Abstract
Background
Triptans selectively agoniste 5-Hydroxytryptamine(5-HT) receptors and are widely used in the treatment of migraine. Nevertheless, there is a dearth of comprehensive real-world clinical research on the safety of triptans. In light of the growing prevalence of migraine, it is imperative to gain a deeper understanding of the true extent of adverse events (AEs) associated with triptans in the clinical management of migraine.
Methods
A database query of AEs reported to the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database for triptans was performed using the online platform Open Vigil 2.1. The query spanned the period from 1 January 2018 to 31 December 2023 and extracted all AEs for ‘sumatriptan’, ‘zolmitriptan’, ‘rizatriptan’, and ‘naratriptan’ from the 15–49 years old population and retrospective quantitative analyses. A proportional reporting ratio (PRR), reporting odds ratio (ROR), and Bayesian Confidence Propagation Neural Network (BCPNN) methodology were utilized to contrast AEs across the four triptans.
Results
A total of 1.272 AEs reports for sumatriptan, 114 for zolmitriptan, 162 for rizatriptan, and 15 for naratriptan were identified. The ratio of females to males was approximately three times higher in all cases, with the highest number of reports originating from the Americas. A review of the FAERS database revealed that nervous system disorders were the primary SOC category for four drugs, with all four drugs exhibiting the AE indicative of reversible cerebral vasoconstriction syndrome, also classified as Nervous system disorders. The most frequently reported AE signal for sumatriptan was dyspnea, which is classified as respiratory, thoracic and mediastinal disorders. The most frequently reported AEs signals for the remaining three drugs were nausea, vomiting and terminal ileitis, all of which are classified as gastrointestinal disorders.
Conclusion
Analyses have demonstrated that AEs are present in a range of systems, including cardiac, nervous, gastrointestinal, and musculoskeletal disorders. It should be noted, however, that the incidence and signal intensity of these AEs vary depending on the specific drug in question. In clinical practice, the selection of an appropriate drug and the monitoring of AEs should be tailored to the individual patient’s and specific characteristics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01913-0.
Keywords: Triptan medications, Adverse events, Food and drug administration adverse event reporting system, Real-world, Pharmacovigilance
Introduction
Migraine is a prevalent chronic -neurological disorder that typically manifests as recurrent episodes of moderate to severe throbbing headaches and concomitant symptoms of autonomic dysfunction, including nausea, vomiting, photophobia, and phonophobia. Headaches may be unilateral, affecting one - side- of the head, or may become a full headache, which is more prevalent in women [1–3]. According to the Global Burden of Disease Study, migraine is the third leading cause of neurological health loss globally and is a major contributor to neurological disability as well as one of the leading causes of disability in people under 50 years of age [4–6]. Triptans are selective 5-hydroxytryptamine (5-HT) receptor agonists that have been developed for the acute treatment of migraine. It relieves migraine attacks by agonizing 5-HTIB/ID receptors on intracranial blood vessels (including arteriovenous anastomoses) and sympathetic nerves in the trigeminal system, constricting blood vessels and inhibiting the release of peripherally active neuropeptides, such as calcitonin gene-related peptide (CGRP) [7–9].
The introduction of the first-generation drug sumatriptan represented a significant advancement in the treatment of acute migraine. However, the second-generation triptan analogues (zolmitriptan, rizatriptan and naratriptan), which exhibit a superior pharmacokinetic profile compared to sumatriptan, demonstrate comparable pharmacodynamic properties and are currently employed for the management of moderate to severe migraine attacks [10–12]. The instructions for the use of drugs such as triptans indicate that they are contraindicated in patients with cardiovascular diseases, including but not limited to heart disease, angina pectoris, myocardial infarction, etc. Adverse reactions are often observed, including but not limited to neurological (headache, dizziness, drowsiness, etc.), cardiac (palpitations, arrhythmias, etc.), gastrointestinal (nausea, vomiting, etc.) and allergic reactions [13]. To date, the majority of clinical trials have demonstrated the safety, efficacy, and tolerability of triptans in the treatment of migraine [10, 14]. Patients with vascular disease due to the vasoconstrictive potential of triptans are frequently excluded from Phase III studies. Furthermore, individuals over the age of 65 are often excluded. In consequence of the aforementioned considerations, the instructions for all Triptan drugs indicate that they are contraindicated in patients with various vascular diseases. Nevertheless, an Austrian study demonstrated that the prevalence of vascular disease in users of the drugs over the age of 50 remained unchanged. This indicates that the use of the drugs does not elevate the risk of vascular events in this age group [15]. Due to the rigorous inclusion criteria for trial populations and constraints on sample size and follow-up duration, the occurrence of AEs and long-term medication safety concerns may be underestimated or overestimated at this juncture. The dearth of post-marketing safety data for triptans in pharmaceuticals underscores the necessity for comprehensive real-world studies.
The FAERS database, which collates data on AEs and medication errors occurring within and outside the United States, represents a significant source of real-world data on AEs, and may provide insight into the occurrence of drug AEs [16]. In previous studies, scholars investigated the drug-related vascular adverse events associated with triptans from 2004 to 2010. Their findings revealed a strong association between ischemic cerebrovascular events, aneurysms and artery dissections, and pregnancy-related vascular events and triptans [17]. And then Sharma [18] et al. explored the data of cardiovascular adverse events of Triptans from 1997 to 2023 in people aged 18–85 years, but no quantitative analyses such as year, region, gender etc. were done to compare with the drugs. The recent advances in pharmacological treatments and the growing prevalence of common adverse reactions have prompted a shift in the way clinicians and related professionals approach drug utilisation in the real world. This necessitates a comprehensive and up-to-date investigation of the subject.The objective of this study was to conduct pharmacovigilance analyses of triptans and AEs - using the FAERS database. This was done with the intention of providing insights into the post-marketing safety of triptans, as well as to inform personalised treatment decisions for clinicians, patients and regulators.
Methods
Data sources
The data for this study were retrieved from the publicly available FAERS database, which collates spontaneous AE reports from a variety of sources, including healthcare professionals, patients, pharmaceutical manufacturers, and others in different regions. This can reflect the true incidence of AEs [19]. This retrospective study used Open Vigil 2.1 to query the FAERS database and retrieve reports of the target drug for the last 6 years between 1 January 2018 and 31 December 2023, which better represents the current real-world AE realities of the drug The generic name of the target drug is the first generation drug: ‘sumatriptan’, with second-generation drugs: ‘zolmitriptan’, ‘rizatriptan’, and ‘naratriptan’. The age limit of 15–49 years was set primarily due to the relatively high incidence and prevalence of migraine in this age group and the fact that AE in this age group has not been extensively investigated in previous studies [20]. In the present study, we selected reported cases defined as AEs in which the reporter identified the target drug as the ‘prime suspect’. We then classified and described the AEs according to the preferred terminology (PT) and the system organ classification (SOC) in the International Medical Dictionary for Regulatory Activities (MedDRA 27.0 Edition) [21]. Data sources are publicly available and therefore do not require ethical approval.
Statistical analysis
Proportional reporting ratio (PRR), reporting odds ratio (ROR) and Bayesian Confidence Propagation Neural Network (BCPNN) methods are commonly used to detect AE signals in pharmacovigilance [22]. PRR [23] can be employed to estimate relative risk; however, PRR methods are susceptible to false-positive signals. In contrast, ROR [24] is a consistent estimate of the ratio, or risk ratio, and is less biased than other indices. BCPNN [25]employs a neural network-supervised learning approach that utilises known adverse drug reactions as a machine-learning training set, which is relatively stable, even with a small number of reports. Therefore, we combined ROR, PRR and BCPNN methods to mine the AE signals of the target drugs, with the calculation of PRR and χ2 based on the ratio imbalance measure quadrangle table. Should the results of all three methods yield a positive outcome, it can be inferred that the criteria set forth in Table 1 have been met, thereby classifying the signal in question as a suspected AE signal [26]. We used Microsoft Office Excel 2021, R 4.3.1 software and online plotting platform (https://www.bioincloud.tech/) (https://www.chiplot.online/) (https://bioincloud.tech/) to statistically analyse and plot the data [27].
Table 1.
