age-matched controls [20]. Given that the DLPFC is important for executive functions [60], these findings indicated that patients with PD may be dependent on executive control processing during normal locomotion to compensate for their impaired automaticity [61–63]. Importantly, dual tasks such as walking with subtraction or generating words normally increase cognitive workload [64]. Perhaps, dual tasks for patients with PD may attenuate executive resources being applied to compensate for impaired automaticity consequently interfering with gait performances [20]. A recent meta-analysis demonstrated that anodal tDCS on the DLPFC reduced dual-task cost on gait speed in older adults, suggesting that increasing DLPFC excitability may improve executive control associated with gait [24]. Taken together, brain stimulation for facilitating DLPFC excitability may contribute to locomotion improvements during dual tasks in patients with PD by maintaining neural resource levels for executive control processing on gait.
In addition to improved motor performance, applying tDCS protocols targeting the DLPFC facilitated cognitive improvements during dual tasks in patients with PD. These findings indicate that increasing DLPFC excitability may enhance dual-task performance including both motor and cognitive tasks. Beneficial effects of NIBS protocols that targeted the DLPFC on dual-task performances appeared in healthy young adults and patients with stroke [65, 66]. DLPFC regions are typically associated with executive functions including shifting and inhibitory control [67, 68]. Furthermore, executive functions may be related to the ability to successfully perform dual tasks [69]. For example, better shifting may decrease task-switch costs and inhibitory control may suppress inappropriate responses consequently contributing to efficient allocation of attention during dual tasks [70]. Importantly, patients with PD often showed impaired executive functions because deficits in the striatal dopamine interfere with normal transmission of information through the frontostriatal circuits leading to dorsolateral frontal-lobe dysfunction [71, 72]. Thus, excitatory stimulation on the DLPFC may improve executive functions of patients with PD by increasing dopamine levels [73–77] via the meso-cortico-limbic pathway and local effects on the nigrostriatal pathway [78–80]. In fact, patients with PD revealed lower functional connectivity patterns across cortico-subcortical areas including DLPFC, caudate, and motor networks [81, 82], whereas NIBS protocols upregulating DLPFC patterns increased functional connectivity across these areas [83, 84]. Taken together, given that patients with PD typically revealed both gait impairments as well as mild cognitive deficits [85, 86], applying NIBS for increasing DLPFC excitability may facilitate improvements in dual-task performances contributing to independent daily living.
Meta-regression analyses revealed that effects of NIBS on motor performances during dual tasks increased with lower age and greater proportion of females for patients with PD. Previous pharmacological studies argued that neuroplasticity facilitated by NIBS protocols may be related to the glutamatergic system [87–91]. Glutamate is the primary excitatory neurotransmitter allowing influx of Ca2+ associated with neuroplasticity via increased sensitivity of the synapse [92–94]. Given that aging normally decreases glutamatergic receptors and glutamate concentration in brain areas such as the frontal, parietal, and temporal cortical regions [95, 96], positive effects of NIBS may be greater for younger patients with PD because of better brain neuroplasticity [97–100]. Moreover, estrogen may protect dopaminergic neurons that presumably induce relatively lower symptom severity in women with PD than men [101–104]. Given that patients with PD who had lower symptom severity showed more neuroplasticity patterns facilitated by brain stimulation [105], women with PD may receive greater therapeutic effects of NIBS protocols due to the neuroprotective effects of estrogen.
Although the current meta-analysis revealed potential effects of NIBS on dual-task performance in patients with PD, these findings should be cautiously interpreted. First, significant positive effects on both motor and cognitive performances during dual tasks were only observed in tDCS studies that targeting the DLPFC, and we found no rTMS studies. Given that potential neurophysiological changes may differ between tDCS and rTMS protocols [106, 107], our findings are still limited to tDCS effects. Thus, additional studies should determine whether rTMS protocols on the DLPFC improve dual-task performances in patients with PD. Importantly, improved cognitive performances during dual tasks after NIBS protocol are still tentative because of prior suggestion that at least five studies may be required for increasing validity of data synthesis [108]. Given that small number of studies may influence reliability of the results, more studies are necessary to determine positive effects of NIBS on cognitive function during dual tasks. In addition, seven out of 10 total studies focused on transient effects of NIBS by providing a single session of stimulation. Given that multiple sessions of NIBS protocols may result in cumulative effects [109, 110], additional studies should investigate long-term effects of NIBS on dual-task performances in patients with PD by administering more sessions of stimulation. Finally, neurophysiological mechanisms underlying NIBS effects on dual-task performance in patients with PD are still inconclusive. Future studies using neuroimaging techniques should investigate how brain activation patterns are changed during and after different NIBS protocols for improving dual-task performance in patients with PD.
