Abstract
The purpose of this study was to investigate the effects of elevated core temperature by exposure to heat stress vs. heat exposure without elevated core temperature (mean skin temperature only) in addition to mental fatigue on aerobic exercise capacity in the heat. Seven highly trained athletes completed two experimental conditions: elevation in core and skin temperatures (hyperthermia: HYP), and skin temperatures (SKIN). Participants performed the AX-Continuous Performance Task and Stroop Task to induce mental fatigue during a warm water immersion at 40 °C (HYP) and a passive seated heat exposure in a climatic chamber at 35 °C and 60% relative humidity (SKIN) for 45 min before exercise. Thereafter, participants performed running trial at 80% maximal oxygen uptake until voluntary exhaustion in the same chamber as the SKIN. Exercise time to exhaustion was significantly shorter in the HYP trial (538 ± 200 s) than in the SKIN trial (757 ± 324 s). Rectal temperature at the end of tasks in the HYP trial increased by 0.86 ± 0.26℃ and was significantly higher (37.69 ± 0.18℃) than that of the SKIN trial (36.96 ± 0.13℃), albeit no significant differences in mean skin temperature. Self-reported mental fatigue using visual analog scale was significantly higher after tasks in both trials, but no significant difference between trials was found. Throughout the trial, salivary cortisol concentration and perceptual responses were not affected by hyperthermia. This study demonstrated that a combination of high core temperature and mean skin temperature, and mental fatigue limit aerobic exercise capacity in highly trained athletes in hot environments compared with heat exposure without an elevation of core temperature.
Introduction
Sports competitive events are often held in hot climate conditions, imposing additional stress on athletes and resulting in high body core temperature (Tc) [1, 2]. Previous studies have reported that the attainment of a high Tc is the main limiting factor inhibiting exercise performance, as evidenced by a reduced central nervous system drive to the skeletal muscle and other adverse effects, including cardiovascular strain and metabolic disturbances [3, 4]. During endurance exercise in a hot environment, high mean skin temperature (Tsk) above 35℃ are also known to cause early fatigue [5]. Therefore, the development of hyperthermia is associated with limitations to aerobic exercise performance. In addition, it has been noted that when external environmental factors, such as radiant heat [6] or air velocity [7], are combined with ambient temperature and relative humidity, the additive and synergistic effects further impair thermoregulatory responses and aerobic exercise capacity.
Regarding the effect of internal environmental factors such as psychological state on exercise-induced fatigue, it is known that prolonged mental exertion leading to mental fatigue reduces endurance exercise performance. Mental exertion refers to the engagement with a demanding cognitive task such as the AX-continuous performance task (AX-CPT) or Stroop task [8–10]. Marcora et al. [8] showed that performing 90-min cognitive task using AX-CPT in 18–22 ℃ air temperature impaired subsequent exercise tolerance. Smith et al. [10] investigated the effects of mental fatigue on soccer-specific physical and technical performance. Their findings demonstrated that the performance of a 30-min modified Stroop task prior to a Yo-Yo intermittent recovery test resulted in a reduction in running distance compared to the control trial. Barzegarpoor et al. [11] showed that performing a 45-min Stroop task during submaximal cycling exercise increased the rating of perceived exertion (RPE), but the physiological responses to exercise were unaffected by mental fatigue. In response to these results, a recent review of previous studies indicates that higher RPE induced by mental fatigue during a certain task may reduce endurance exercise performance [12, 13]. Furthermore, the elevation of RPE is observed more frequently in hot than in thermoneutral conditions, leading to reduced endurance exercise performance [14].