Formulas and signal detection criterias for reporting odds ratio (ROR), proportional reporting ratio (PRR) and bayesian confidence propagation neural network (BCPNN)
| Algorithms | Equation | Criteria | |
|---|---|---|---|
| ROR | ROR=ad/bc | lower limitof 95%CI>1,a≥3 | |
| 95%CI=eIn(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | |||
| PRR | PRR=a(c+d)/c/(a+b) | PRR>2,χ²>4,a>3 | |
| χ²=[(ad-bc)^2](a+b+c+d)/[(a+b)(c+d)(a+c)(b+d)] | |||
| BCPNN | IC=log2a(a+b+c+d)(a+c)(a+b) | IC025>0 | |
| 95%CI=E(IC)±2V(IC)^0.5 | |||
| Fourfold table of disproportionality measures. | Target AE | OtherAE | Total |
| Target drugs | a | b | a+b |
| Other drugs | c | d | c + d |
| Total | a+c | b + d | a+b + c + d |
ROR reporting odds ratio, CI confidence interval, PRR proportional reporting ratio, χ2 chi-squared, BCPNN bayesian confidence propagation neural network, IC information component, IC025 the lower limit of 95%CI, of the IC
Results
Demographic information on the AE reports
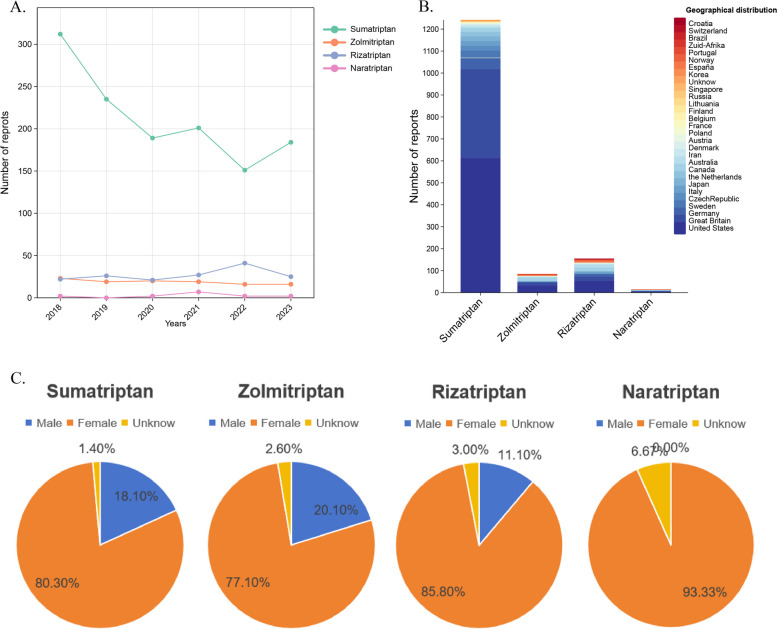
A total of 968.550 AEs reports were identified from the FAERS database between 1 January 2018 and 31 December 2023. Of the four triptans, sumatriptan had the highest number of AEs reports, with 1,272 reports, followed by zolmitriptan with 114 reports, rizatriptan with 162 reports, and naratriptan with the lowest number of reports, at 15. With the exception of unknown reports, the ratio of females to males was approximately 3 times greater for all four drugs (Fig. 1C), demonstrating a wide range of differences. It is hypothesised that this may be due to the fact that migraines are more prevalent in women. Consequently, the population using triptans in the real world is predominantly female, which results in a higher number of AEs in women. The number of reports of sumatriptan was concentrated in 2018, with an overall downward trend (Fig. 1A). Furthemore, the most frequently reported age group was between 31 and 49 years of age, with the highest number of reports originating from - America- (Fig. 1B). The primary indication for the drug is the treatment of migraine. Please refer to Table 2 for further details.
Fig. 1.
Demographic information reported by AE in FAERS. A Number of four drugs reported per year; B Number of reports by region for the four drugs; C Rates were reported by sex for the four drugs
Table 2.
Demographic information reported by AE in FAERS
| Sumatriptan(n=1172) | Zolmitriptan(n=114) | Rizatriptan(n=106) | Naratriptan(n=15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||||
| Sex | Male | 231 | 18.1 | Male | 23 | 20.1 | Male | 18 | 11.1 | Male | 0 | 0.0 |
| Female | 1022 | 80.3 | Female | 88 | 77.1 | Female | 139 | 85.8 | Female | 14 | 93.3 | |
| Unknow | 19 | 1.4 | Unknow | 3 | 2.6 | Unknow | 5 | 3.0 | Unknow | 1 | 6.7 | |
| Age (years) | 15-18 | 74 | 5.82 | 15-18 | 5 | 4.4 | 15-18 | 4 | 2.5 | 15-18 | 0 | 0.0 |
| 19-30 | 334 | 26.26 | 19-30 | 25 | 21.9 | 19-30 | 27 | 14.1 | 19-30 | 1 | 6.7 | |
| 31-49 | 864 | 67.92 | 31-49 | 84 | 73.7 | 31-49 | 131 | 67.9 | 31-49 | 14 | 93.3 | |
| Reporting year | 2018 | 312 | 24.5 | 2018 | 23 | 20.1 | 2018 | 22 | 13.5 | 2018 | 2 | 13.3 |
| 2019 | 235 | 18.4 | 2019 | 19 | 16.6 | 2019 | 26 | 16.0 | 2019 | 0 | 0.0 | |
| 2020 | 189 | 14.8 | 2020 | 20 | 17.5 | 2020 | 21 | 12.9 | 2020 | 2 | 13.3 | |
| 2021 | 201 | 15.8 | 2021 | 19 | 16.6 | 2021 | 27 | 16.6 | 2021 | 7 | 46.6 | |
| 2022 | 151 | 11.8 | 2022 | 16 | 14.0 | 2022 | 41 | 25.3 | 2022 | 2 | 13.3 | |
| 2023 | 184 | 14.4 | 2023 | 16 | 14.9 | 2023 | 25 | 16.4 | 2023 | 2 | 13.3 | |
| Geographical distribution | United States | 613 | 48.1 | United States | 33 | 28.9 | United States | 54 | 33.3 | United States | 5 | 33.3 |
| Great Britain | 405 | 31.8 | France | 29 | 25.4 | Great Britain | 18 | 11.1 | Germany | 3 | 20.0 | |