Conclusion
This systematic review and meta-analysis found positive effects of NIBS on dual-task performances in patients with PD. Specifically, applying tDCS on the DLPFC effectively improved motor and cognitive performances during dual tasks. Furthermore, the meta-regression analysis identified a significant relationship between greater improvements in motor performance during dual tasks after NIBS and younger age as well as a higher proportion of females in patients with PD. These findings suggest that NIBS protocols increasing DLPFC excitability may be a viable option for improving dual-task performance in patients with PD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- PD
Parkinson’s disease
- NIBS
Non-invasive brain stimulation
- tDCS
Transcranial direct current stimulation
- rTMS
Repetitive transcranial magnetic stimulation
- DLPFC
Dorsolateral prefrontal cortex
- SMA
Supplementary motor area
- M1
Primary motor cortex
- TUG
Timed up and go
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- RCT
Randomized control trials
- SMD
Standardized mean difference
- CI
Confidence interval
- H&Y
Hoehn and Yahr scale
- UPDRS-III
Unified Parkinson’s disease rating scale part III
- MMSE
Mini-mental state examination
- MoCA
Montreal cognitive assessment
Author contributions
Conceptualization: H.L.; Systematic review and meta-analysis: H.L.; Writing - original draft: H.L. and B.J.C.; Writing—review and editing: N.K.; Supervision: N.K. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019;18(7):697–708. [DOI] [PubMed] [Google Scholar]
- 2.Bock MA, Vittinghoff E, Bahorik AL, Leng Y, Fink H, Yaffe K. Cognitive and functional trajectories in older adults with prediagnostic Parkinson disease. Neurology. 2023;100(13):e1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–45. [DOI] [PubMed] [Google Scholar]
- 4.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):1–21. [DOI] [PubMed] [Google Scholar]
- 5.Schapira AH, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–50. [DOI] [PubMed] [Google Scholar]
- 6.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012. [DOI] [PMC free article] [PubMed]
- 7.Zhang X, Fan W, Yu H, Li L, Chen Z, Guan Q. Single-and dual-task gait performance and their diagnostic value in early-stage Parkinson’s disease. Front Neurol. 2022;13:974985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar RD, Ren X, Ellis TD, Toraif N, Barthelemy OJ, Neargarder S, et al. Dual tasking in Parkinson’s disease: cognitive consequences while walking. Neuropsychology. 2017;31(6):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleiner AFR, Pagnussat AS, Prisco Gd, Vagnini A, Stocchi F, De Pandis MF, et al. Analyzing gait variability and dual-task interference in patients with Parkinson’s disease and freezing by means of the word-color Stroop test. Aging Clin Exp Res. 2018;30(9):1137–42. [DOI] [PubMed] [Google Scholar]
- 10.Morrison S, Moxey J, Reilly N, Russell DM, Thomas KM, Grunsfeld AA. The relation between falls risk and movement variability in Parkinson’s disease. Exp Brain Res. 2021;239(7):2077–87. [DOI] [PubMed] [Google Scholar]
- 11.Hariz GM, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand. 2011;123(1):20–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Manor B, Yu W, Lo OY, Gouskova N, Salvador R, et al. Targeted tDCS mitigates dual-task costs to gait and balance in older adults. Ann Neurol. 2021;90(3):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljubisavljevic MR, Oommen J, Filipovic S, Bjekic J, Szolics M, Nagelkerke N. Effects of tDCS of dorsolateral prefrontal cortex on dual-task performance involving manual dexterity and cognitive task in healthy older adults. Front Aging Neurosci. 2019;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong P-L, Yang Y-R, Huang S-F, Fuh J-L, Chiang H-L, Wang R-Y. Transcranial direct current stimulation on different targets to modulate cortical activity and dual-task walking in individuals with Parkinson’s disease: a double blinded randomized controlled trial. Front Aging Neurosci. 2022;14:807151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno MEB, da Silva TCO, de Souza RJ, Volpe RP, Moura FA, Smaili SM. Acute effects of transcranial direct current stimulation combined with physical therapy on the balance and gait in individuals with Parkinson’s disease: a randomized controlled trial. Clin Neurol Neurosurg. 2023;226:107604. [DOI] [PubMed] [Google Scholar]
- 16.Tombu M, Jolicœur P. A central capacity sharing model of dual-task performance. J Exp Psychol-Hum Percept Perform. 2003;29(1):3. [DOI] [PubMed] [Google Scholar]
- 17.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35(3):715–28. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis. 2015;82:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilat M, Bell PT, Martens KAE, Georgiades MJ, Hall JM, Walton CC, et al. Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. NeuroImage. 2017;152:207–20. [DOI] [PubMed] [Google Scholar]
- 20.Ranchet M, Hoang I, Cheminon M, Derollepot R, Devos H, Perrey S, et al. Changes in prefrontal cortical activity during walking and cognitive functions among patients with Parkinson’s disease. Front Neurol. 2020;11:601686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol. 2010;23(4):407–12. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SJ, Ng B, Abugharbieh R, Eigenraam L, McKeown MJ. Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson’s disease. Eur J Neurosci. 2009;29(11):2187–96. [DOI] [PubMed] [Google Scholar]
- 23.Valentino F, Cosentino G, Brighina F, Pozzi NG, Sandrini G, Fierro B, et al. Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord. 2014;29(8):1064–9. [DOI] [PubMed] [Google Scholar]
- 24.Usman JS, Wong TW-L, NG SSM. Effects of transcranial direct current stimulation on dual-task performance in older and young adults: a systematic review and meta-analysis. Arch Gerontol Geriatr Plus. 2024:100047.