Recently, the combined effects of mental fatigue from a prolonged cognitive task and exposure to heat stress have been investigated as a possible additional negative effect [13]. Otani et al. [15] demonstrated that heat stress-induced increase in core and skin temperatures, using warm water immersion during cognitive tasks, reduced subsequent aerobic exercise capacity compared to mental fatigue alone. Van Cutsem et al. [16] reported that mild mental fatigue caused by a cognitive task performed disappears after 30 min, and subsequent self-paced cycling effort at 30℃ did not affect time trial performance. The results of these two studies suggest that the combined negative effects on endurance exercise performance are likely to be due to the degree of intensity of heat stress and mental fatigue. In the focus on exposure to heat stress, it is not clear whether the effects of elevation of Tc and Tsk (hyperthermia) or heat exposure without elevation of Tc (Tsk only) during a cognitive task performed induce impairment of exercise capacity. It is possible that simultaneous exposure to mental fatigue and heat exposure that increases Tc would attenuate aerobic exercise capacity. The purpose of this study was to investigate the effects of elevated core temperature by exposure to heat stress vs. heat exposure without elevated core temperature (Tsk only) in addition to mental fatigue on aerobic exercise capacity in the heat. We hypothesized that aerobic exercise capacity in hot environments would be impaired by a combination of high Tc and Tsk, and mental fatigue compared with high Tsk and mental fatigue.
Methods
Participants
Eight non-heat-acclimatized highly trained male athletes were recruited for this study. However, one participant dropped out due to heat-related illness in the experimental trial. Therefore, seven non-heat-acclimatized highly trained male athletes (age: 22 ± 1 years, height: 173.2 ± 2.4 cm, body mass [BM]: 67.11 ± 5.2 kg, maximal oxygen uptake [●VO2max]: 63.7 ± 7.1 mL/kg/min) were participated for this study. They were categorized according to “tier 3” as highly trained by a previous study [17]. All the participants were non-smokers and normotensive, had no known autonomic dysfunction or cardiovascular disease, and were not taking any medications. The study protocol was approved by the National Institute of Fitness and Sports in Kanoya Ethics Committee (Permission number: 22–1–14), and all participants provided their informed consent to participate prior to commencing the study. The study complied with the latest version of the Declaration of Helsinki and was conducted according to international standards.
Preliminary measurements
In order to determine the ●VO2max, on their first visit to the laboratory, each participant performed a progressive exercise test by treadmill (h/p/cosmos para graphics; h/p/cosmos, Germany) at room temperature (25 °C and 50% relative humidity [RH]). Their height and BM were measured to the nearest 0.1 cm and 10 g, respectively (TBF-210; Tanita, Japan). The protocol consisted of progressive exercise beginning at 8 km/h for 3 min, followed by increments of 2 km/h every 3 min until 16 km/h. And then speed was increased by 2 km/h every 2 min until volitional exhaustion [18]. Respiratory gases were measured every 30 s during the test using a pre-calibrated automatic gas analyzer (AE-310 s; Minato Medical Science, Japan). Heart rate (HR) was monitored continuously via telemetry using an HR monitor (RC3 GPS, Polar, Finland). The test was considered to be valid if two of the following three criteria were met: 1) oxygen consumption reached a plateau, 2) HR remained within 5% of the predicted maximum (220–age), or 3) the respiratory exchange ratio was above 1.10 [18].
Experimental protocol
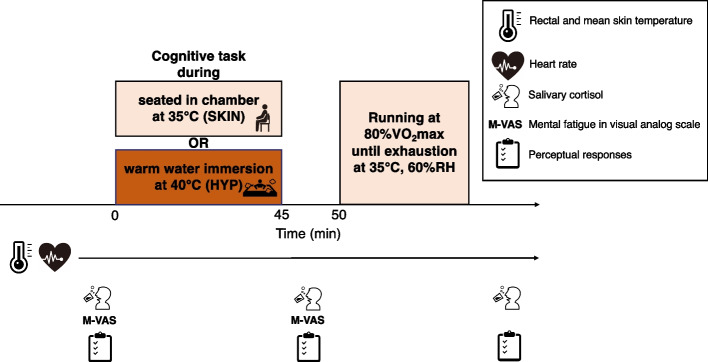
The participants completed two trials in a randomized design: one with elevation in core and skin temperatures (hyperthermia: HYP), and another with just skin temperature (SKIN). All experimental trials were separated by at least 2 days to avoid an order effect and commenced at the same time to control for circadian variations in the core temperature. To avoid the influence of natural heat acclimatization all trials took place during the winter season. Throughout the study period, all participants were asked to keep their normal lifestyle activities at a stable level, including their physical activity and nutritional habits. During the 24-h period before the experimental trial, the participants were instructed to refrain from strenuous exercise and the consumption of alcohol, caffeine, and nutritional supplements. Each participant arrived at the laboratory after an overnight fast and refraining drinking any type of beverage for 2 h. Upon arrival at the laboratory, the participants had urine samples collected and were weighed. The participants were dressed in shorts and athletic shoes in all trials. The intrinsic clothing insulation value for this ensemble was 0.08 clo. [19] The participants were fitted with measuring equipment and asked to perform cognitive tasks, including the AX-CPT and Stroop task, for 45 min in order to induce mental fatigue. This was carried out while they were either immersed in warm water at 40 °C (HYP) or seated in a chamber at 35 °C with 60% RH (SKIN) for the same duration. Participants in the HYP trial were immersed to the upper chest at a seated posture in bathtub (1.5 × 1.0 × 0.5 m). The participants' perceptual responses and saliva samples were collected before and after the 45-min cognitive task. During the HYP trial, participants moved to the climatic chamber and performed the running exercise at an intensity equivalent to 80%●VO2max until reaching voluntary exhaustion (TTE) in the same hot environment as the SKIN trial. After the exercise, the participants dried themselves with a towel and were weighed again to determine their BM before the collection of saliva and urine samples (Fig. 1).