| Germany | 50 | 3.9 | Canada | 15 | 13.2 | Canada | 17 | 10.5 | Norway | 3 | 20.0 | |
| France | 32 | 2.5 | Great Britain | 13 | 11.4 | Germany | 13 | 8.0 | Canada | 2 | 13.3 | |
| Sweden | 32 | 2.5 | Australia | 5 | 4.4 | Australia | 13 | 8.0 | Australia | 1 | 6.7 | |
| CzechRepublic | 23 | 1.8 | Germany | 4 | 3.5 | France | 10 | 6.2 | Japan | 1 | 6.7 | |
| Italy | 22 | 1.7 | España | 3 | 2.6 | Italy | 8 | 4.9 | ||||
| Japan | 20 | 1.5 | Austria | 2 | 1.8 | Japan | 5 | 3.1 | ||||
| the Netherlands | 20 | 1.5 | Norway | 2 | 1.8 | Norway | 5 | 3.1 | ||||
| Canada | 18 | 1.4 | Portugal | 2 | 1.8 | Austria | 3 | 1.9 | ||||
| Australia | 7 | 0.6 | Italy | 1 | 0.9 | España | 3 | 1.9 | ||||
| Iran | 7 | 0.6 | Japan | 1 | 0.9 | India | 3 | 1.9 | ||||
| Denmark | 4 | 0.3 | Korea | 1 | 0.9 | Unkown | 2 | 1.2 | ||||
| Austria | 3 | 0.2 | the Netherlands | 1 | 0.9 | Croatia | 2 | 1.2 | ||||
| Poland | 3 | 0.2 | Poland | 1 | 0.9 | Portugal | 2 | 1.2 | ||||
| Switzerland | 2 | 0.2 | Zuid-Afrika | 1 | 0.9 | Brazil | 1 | 0.6 | ||||
| France | 2 | 0.2 | Switzerland | 1 | 0.6 | |||||||
| Belgium | 1 | <0.1 | Finland | 1 | 0.6 | |||||||
| Finland | 1 | <0.1 | Zuid-Afrika | 1 | 0.6 | |||||||
| Lithuania | 1 | <0.1 | ||||||||||
| Russia | 1 | <0.1 | ||||||||||
| Singapore | 1 | <0.1 | ||||||||||
| Unknow | 6 | 0.5 | ||||||||||
| Indication | Migraine | 788 | 62.0 | Migraine | 54 | 60.7 | Migraine | 98 | 60.5 | Migraine | 8 | 53.3 |
| Unknow | 305 | 24.0 | Headache | 2 | 2.3 | Headache | 5 | 3.1 | Headache | 2 | 13.3 | |
| Headache | 65 | 5.1 | Multiple sclerosis relapse | 2 | 2.3 | Cluster headache | 3 | 1.9 | Unknow | 5 | 33.3 | |
| Cluster headache | 37 | 2.9 | Tinnitus | 2 | 2.3 | Drug abuse | 3 | 1.9 | ||||
| Ill-defined disorder | 34 | 2.7 | Cluster headache | 1 | 1.1 | Suicidal ideation | 3 | 1.9 | ||||
| Other | 43 | 3.4 | Diaphragmatic hernia | 1 | 1.1 | Unknow | 50 | 30.9 | ||||
| Unknow | 22 | 30.2 | ||||||||||
Signal AE mining and analysis of AE signals at the PT level
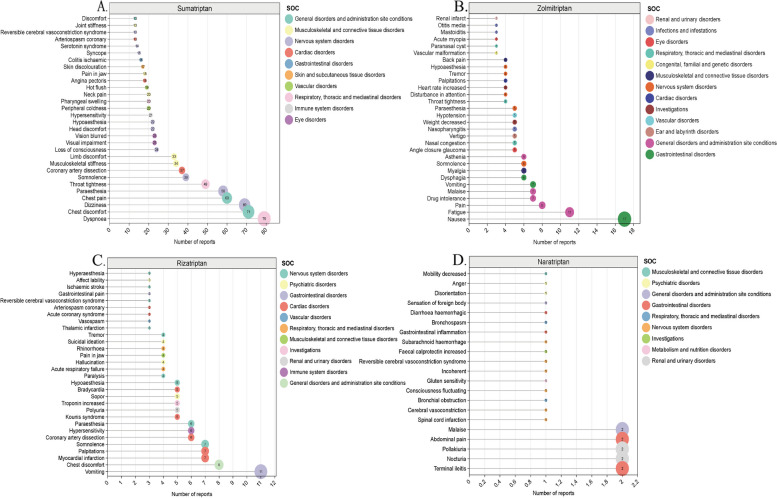
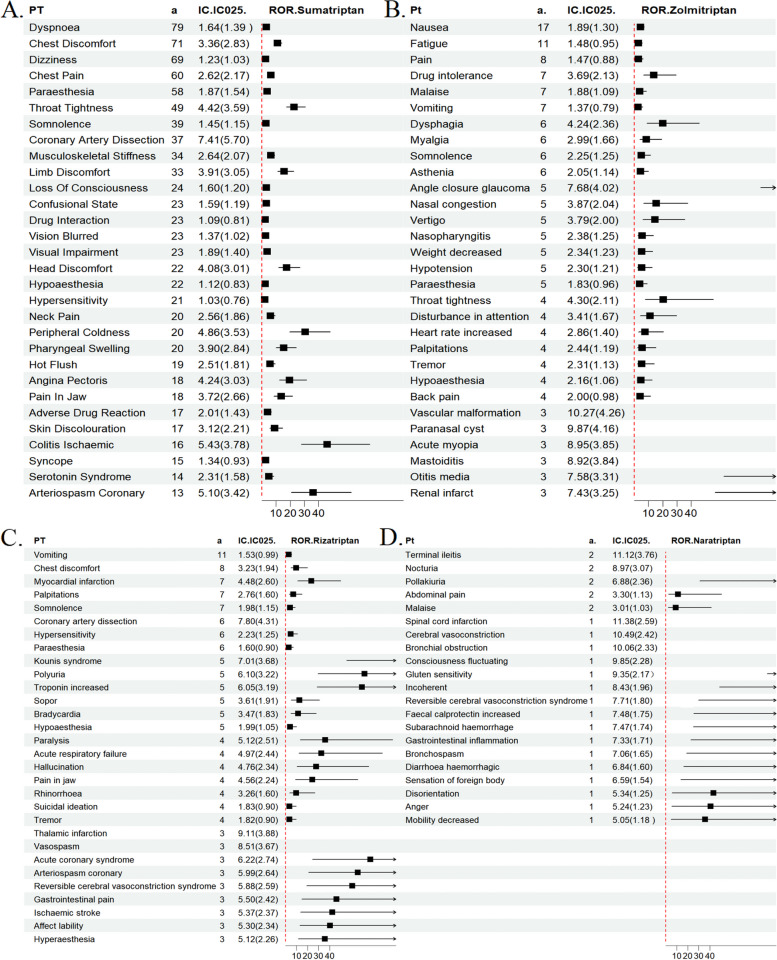
In this study, the analysis of AE signals was conducted using ROR, PRR and BCPNN. A total of 164 AE signals were obtained for sumatriptan, following the removal of entry errors, incomplete information, and the screening and exclusion of signals pertaining to product quality, use problems, and drug indications. A total of 101 positive AE signals were obtained for zolmitriptan, 97 AE signals were obtained for rizatriptan, and 21 positive signals were obtained for naratriptan. All AE signals are presented in the Supplementary Material Table S1. We next analysed all signals at the PT level, focusing on the top 30 most frequent and highest signal intensity detections that occurred (Table 3; Fig. 2). The three most frequently reported AE associated with sumatriptan were dyspnoea, chest discomfort and dizziness- and the top 3 AE signals with PRR values were migrainous infarction, pityriasis lichenoides et varioliformis acuta, and subclavian artery thrombosis. The three most frequently reported AE signals associated with zolmitriptan were nausea, fatigue and pain. and the top 3 AE signals with PRR values were paraesthesia ear, vascular malformation and paranasal cyst. The three most frequently reported AE signals for rizatriptan were vomiting, chest discomfort, and myocardial infarction and the top 3 AE signals in terms of PRR values were broad ligament tear, hemiataxia, and thalamic infarction. The three most frequently reported AEs associated with naratriptan were terminal ileitis, nocturia, and abdominal pain. Among the AEs with the highest PRR, the top three were spinal cord infarction, terminal ileitis, and cerebral vasoconstriction.
Table 3.