- 25.Lin X, Zhang Y, Chen X, Wen L, Duan L, Yang L. Effects of noninvasive brain stimulation on dual-task performance in different populations: a systematic review. Front Neurosci. 2023;17:1157920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung CLH, Mak MKY, Hallett M. Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann Neurol. 2020;88(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra RK, Thrasher AT. Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: a double blind, sham-controlled study. Gait Posture. 2021;84:11–6. [DOI] [PubMed] [Google Scholar]
- 28.Santos Ferreira I, Teixeira Costa B, Lima Ramos C, Lucena P, Thibaut A, Fregni F. Searching for the optimal tDCS target for motor rehabilitation. J NeuroEng Rehabil. 2019;16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131(2):474–528. [DOI] [PubMed] [Google Scholar]
- 30.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amir-Behghadami M, Janati A, Population. Intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020. [DOI] [PubMed]
- 32.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis: Wiley; 2021.
- 33.Lee JH, Lee TL, Kang N. Transcranial direct current stimulation decreased cognition-related reaction time in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2021;70:101377. [DOI] [PubMed] [Google Scholar]
- 34.Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2011;40(6):1732–4. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random‐effects models for meta‐analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;2019:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane Handb Syst Reviews Interventions. 2019;205:28. [Google Scholar]
- 41.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane. 2023.
- 42.Lench DH, DeVries W, Kearney-Ramos TE, Chesnutt A, Monsch ED, Embry AE, et al. Paired inhibitory stimulation and gait training modulates supplemental motor area connectivity in freezing of gait. Parkinsonism Relat Disord. 2021;88:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra RK, Thrasher AT. Effect of concurrent transcranial direct current stimulation on instrumented timed up and go task performance in people with Parkinson’s disease: a double-blind and cross-over study. J Clin Neurosci. 2022;100:184–91. [DOI] [PubMed] [Google Scholar]
- 44.Putzolu M, Pelosin E, Ogliastro C, Lagravinese G, Bonassi G, Ravaschio A, et al. Anodal tDCS over prefrontal cortex improves dual-task walking in parkinsonian patients with freezing. Mov Disord. 2018;33(12):1972–3. [DOI] [PubMed] [Google Scholar]
- 45.Schabrun SM, Lamont RM, Brauer SG. Transcranial direct current stimulation to enhance dual-task gait training in Parkinson’s disease: a pilot RCT. PLoS ONE. 2016;11(6):e0158497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swank C, Mehta J, Criminger C. Transcranial direct current stimulation lessens dual task cost in people with Parkinson’s disease. Neurosci Lett. 2016;626:1–5. [DOI] [PubMed] [Google Scholar]
- 47.Criminger C, Swank C, Almutairi S, Mehta J. Transcranial direct current stimulation plus concurrent activity may influence task prioritization during walking in people with Parkinson’s disease–initial findings. Res Rev Parkinsonism. 2018:25–32.
- 48.Broeder S, Vandendoorent B, Hermans P, Nackaerts E, Verheyden G, Meesen R, et al. Transcranial direct current stimulation enhances motor learning in Parkinson’s disease: a randomized controlled trial. J Neuro. 2023;270(7):3442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427. [DOI] [PubMed] [Google Scholar]
- 50.Fahn S. Unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. 1987:153 – 63.