Top 30 AEs with the highest percentage of signal detection in the FAERS database
| Sumatriptan | Zolmitriptan | Rizatriptan | Naratriptan | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | PT | n(%) | PRR | χ2 | ROR(95%CI) | PT | n(%) | PRR | χ2 | ROR(95%CI) | PT | n(%) | PRR | χ2 | ROR(95%CI) | PT | n(%) | PRR | χ2 | ROR(95%CI) |
| 1 | Dyspnoea | 79(5.36) | 3.125 | 113.98 | 3.27(2.60 to 4.11) | Nausea | 17(5.86) | 3.7 | 32.213 | 4.21(2.51 to 7.07) | Vomiting | 11(4.28) | 2.89 | 12.07 | 3.04(1.64 to 5.62) | Terminal ileitis | 2(7.69) | 2306.038 | 2507.84 | 2690.21(589.43 to 12278.26) |
| 2 | Chest discomfort | 71(4.82) | 10.411 | 590.343 | 11.00(8.64 to 14.00) | Fatigue | 11(3.79) | 2.787 | 11.291 | 2.99(1.60 to 5.58) | Chest discomfort | 8(3.11) | 9.365 | 51.922 | 9.84(4.82 to 20.06) | Nocturia | 2(7.69) | 506.822 | 563.208 | 591.12(131.68 to 2653.66) |
| 3 | Dizziness | 69(4.68) | 2.346 | 53.084 | 2.43(1.90 to 3.10) | Pain | 8(2.76) | 2.78 | 7.628 | 2.92(1.42 to 6.01) | Myocardial infarction | 7(2.72) | 22.372 | 122.176 | 23.42(10.95 to 50.07) | Pollakiuria | 2(7.69) | 117.655 | 129.315 | 137.10(30.65 to 613.24) |
| 4 | Chest pain | 60(4.07) | 6.186 | 255.801 | 6.46(4.98 to 8.38) | Drug intolerance | 7(2.41) | 12.906 | 65.679 | 13.74(6.38 to 29.58) | Palpitations | 7(2.72) | 6.765 | 29.037 | 7.05(3.30 to 15.06) | Abdominal pain | 2(7.69) | 9.857 | 8.413 | 11.33(2.54 to 50.64) |
| 5 | Paraesthesia | 58(3.93) | 3.657 | 110.249 | 3.79(2.91 to 4.94) | Malaise | 7(2.41) | 3.694 | 11.385 | 3.88(1.80 to 8.36) | Somnolence | 7(2.72) | 3.947 | 12.738 | 4.09(1.92 to 8.74) | Malaise | 2(7.69) | 8.064 | 6.433 | 9.24(2.07 to 41.30) |
| 6 | Throat tightness | 49(3.32) | 21.97 | 935.993 | 22.86(17.11 to 30.53) | Vomiting | 7(2.41) | 2.578 | 5.409 | 2.69(1.25 to 5.79) | Coronary artery dissection | 6(2.33) | 230.571 | 1111.952 | 240.14(104.65 to 551.01) | Spinal cord infarction | 1(3.85) | 2767.246 | 664.241 | 2980.03(375.50 to 23649.77) |
| 7 | Somnolence | 39(2.65) | 2.731 | 41.413 | 2.79(2.03 to 3.84) | Dysphagia | 6(2.07) | 18.994 | 85.152 | 20.06(8.80 to 45.76) | Hypersensitivity | 6(2.33) | 4.709 | 14.125 | 4.86(2.15 to 11.01) | Cerebral vasoconstriction | 1(3.85) | 1471.939 | 359.347 | 1585.09(203.25 to 12361.95) |
| 8 | Coronary artery dissection | 37(2.51) | 215.911 | 6064.65 | 222.69(154.19 to 321.63) | Myalgia | 6(2.07) | 7.973 | 30.136 | 8.39(3.68 to 19.12) | Paraesthesia | 6(2.33) | 3.042 | 6.391 | 3.13(1.38 to 7.08) | Bronchial obstruction | 1(3.85) | 1080.955 | 265.108 | 1164.03(150.05 to 9030.24) |
| 9 | Musculoskeletal stiffness | 34(2.31) | 6.285 | 145.362 | 6.44(4.57 to 9.06) | Somnolence | 6(2.07) | 4.742 | 14.337 | 4.96(2.18 to 11.31) | Kounis syndrome | 5(1.95) | 131.22 | 512.184 | 135.71(55.16 to 333.87) | Consciousness fluctuating | 1(3.85) | 934.88 | 229.629 | 1006.72(130.03 to 7794.40) |
| 10 | Limb discomfort | 33(2.24) | 15.347 | 421.055 | 15.75(11.11 to 22.33) | Asthenia | 6(2.07) | 4.147 | 11.503 | 4.33(1.90 to 9.88) | Polyuria | 5(1.95) | 69.419 | 269.446 | 71.78(29.30 to 175.87) | Gluten sensitivity | 1(3.85) | 658.868 | 162.187 | 709.47(91.98 to 5472.47) |
| 11 | Loss of consciousness | 24(1.63 | 3.041 | 30.944 | 3.08(2.06 to 4.62) | Angle closure glaucoma | 5(1.72) | 210.485 | 824.486 | 220.75(89.07 to 547.14) | Troponin increased | 5(1.95) | 67.11 | 260.288 | 69.39(28.32 to 169.99) | Incoherent | 1(3.85) | 345.906 | 85.069 | 372.44(48.49 to 2860.56) |
| 12 | Visual impairment | 23(1.56) | 3.711 | 42.897 | 3.06(2.03 to 4.63) | Nasal congestion | 5(1.72) | 14.669 | 50.83 | 15.34(6.25 to 37.67) | Sopor | 5(1.95) | 12.25 | 41.055 | 12.64(5.18 to 30.85) | Reversible cerebral vasoconstriction syndrome | 1(3.85) | 210.277 | 51.434 | 226.38(29.53 to 1735.50) |
| 13 | Vision blurred | 23(1.56) | 2.581 | 20.807 | 2.16(1.43 to 3.26) | Vertigo | 5(1.72) | 13.894 | 47.719 | 14.53(5.92 to 35.67) | Bradycardia | 5(1.95) | 11.062 | 36.301 | 11.41(4.67 to 27.85) | Faecal calprotectin increased | 1(3.85) | 178.763 | 43.6 | 192.44(25.11 to 1474.68) |
| 14 | Head discomfort | 22(1.49) | 17.26 | 314.468 | 2.61(1.73 to 3.95) | Nasopharyngitis | 5(1.72) | 5.196 | 13.115 | 5.40(2.20 to 13.26) | Hypoaesthesia | 5(1.95) | 3.976 | 8.427 | 4.08(1.67 to 9.95) | Subarachnoid haemorrhage | 1(3.85) | 177.844 | 43.371 | 191.45(24.98 to 1467.08) |
| 15 | Hypoaesthesia | 22(1.49) | 2.171 | 12.822 | 3.76(2.49 to 5.69) | Weight decreased | 5(1.72) | 5.048 | 12.545 | 5.25(2.14 to 12.88) | Paralysis | 4(1.56) | 34.945 | 99.665 | 35.88(13.25 to 97.12) | Gastrointestinal inflammation | 1(3.85) | 161.638 | 39.34 | 174.00(22.71 to 1333.05) |
| 16 | Hypersensitivity | 21(1.42) | 2.045 | 10.248 | 17.56(11.47 to 26.89) | Hypotension | 5(1.72) | 4.915 | 12.031 | 5.11(2.08 to 12.54) | Acute respiratory failure | 4(1.56) | 31.531 | 89.327 | 32.37(11.96 to 87.61) | Bronchospasm | 1(3.85) | 134.072 | 32.479 | 144.31(18.84 to 1105.19) |
| 17 | Peripheral coldness | 20(1.36) | 30.168 | 516.03 | 2.19(1.44 to 3.35) | Paraesthesia | 5(1.72) | 3.554 | 6.888 | 3.68(1.50 to 9.03) | Hallucination | 4(1.56) | 27.183 | 76.147 | 27.90(10.31 to 75.49) | Diarrhoea haemorrhagic | 1(3.85) | 114.728 | 27.661 | 123.48(16.13 to 945.40) |
| 18 | Pharyngeal swelling | 20(1.36) | 15.213 | 247.115 | 2.06(1.34 to 3.18) | Throat tightness | 4(1.38) | 19.805 | 53.837 | 20.54(7.55 to 55.81) | Pain in jaw | 4(1.56) | 23.648 | 65.424 | 24.27(8.97 to 65.65) | Sensation of foreign body | 1(3.85) | 96.353 | 23.083 | 103.69(13.55 to 793.68) |
| 19 | Neck pain | 20(1.36) | 5.933 | 76.811 | 6.02(3.86 to 9.37) | Disturbance in attention | 4(1.38) | 10.604 | 25.914 | 10.98(4.04 to 29.82) | Rhinorrhoea | 4(1.56) | 9.604 | 22.856 | 9.84(3.64 to 26.59) | Disorientation | 1(3.85) | 40.41 | 9.138 | 43.44(5.68 to 332.27) |
| 20 | Hot flush | 19(1.29) | 5.736 | 69.33 | 30.66(19.55 to 48.09) | Heart rate increased | 4(1.38) | 7.268 | 15.878 | 7.51(2.77 to 20.40) | Suicidal ideation | 4(1.56) | 3.55 | 5.034 | 3.62(1.34 to 9.78) | Anger | 1(3.85) | 37.825 | 8.494 | 40.66(5.32 to 310.97) |
| 21 | Angina pectoris | 18(1.22) | 19.29 | 287.403 | 15.45(9.89 to 24.14) | Palpitations | 4(1.38) | 5.417 | 10.394 | 5.59(2.06 to 15.18) | Tremor | 4(1.56) | 3.539 | 5.002 | 3.61(1.34 to 9.74) | Mobility decreased | 1(3.85) | 33.244 | 7.353 | 35.73(4.67 to 273.22) |
| 22 | Pain in jaw | 18(1.22) | 13.349 | 190.49 | 5.81(3.69 to 9.16) | Tremor | 4(1.38) | 4.961 | 9.062 | 5.12(1.88 to 13.89) | Thalamic infarction | 3(1.17) | 605.25 | 1148.26 | 617.58(187.05 to 2039.10) | |||||
| 23 | Skin discolouration | 17(1.15) | 8.797 | 108.778 | 19.57(12.22 to 31.34) | Hypoaesthesia | 4(1.38) | 4.459 | 7.613 | 4.59(1.69 to 12.47) | Vasospasm | 3(1.17) | 387.36 | 756.605 | 395.25(121.91 to 1281.43) | |||||
| 24 | Colitis ischaemic | 16(1.09) | 45.359 | 615.664 | 13.54(8.47 to 21.64) | Back pain | 4(1.38) | 4.015 | 6.348 | 4.13(1.52 to 11.22) | Acute coronary syndrome | 3(1.17) | 75.362 | 150.32 | 76.88(24.35 to 242.71) | |||||
| 25 | Syncope | 15(1.02) | 2.528 | 12.385 | 4.10(2.54 to 6.62) | Vascular malformation | 3(1.03) | 1429.085 | 2566.898 | 1470.28(428.57 to 5044.00) | Arteriospasm coronary | 3(1.17) | 64.346 | 127.858 | 65.64(20.81 to 207.02) | |||||
| 26 | Serotonin syndrome | 14(0.95) | 4.97 | 40.355 | 8.91(5.50 to 14.41) | Paranasal cyst | 3(1.03) | 1044.331 | 1946.11 | 1074.43(320.24 to 3604.76) | Reversible cerebral vasoconstriction syndrome | 3(1.17) | 59.229 | 117.401 | 60.42(19.16 to 190.47) | |||||
| 27 | Arteriospasm coronary | 13(0.88) | 35.714 | 387.403 | 45.95(27.68 to 76.29) | Acute myopia | 3(1.03) | 522.166 | 1023.799 | 537.20(165.14 to 1747.47) | Gastrointestinal pain | 3(1.17) | 45.465 | 89.19 | 46.37(14.73 to 146.02) | |||||
| 28 | Reversible cerebral vasoconstriction syndrome | 13(0.88) | 32.785 | 354.98 | 2.55(1.53 to 4.24) | Mastoiditis | 3(1.03) | 512.313 | 1005.429 | 527.06(162.12 to 1713.48) | Ischaemic stroke | 3(1.17) | 41.562 | 81.172 | 42.39(13.47 to 133.43) | |||||
| 29 | Joint stiffness | 13(0.88) | 9.917 | 94.427 | 5.02(2.96 to 8.51) | Otitis media | 3(1.03) | 195.342 | 393.527 | 200.95(63.01 to 640.88) | Affect lability | 3(1.17) | 39.607 | 77.152 | 40.40(12.84 to 127.13) | |||||
| 30 | Discomfort | 13(0.88) | 2.676 | 12.026 | 36.09(20.65 to 63.09) | Renal infarct | 3(1.03) | 176.316 | 355.459 | 181.37(56.94 to 577.77) | Hyperaesthesia | 3(1.17) | 34.96 | 67.588 | 35.65(11.33 to 112.17) | |||||
Fig. 2.
The names and numbers of the top 30 AEs with the highest percentage of 4 drug signals detected in the FAERS database and their corresponding SOCs
AE report stratified analysis by SOC and relationship between main AE signals detection and SOCs
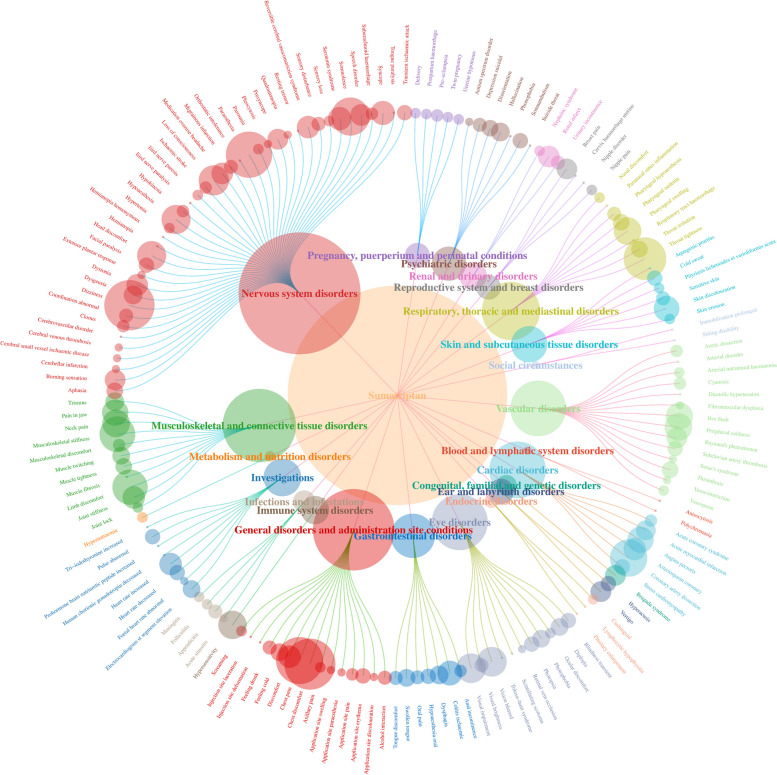
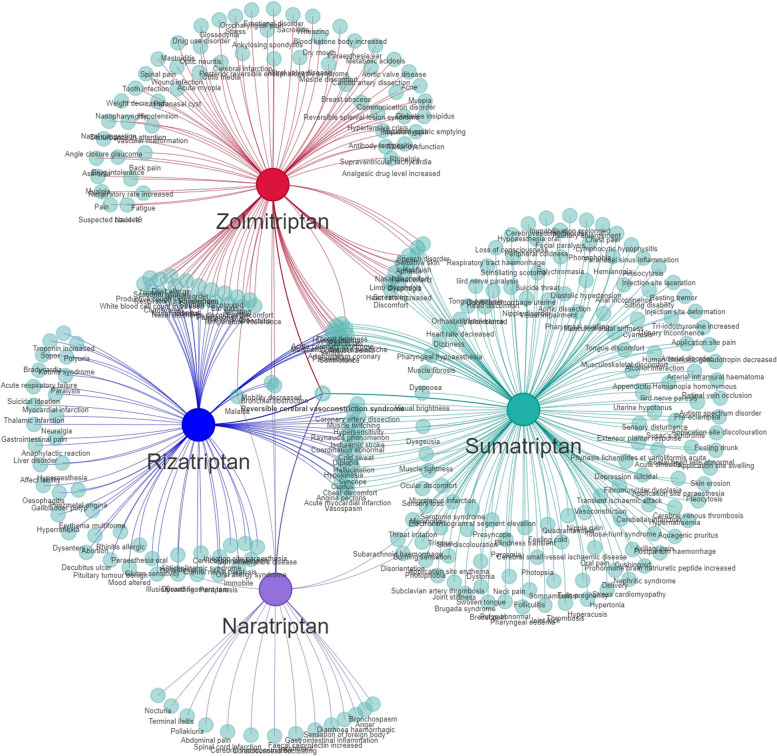
The AE signals of the four drugs were classified according to the SOC for the involved organs and systems using the MedDRA27.0. Additionally, a visual analysis was conducted to examine the PT signals and their corresponding SOCs. Our findings revealed that nervous system disorders constituted the primary SOC category for the four triptans within the FAERS database (Table 4; Fig. 3, Figure S1-S3).
Table 4.