- 51.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. [DOI] [PubMed] [Google Scholar]
- 52.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 53.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 54.Clark D. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci. 2015; 9: 246. 2015. [DOI] [PMC free article] [PubMed]
- 55.Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–6. [DOI] [PubMed] [Google Scholar]
- 56.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? NeuroImage. 2007;37(4):1338–45. [DOI] [PubMed] [Google Scholar]
- 57.Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci. 2005;102(35):12566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to automatic movements in Parkinson’s disease: modified automatic mode in the striatum. Cereb Cortex. 2015;25(10):3330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks D, Ibanez V, Sawle Gea, Quinn N, Lees A, Mathias C, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28(4):547–55. [DOI] [PubMed] [Google Scholar]
- 60.Panikratova YR, Vlasova RM, Akhutina TV, Korneev AA, Sinitsyn VE, Pechenkova EV. Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. Int J Psychophysiol. 2020;151:70–9. [DOI] [PubMed] [Google Scholar]
- 61.de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, King LA, Nutt JG, Chagas EF, et al. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture. 2017;56:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Deutsch J, Hausdorff J, et al. Altered brain activation in complex walking conditions in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:91–6. [DOI] [PubMed] [Google Scholar]
- 63.Stuart S, Vitorio R, Morris R, Martini DN, Fino PC, Mancini M. Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: a structured review. Maturitas. 2018;113:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoang I, Ranchet M, Derollepot R, Moreau F, Paire-Ficout L. Measuring the cognitive workload during dual-task walking in young adults: a combination of neurophysiological and subjective measures. Front Hum Neurosci. 2020;14:592532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goh H-T, Connolly K, Hardy J, McCain K, Walker-Batson D. Single session of repetitive transcranial magnetic stimulation to left dorsolateral prefrontal cortex increased dual-task gait speed in chronic stroke: a pilot study. Gait Posture. 2020;78:1–5. [DOI] [PubMed] [Google Scholar]
- 66.Wrightson JG, Twomey R, Ross EZ, Smeeton NJ. The effect of transcranial direct current stimulation on task processing and prioritisation during dual-task gait. Exp Brain Res. 2015;233:1575–83. [DOI] [PubMed] [Google Scholar]
- 67.Strobach T, Antonenko D. tDCS-induced effects on executive functioning and their cognitive mechanisms: a review. J Cogn Enhance. 2017;1:49–64. [Google Scholar]
- 68.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. [DOI] [PubMed] [Google Scholar]
- 69.Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35(6):619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strobach T, Salminen T, Karbach J, Schubert T. Practice-related optimization and transfer of executive functions: a general review and a specific realization of their mechanisms in dual tasks. Psychol Res. 2014;78:836–51. [DOI] [PubMed] [Google Scholar]
- 71.Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology. 2010;35(1):70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41(6):645–54. [DOI] [PubMed] [Google Scholar]
- 73.Bunai T, Hirosawa T, Kikuchi M, Fukai M, Yokokura M, Ito S, et al. tDCS-induced modulation of GABA concentration and dopamine release in the human brain: a combination study of magnetic resonance spectroscopy and positron emission tomography. Brain Stimul. 2021;14(1):154–60. [DOI] [PubMed] [Google Scholar]
- 74.Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, Suaud-Chagny M-F, et al. Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb Cortex. 2018;28(7):2636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukai M, Bunai T, Hirosawa T, Kikuchi M, Ito S, Minabe Y, et al. Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: a study with positron emission tomography. Transl Psychiatr. 2019;9(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunelin J, Szekely D, Costes N, Mondino M, Bougerol T, Saoud M. Theta burst stimulation in the negative symptoms of schizophrenia and striatal dopamine release. An iTBS-[11 C] raclopride PET case study. Schizophr Res. 2011;131(1–3):264–5. [DOI] [PubMed] [Google Scholar]
- 77.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Foly KN, Hamdy A. Dopamine levels after repetitive transcranial magnetic stimulation of motor cortex in patients with Parkinson’s disease: preliminary results. Wiley Online Library; 2007. [DOI] [PubMed]