Distribution of AE signals in each SOC
| SOC | Sumatriptan | Zolmitriptan | Rizatriptan | Naratriptan |
|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | |
| Nervous system disorders | 42(25.6) | 16(15.8) | 22(24.2) | 6(28.6) |
| General disorders and administration site conditions | 15(9.1) | 8(7.9) | 3(3.3) | 2(9.5) |
| Vascular disorders | 14(8.5) | 3(3.0) | 2(2.2) | 0(0) |
| Eye disorders | 11(6.7) | 4(4.0) | 1(1.1) | 0(0) |
| Musculoskeletal and connective tissue disorders | 11(6.7) | 9(8.9) | 4(4.4) | 1(4.8) |
| Respiratory, thoracic and mediastinal disorders | 9(5.5) | 14(13.9) | 10(11.0) | 2(9.5) |
| Investigations | 8(4.9) | 7(6.9) | 2(2.2) | 1(4.8) |
| Gastrointestinal disorders | 7(4.3) | 7(6.9) | 5(5.5) | 4(19.0) |
| Psychiatric disorders | 7(4.3) | 5(5.0) | 8(8.8) | 2(9.5) |
| Cardiac disorders | 6(3.7) | 6(5.9) | 10(11.0) | 0(0) |
| Skin and subcutaneous tissue disorders | 6(3.7) | 2(2.0) | 3(3.3) | 0(0) |
| Pregnancy, puerperium and perinatal conditions | 5(3.0) | 0(0) | 1(1.1) | 0(0) |
| Infections and infestations | 4(2.4) | 9(8.9) | 3(3.3) | 0(0) |
| Reproductive system and breast disorders | 4(2.4) | 0(0) | 1(1.1) | 0(0) |
| Endocrine disorders | 3(1.8) | 1(1.0) | 0(0) | 0(0) |
| Renal and urinary disorders | 3(1.8) | 1(1.0) | 1(1.1) | 2(9.5) |
| Blood and lymphatic system disorders | 2(1.2) | 0(0) | 0(0) | 0(0) |
| Ear and labyrinth disorders | 2(1.2) | 5(5.0) | 4(4.4) | 0(0) |
| Social circumstances | 2(1.2) | 0(0) | 1(1.1) | 0(0) |
| Congenital, familial and genetic disorders | 1(0.6) | 1(1.0) | 1(1.1) | 0(0) |
| Immune system disorders | 1(0.6) | 2(2.0) | 5(5.5) | 0(0) |
| Metabolism and nutrition disorders | 1(0.6) | 1(1.0) | 1(1.1) | 1(4.8) |
| Hepatobiliary disorders | 0(0) | 0(0) | 2(2.2) | 0(0) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 0(0) | 0(0) | 1(1.1) | 0(0) |
Fig. 3.
Distribution network plot of AE signals in each SOC for the Sumatriptan. The root node represents the name of the drug and the number of AE signals, with the SOC in the inner ring and the AE signal name in the outer ring
Subsequently, we concentrated on the ROR 95% confidence interval (CI) intensity of the signal among the top 30 most frequent AEs where the drug appeared (Table 3) for the purpose of forest plot visualisation analysis (Fig. 4). With regard to sumatriptan, the strongest signal (ROR = 222.69 (154.19 to 321.63)) was coronary artery dissection, which is classified as a cardiac disorder. The clinical use of sumatriptan has yielded a robust signal for the heart, which aligns with its contraindication in patients with preexisting cardiovascular disease and adverse cardiac effects. For zolmitriptan, the strongest signal was vascular malformation [ROR = 1470.28 (428.57 to 5044.00)]. The highest AE associated with rizatriptan was thalamic stroke no infarction (ROR = 617.58 [187.05 to 2039.10]), a neurological disorder. This was followed by vasospasm (ROR = 395.25 [121.91 to 1281.43]) and coronary artery dissection (ROR = 240.14 [104.65 to 551.01]). This indicates that it can cause a range of neurological disorders, as well as the possibility of cardio-cerebral adverse effects. Naratriptan has been associated with a range of neurological disorders, with the highest incidence being spinal cord infarction (ROR = 2980.03 (375.50 to 23649.77)). It is speculated that cell death may be caused by ischaemia in the spinal cord due to excessive vasoconstriction of the blood vessels. Additionally, it has been linked to adverse reactions affecting the gastrointestinal system. Still awaiting further confirmation. The main AEs of the drugs are related to cardiac disorders, nervous system disorders, gastrointestinal disorders and respiratory system, and patients suffering from related system disorders should be closely monitored for adverse effects during the clinical use of triptans.
Fig. 4.
Signal detection results of AE reports and ROR (95%CI)
Comparison of SOCs
A comparative analysis was conducted to evaluate the AE signals associated with the four triptans. According to the FAERS database, sumatriptan exhibited the highest frequency of positive AE signals among the four triptans. A total of one AE was reported for all four drugs, namely reversible cerebral vasoconstriction syndrome, a disorder of the nervous system (Fig. 5). Consistent with the presence of adverse events in the drug insert. This comparison elucidates the interrelated nature of some of the AEs associated with these drugs, while also underscoring the distinctive attributes of each drug. It thus offers a comprehensive and nuanced perspective on their safety in clinical settings.
Fig. 5.
Network Venn diagrams of PT-positive signals in the FAERS database for the four triptans
Discussion
The introduction of triptan medication has broadened the spectrum of potential migraine treatments, offering a greater array of options for clinicians and patients alike. In addition to evaluating the efficacy of these drugs, it is imperative to ensure their safety. This study explored the risk status of its AEs from the perspective of signalling risk by accessing triptan data from the FAERS database and performing signal mining. By undertaking a comparative and analytical examination of the AEs of sumatriptan, zolmitriptan, rizatriptan, and naratriptan as reported in the FAERS database, this study offers a comprehensive understanding of the similarities and differences in the safety profiles of these four drugs in common, novel, and rare AEs. In clinical practice, AEs associated with triptans affect multiple organ systems, including the neurological, gastrointestinal, and cardiac systems. Our data mining process identified all of the AEs listed in the drug’s package insert. The four drugs share an AE, which is Reversible cerebral vasoconstriction syndrome.
The initial triptan developed by GlaxoSmithKline ® and introduced to the market in 1991 was sumatriptan (GR43175). While it demonstrated efficacy in the acute treatment of migraine, particularly when administered parenterally, the low oral bioavailability of sumatriptan prompted the development of a second generation of triptans [28]. In the present study, the highest number of AEs was reported for the first generation of sumatriptan. This may be attributed to the fact that it is the earliest triptans to be introduced on the market and therefore more widely used in clinical practice. With regard to age and gender, the data revealed a higher incidence of AEs in female patients. This finding may be attributable to the elevated prevalence of migraine in women, which may result in a greater utilization of triptans in women in clinical practice. This finding indicates that, in the future development of new drugs, consideration could be given to the creation of a new generation of drugs for different genders and age groups. For example, a formulation of the triptans class specifically targeting premenstrual migraine in women could be developed and could be combined with the mechanism of action of non-steroidal anti-inflammatory drugs (NSAIDs) with a view to reducing the incidence of associated AEs.
In the course of our study, AEs associated with triptans were observed to affect multiple organ systems, including the nervous system and the heart, in actual clinical practice. In the context of cardiac disorders and cardiovascular disease AEs, clinical trials and drug inserts for triptans indicate that the use of this medication is contraindicated in patients with risk factors for cardiovascular disease and the cardiac system. These risk factors include, but are not limited to, the following: ischemic coronary artery disease (e.g., angina pectoris, history of myocardial infarction) and a history of stroke or transient ischemic attack [29–31]. Our study also confirms that sumatriptan may lead to adverse events such as Coronary artery dissection (n = 37), Angina pectoris (n = 18), Arteriospasm coronary (n = 13), rizatriptan may lead to Myocardial infarction (n = 5) and zolmitriptan may lead to Palpitations (n = 4). The aforementioned data collectively indicate the presence of a potential risk factor for cardiac disease in the context of triptans utilized in actual clinical practice. Triptan has vasoconstrictive properties and exerts its pharmacological effects mainly by acting on the 5-HT receptor. The affinity for different subtypes of 5-HT receptors varies among the different triptan analogues. In the cerebral vasculature, some triptans selectively constrict dilated intracranial blood vessels, thereby relieving migraine symptoms. However, in the peripheral vasculature, such as the coronary arteries, their effects are more complex [10, 32, 33]. Clinical trials and research reports have identified the potential effects of triptan on cardiac function. In a small number of cases, cardiovascular ischaemic events ( myocardial infarction, cerebral vasoconstriction ), as well as other symptoms including palpitations and arrhythmias, have been reported. However, the incidence of these events is relatively low [34–37]. It has been postulated that Tretinoin may also induce related cardiac adverse effects via its influence on neurotransmitter release and vascular endothelial function, among other mechanisms. Further investigation is required to elucidate the precise mechanism of action. The data presented herein underscore the potential risks associated with triptans in individuals with pre-existing cardiac disorders. Consequently, it is imperative that future drug development prioritise the creation of more selective and relevant analogues, with the aim of reducing vascular-related AEs.
Given the expression of 5-HT1B and 5-HT1D receptors in multiple regions of the central nervous system (CNS), it is reasonable to hypothesise that triptan may induce adverse central effects, which may depend on lipid solubility [38]. The present study identified nervous system disorders as the primary SOC category for four drugs in the FAERS database. Additionally, it was determined that the AE “reversible cerebral vasoconstriction syndrome,” which is common to all four drugs, also belongs to the Nervous system disorders category. It is hypothesised that the lipid solubility of some triptans and the disruption of the blood-brain barrier during a migraine attack may facilitate the penetration of anti-migraine drugs into the CNS [39, 40]. It is particularly important in the future to update the new generation of tretinoin based on pharmacological mechanisms. A further avenue for investigation is the potential rational adjustment of the lipid solubility of the new generation of triptans, without compromising their pharmacological efficacy or pharmacokinetics, or whether 5-HT should be highly selected for the treatment of migraine through targeting.
We also observed that the first 30 most frequent and highest signal intensity detections for all four drugs involved gastrointestinal disorders. The AEs with the highest frequency associated with zolmitriptan, rizatriptan, and naratriptan medications are all classified as gastrointestinal disorders. It has been reported that triptans may cause gastrointestinal complications, including ischemic colitis, a finding that was also confirmed in our study [41]. Respiratory, thoracic and mediastinal disorders ( dyspnoea, chest pain, etc.) had the first frequency of sumatriptan, which is consistent with AE reports from previous studies [40]. AEs for gastrointestinal disorders are a common occurrence in a diverse range of pharmaceutical agents employed for the management of pain, including NSAIDs such as ibuprofen and acetylsalicylic acid, as well as opioids [42, 43]. In clinical practice, a significant proportion of patients express concern that oral medications may cause damage to the gastrointestinal tract, which can lead to AEs such as nausea and vomiting. In the case of triptans for migraine, AEs on the gastrointestinal system have also been observed, which provides a valuable reference point for clinical use. It may be advisable for patients with digestive disorders to be administered drugs with a lesser propensity for side effects or the early development of new dosage forms that do not need to be absorbed through the gastrointestinal tract.
The results of a recent meta-analysis have demonstrated that four of the tretinoin analogues (sumatriptan, zolmitriptan, rizatriptan, and eletriptan) are more efficacious in the treatment of migraines than the recently marketed and more expensive drugs lasmiditan, rimegepant and ubrogepant. Consequently, these four tretinoin analogues should be considered as the drugs of choice for the treatment of migraines [44]. Previous studies have explored the adverse effects associated with gepants, which are used for the prophylactic and acute treatment of migraine, and which have a different mechanism of action than triptans. Researchers have also identified AEs related to the gastrointestinal system and have shown that it is more suitable for patients with a history of cardiovascular disease compared to triptans cardiovascular side effect risk, however comparative and real-world studies between triptans in recent years remain unexplored [45]. The safety and efficacy of migraine treatment are contingent upon the characteristics of the drug in question, as well as the patient’s specific circumstances, including the use of multiple medications. Consequently, further investigation into the adverse effects associated with the triptans is currently a priority. Our study addresses a current gap in the literature by analysing AE signals at multiple levels. The findings can be used to inform treatment choices and facilitate informed decision-making between patients and clinicians. Future research should focus on a detailed characterisation of the specific mechanisms of action of 5-HT receptors in migraine and other related pain conditions. Additionally, efforts should be made to minimise potential side effects in response to current real-world adverse effects, and the development of more specific drugs to optimise migraine treatment strategies by precisely targeting therapy should be a priority.
Limitations
Limitations of this study include the fact that FAERS is a self-reporting database in the U.S., which has limitations such as incomplete reporting information and uncertainty about the causal relationship between reported events and medications. Nevertheless, the results of this comprehensive data mining exercise offer guidance on the safe and rational use of medications. It is important to note that all signal detection results are only indicative of statistical associations. They do not determine prevalence or confirm causality. Consequently, future research should build on the AE signals identified in this paper and conduct further high-quality studies to elucidate the prevalence of these AEs in real-world use scenarios. Further evaluation is required to ascertain whether there are clear causal associations.
Conclusion
This study employed the FAERS database to examine the potential AEs of representative treprostinil analogues utilized for the treatment of migraine. Analyses have demonstrated that AE is present in a range of systems, including those pertaining to the cardiac, nervous, gastrointestinal, and musculoskeletal and connective tissue disorders. The clinical significance of this warrants careful consideration. It is recommended that future studies should aim to develop a new generation of highly selective analogues based on the possible mechanisms underlying the generation of these AEs, with a view to developing targeted therapies to reduce patient suffering.
Supplementary Information
Acknowledgements
We sincerely thank the researchers, including clinicians, patients, and regulatory agencies, who contributed to the FAERS data.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- AEs
Adverse events
- FDA
Food and Drug Administration
- FAERS
U.S. Food and Drug Administration Adverse Event Reporting System
- PRR
Proportional reporting ratio
- ROR
Reporting odds ratio
- BCPNN
Bayesian Confidence Propagation Neural Network
- PT
Preferred terminology
- SOC
System organ classification
- NSAIDs
Non-steroidal anti-inflammatory drugs
- CNS
Central nervous system
Authors' contributions
In this study, WL: Data curation, Funding acquisition, Project administration, Resources, Investigation, Supervision, Writing review & editing. HH: Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. CL: Conceptualization, Investigation, Visualization, Writing – original draft. QS and CL: Conceptualization, Formal analysis, Methodology, Project administration, Writing – review & editing. AL、YL and YZ: Formal analysis, Methodology, Visualization, Writing – review & editing. BF and PM: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft.
Funding
This work was supported by Capital Health Development Research Special Project (2022-1-4061) and National High Level Hospital Clinical Research Funding (2024-NHLHCRF-PYII-22).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peng Mao, Email: doctormaopeng@126.com.
Bi-Fa Fan, Email: fbf1616@yeah.net.