- 78.Cai J, Tong Q. Anatomy and function of ventral tegmental area glutamate neurons. Front Neural Circuits. 2022;16:867053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31(28):10340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):Rc157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seibert TM, Murphy EA, Kaestner EJ, Brewer JB. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology. 2012;263(1):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao L-l, Wu T. The study of brain functional connectivity in Parkinson’s disease. Transl Neurodegener. 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alkhasli I, Sakreida K, Mottaghy FM, Binkofski F. Modulation of fronto-striatal functional connectivity using transcranial magnetic stimulation. Front Hum Neurosci. 2019;13:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial direct current stimulation modulates neuronal networks in attention deficit hyperactivity disorder. Brain Topogr. 2017;30:656–72. [DOI] [PubMed] [Google Scholar]
- 85.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn P. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–24. [DOI] [PubMed] [Google Scholar]
- 86.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21(9):1343–9. [DOI] [PubMed] [Google Scholar]
- 87.Nitsche M, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–32. [DOI] [PubMed] [Google Scholar]
- 89.Brown JC, DeVries WH, Korte JE, Sahlem GL, Bonilha L, Short EB, et al. NMDA receptor partial agonist, d-cycloserine, enhances 10 hz rTMS-induced motor plasticity, suggesting long-term potentiation (LTP) as underlying mechanism. Brain Stimul. 2020;13(3):530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004;29(8):1573–8. [DOI] [PubMed] [Google Scholar]
- 91.Kricheldorff J, Göke K, Kiebs M, Kasten FH, Herrmann CS, Witt K, et al. Evidence of neuroplastic changes after transcranial magnetic, electric, and deep brain stimulation. Brain Sci. 2022;12(7):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deutschenbaur L, Beck J, Kiyhankhadiv A, Mühlhauser M, Borgwardt S, Walter M, et al. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;64:325–33. [DOI] [PubMed] [Google Scholar]
- 93.Kumar A, Foster TC. Alteration in NMDA receptor mediated glutamatergic neurotransmission in the hippocampus during senescence. Neurochem Res. 2019;44:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rozisky JR, Antunes LC, Brietzke AP, de Sousa AC, Caumo W. Transcranial direct current stimulation and neuroplasticity. In: Rogers L, editor. Transcranial Direct Current Stimulation (tDCS): emerging used, Safety and Neurobiological effects. New York, NY: Nova Science Publishers Inc; 2016. pp. 1–26. [Google Scholar]
- 95.Roalf DR, Sydnor VJ, Woods M, Wolk DA, Scott JC, Reddy R, et al. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol Aging. 2020;95:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122(1):1–29. [DOI] [PubMed] [Google Scholar]
- 97.Ghasemian-Shirvan E, Farnad L, Mosayebi-Samani M, Verstraelen S, Meesen RL, Kuo M-F, et al. Age-related differences of motor cortex plasticity in adults: a transcranial direct current stimulation study. Brain Stimul. 2020;13(6):1588–99. [DOI] [PubMed] [Google Scholar]
- 98.Todd G, Kimber TE, Ridding MC, Semmler JG. Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol. 2010;121(3):441–7. [DOI] [PubMed] [Google Scholar]
- 99.Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Málly J, Stone TW, Sinkó G, Geisz N, Dinya E. Long term follow-up study of non-invasive brain stimulation (NBS)(rTMS and tDCS) in Parkinson’s disease (PD). Strong age-dependency in the effect of NBS. Brain Res Bull. 2018;142:78–87. [DOI] [PubMed] [Google Scholar]
- 101.Song Y-j, Li S-r, Li X-w, Chen X, Wei Z-x, Liu Q-s, et al. The effect of estrogen replacement therapy on Alzheimer’s disease and Parkinson’s disease in postmenopausal women: a meta-analysis. Front Neurosci. 2020;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawada H, Shimohama S. Estrogens and Parkinson disease: novel approach for neuroprotection. Endocrine. 2003;21:77–9. [DOI] [PubMed] [Google Scholar]
- 103.Picillo M, LaFontant D-E, Bressman S, Caspell-Garcia C, Coffey C, Cho HR, et al. Sex-related longitudinal change of motor, non-motor, and biological features in early Parkinson’s disease. J Parkinsons Dis. 2022;12(1):421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwaki H, Blauwendraat C, Leonard HL, Makarious MB, Kim JJ, Liu G, et al. Differences in the presentation and progression of Parkinson’s disease by sex. Mov Disord. 2021;36(1):106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moriyasu S, Shimizu T, Honda M, Ugawa Y, Hanajima R. Motor cortical plasticity and its correlation with motor symptoms in Parkinson’s disease. eNeurologicalSci. 2022;29:100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128(11):2318–29. [DOI] [PubMed] [Google Scholar]
- 107.Chisari C, Fanciullacci C, Lamola G, Rossi B, Cohen LG. NIBS-driven brain plasticity. Arch Ital Biol. 2014;152(4):247–58. [DOI] [PubMed] [Google Scholar]
- 108.Myung S-K. How to review and assess a systematic review and meta-analysis article: a methodological study (secondary publication). J Educ Eval Health Prof. 2023;20. [DOI] [PMC free article] [PubMed]
- 109.Ho K-A, Taylor JL, Chew T, Gálvez V, Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. 2016;9(1):1–7. [DOI] [PubMed] [Google Scholar]
- 110.Christova M, Rafolt D, Gallasch E. Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Behav Brain Res. 2015;287:27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.