References
- 1.Silberstein SD (2004) Migraine. Lancet 363(9406):381–391. 10.1016/s0140-6736(04)15440-8 [DOI] [PubMed] [Google Scholar]
- 2.Tam ACT, Naik H, Trenaman L, Lynd L, Zhang W (2024) Health-related quality of life among women and men living with migraine: a Canada-wide cross-sectional study. J Headache Pain 25(1):170. 10.1186/s10194-024-01882-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashina M, Katsarava Z, Do TP et al (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495. 10.1016/s0140-6736(20)32160-7 [DOI] [PubMed] [Google Scholar]
- 4.Global regional, national burden of neurological disorders 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480. 10.1016/s1474-4422(18)30499-x [DOI] [PMC free article] [PubMed]
- 5.Steiner TJ, Stovner LJ, Vos T (2016) GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain 17(1):104. 10.1186/s10194-016-0699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(4):344–381. 10.1016/s1474-4422(24)00038-3 [DOI] [PMC free article] [PubMed]
- 7.Labastida-Ramírez A, Rubio-Beltrán E, Haanes KA et al (2020) Lasmiditan inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 161(5):1092–1099. 10.1097/j.pain.0000000000001801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey PP, Feniuk W (1991) Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol Sci 12(12):444–446. 10.1016/0165-6147(91)90630-b [DOI] [PubMed] [Google Scholar]
- 9.Humphrey PP, Feniuk W, Perren MJ, Beresford IJ, Skingle M, Whalley ET (1990) Serotonin and migraine. Ann N Y Acad Sci 600:587–98; discussion 598-600. 10.1111/j.1749-6632.1990.tb16912.x [DOI] [PubMed] [Google Scholar]
- 10.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB (2002) Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 22(8):633–658. 10.1046/j.1468-2982.2002.00404.x [DOI] [PubMed] [Google Scholar]
- 11.Sacco S, Lampl C, Amin FM et al (2022) European Headache Federation (EHF) consensus on the definition of effective treatment of a migraine attack and of triptan failure. J Headache Pain 23(1):133. 10.1186/s10194-022-01502-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingram EE, Bocklud BE, Corley SC et al (2023) Non-CGRP Antagonist/Non-Triptan options for Migraine Disease Treatment: clinical considerations. Curr Pain Headache Rep 27(10):497–502. 10.1007/s11916-023-01151-0 [DOI] [PubMed] [Google Scholar]
- 13.Snow V, Weiss K, Wall EM, Mottur-Pilson C (2002) Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med 137(10):840–849. 10.7326/0003-4819-137-10-200211190-00014 [DOI] [PubMed] [Google Scholar]
- 14.Cameron C, Kelly S, Hsieh SC et al (2015) Triptans in the Acute treatment of migraine: a systematic review and network Meta-analysis. Headache 55(Suppl 4):221–235. 10.1111/head.12601 [DOI] [PubMed] [Google Scholar]
- 15.Zebenholzer K, Gall W, Gleiss A, Pavelic AR, Wöber C (2022) Triptans and vascular comorbidity in persons over fifty: findings from a nationwide insurance database - A cohort study. Headache 62(5):604–612. 10.1111/head.14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass-Hout TA, Xu Z, Mohebbi M et al (2016) OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inf Assoc 23(3):596–600. 10.1093/jamia/ocv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberto G, Piccinni C, D’Alessandro R, Poluzzi E (2014) Triptans and serious adverse vascular events: data mining of the FDA adverse event reporting System database. Cephalalgia 34(1):5–13. 10.1177/0333102413499649 [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Varghese Gupta S, Bhatt P et al (2024) Cardiovascular adverse events associated with triptans for treatment of migraine: a pharmacovigilance study of the FDA adverse event reporting system (FAERS). Can J Physiol Pharmacol. 10.1139/cjpp-2024-0117 [DOI] [PubMed]
- 19.Wei L, Tian Y, Chen X et al (2023) Data mining and analysis for emicizumab adverse event signals based on the Food and Drug Administration adverse event reporting System database. Int J Clin Pharm 45(3):622–629. 10.1007/s11096-022-01514-4 [DOI] [PubMed] [Google Scholar]
- 20.Böhm R, Höcker J, Cascorbi I, Herdegen T (2012) OpenVigil–free eyeballs on AERS pharmacovigilance data. Nat Biotechnol 30(2):137–138. 10.1038/nbt.2113 [DOI] [PubMed] [Google Scholar]
- 21.Simon TA, Simon JH, Heaning EG, Gomez-Caminero A, Marcu JP (2023) Delta-8, a Cannabis-Derived Tetrahydrocannabinol Isomer: evaluating Case Report Data in the Food and Drug Administration adverse event reporting system (FAERS) database. Drug Healthc Patient Saf 15:25–38. 10.2147/dhps.S391857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noguchi Y, Tachi T, Teramachi H (2021) Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform 22(6). 10.1093/bib/bbab347 [DOI] [PubMed]
- 23.Evans SJ, Waller PC, Davis S (2001) Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 10(6):483–486. 10.1002/pds.677 [DOI] [PubMed] [Google Scholar]
- 24.Noguchi Y, Tachi T, Teramachi H (2020) Comparison of Signal Detection Algorithms based on frequency statistical model for drug-drug Interaction using spontaneous Reporting systems. Pharm Res 37(5):86. 10.1007/s11095-020-02801-3 [DOI] [PubMed] [Google Scholar]
- 25.Robert M, Jouanjus E, Khouri C, Fouilhé Sam-Laï N, Revol B (2023) The opioid epidemic: a worldwide exploratory study using the WHO pharmacovigilance database. Addiction 118(4):771–775. 10.1111/add.16081 [DOI] [PubMed] [Google Scholar]
- 26.Shu Y, Zhang Q, He X, Liu Y, Wu P, Chen L (2022) Fluoroquinolone-associated suspected tendonitis and tendon rupture: a pharmacovigilance analysis from 2016 to 2021 based on the FAERS database. Front Pharmacol 13:990241. 10.3389/fphar.2022.990241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang D, Chen M, Huang X et al (2023) SRplot: a free online platform for data visualization and graphing. PLoS One 18(11):e0294236. 10.1371/journal.pone.0294236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalón CM, Centurión D, Valdivia LF, de Vries P, Saxena PR (2003) Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol 1(1):71–84. 10.2174/1570161033386826 [DOI] [PubMed] [Google Scholar]
- 29.Vanmolkot FH, de Hoon JN (2006) Acute effects of sumatriptan on aortic blood pressure, stiffness, and pressure waveform. Clin Pharmacol Ther Jul 80(1):85–94. 10.1016/j.clpt.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 30.Dodick DW, Shewale AS, Lipton RB et al (2020) Migraine patients with Cardiovascular Disease and contraindications: an analysis of real-world Claims Data. J Prim Care Community Health 11:2150132720963680. 10.1177/2150132720963680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diener HC (2020) The risks or Lack Thereof of Migraine treatments in Vascular Disease. Headache 60(3):649–653. 10.1111/head.13749 [DOI] [PubMed] [Google Scholar]
- 32.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ (2001) Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 358(9294):1668–1675. 10.1016/s0140-6736(01)06711-3 [DOI] [PubMed] [Google Scholar]
- 33.MaassenVanDenBrink A, Reekers M, Bax WA, Ferrari MD, Saxena PR (1998) Coronary side-effect potential of current and prospective antimigraine drugs. Circulation. 98(1):25–30. 10.1161/01.cir.98.1.25 [DOI] [PubMed] [Google Scholar]
- 34.Somers-Edgar TJ, Shah J, Kueh A, Kasargod Prabhakar C (2023) Triptan-induced takotsubo syndrome: a case report. Eur Heart J Case Rep 7(8):ytad221. 10.1093/ehjcr/ytad221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen CL, Hougaard A, Gaist D, Hallas J (2024) Risk of stroke and myocardial infarction among initiators of triptans. JAMA Neurol 1(3):248–254. 10.1001/jamaneurol.2023.5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor P, Gladstone P (1995) Oral sumatriptan-associated transmural myocardial infarction. Neurology 45(12):2274–2276. 10.1212/wnl.45.12.2274 [DOI] [PubMed] [Google Scholar]
- 37.Ottervanger JP, Paalman HJ, Boxma GL, Stricker BH (1993) Transmural myocardial infarction with sumatriptan. Lancet 341(8849):861–862. 10.1016/0140-6736(93)93064-8 [DOI] [PubMed] [Google Scholar]
- 38.Lucaites VL, Krushinski JH, Schaus JM, Audia JE, Nelson DL (2005) [3H]LY334370, a novel radioligand for the 5-HT1F receptor. II. Autoradiographic localization in rat, guinea pig, monkey and human brain. Naunyn Schmiedebergs Arch Pharmacol 371(3):178–184. 10.1007/s00210-005-1036-8 [DOI] [PubMed] [Google Scholar]
- 39.Hoskin KL, Goadsby PJ (1998) Comparison of more and less lipophilic serotonin (5HT1B/1D) agonists in a model of trigeminovascular nociception in cat. Exp Neurol 150(1):45–51. 10.1006/exnr.1997.6749 [DOI] [PubMed] [Google Scholar]
- 40.González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM (2018) Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol 14(1):25–41. 10.1080/17425255.2018.1416097 [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TQ, Lewis JH (2014) Sumatriptan-associated ischemic colitis: case report and review of the literature and FAERS. Drug Saf 37(2):109–121. 10.1007/s40264-013-0134-7 [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Zhang X, Chen M, Wang Y (2023) A real-world data analysis of acetylsalicylic acid in FDA adverse event reporting System (FAERS) database. Expert Opin Drug Metab Toxicol 19(6):381–387. 10.1080/17425255.2023.2235267 [DOI] [PubMed] [Google Scholar]
- 43.Le H, Hong H, Ge W et al (2023) A systematic analysis and data mining of opioid-related adverse events submitted to the FAERS database. Exp Biol Med (Maywood) 248(21):1944–1951. 10.1177/15353702231211860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsson WK, Ostinelli EG, Zhuang ZA et al (2024) Comparative effects of drug interventions for the acute management of migraine episodes in adults: systematic review and network meta-analysis. BMJ 386:e080107. 10.1136/bmj-2024-080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Q, Liao X, Wu H, Huang Y, Liang T, Li H (2024) Real-world study of adverse events associated with gepant use in migraine treatment based on the VigiAccess and U.S. Food and Drug Administration’s adverse event reporting system databases. Front Pharmacol 15:1431562. 10.3389/fphar.2024.1431562 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.