Fig. 1.
Schematic representation of the experimental protocol
Cognitive task
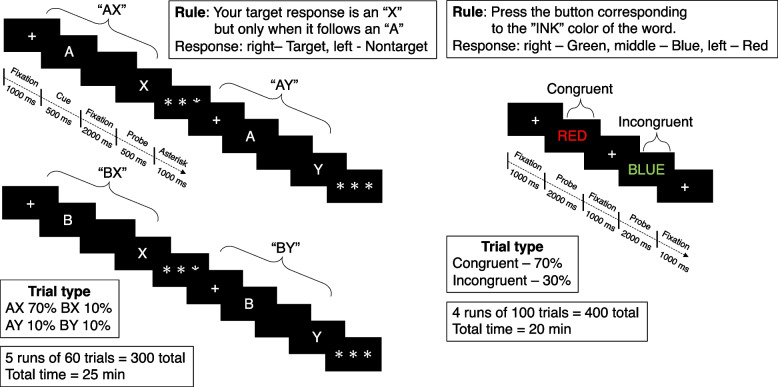
The 45-min task used as experimental manipulation in the present study is similar to those used by Lesh et al. [20]. Previous studies have demonstrated that the execution of the Stroop task for a duration of 30 to 45 min results in the induction of mental fatigue in physically active subjects [21, 22]. Recently, a previous study observed that the AX-CPT or Stroop task for a duration of 10 min also caused mental fatigue [23]. It is notable that the longer the task, the greater the mental fatigue. Accordingly, we attempted to enhance the mental fatigue for highly trained athletes by employing the 25-min AX-CPT (300 trials) and the 20-min Stroop task (400 trials), when the combination of fixation, cue and probe times for each task were taken into consideration. Task parameters and visual depictions of the AX-CPT and the Stroop task are presented in Fig. 2. Briefly, in the AX-CPT, participants make a target response (index finger button press) to the probe letter X only if it was preceded by the cue letter A. All cues and non-target probes require non-target responses (middle finger button press). Target sequence trials are frequent and set up a prepotent tendency to make a target response when the probe letter X occurs. As a result, non-target sequence trials where any non-A cue (collectively called B-cues) is presented and followed by a probe letter X, require the most cognitive control. The frequency of occurrence was 70% AX, 10% AY, 10% BX, and 10% BY, respectively.
Fig. 2.
Schematic representation of the AX-CPT (A) and Stroop tasks (B) protocol
In the Stroop task, stimuli consisted of one of three color words (RED, GREEN, and BLUE) that were written in one of three color inks (red, green, and blue). Stimuli could be Congruent (word and ink match) or Incongruent (word and ink do NOT match). Participants respond with a button press corresponding to the color of the ink of the word. The trials were arranged in pseudorandom sequence with 70% of trials being congruent (matched word and color), whereas 30% were incongruent, with all incongruent word–color combinations being equally common. Participants were instructed to respond as quickly and accurately as possible in both tasks. Cognitive task performance was assessed reaction time, lapses of attention, and accuracy by standard psychology software (Multi-PAS2, DKH, Japan). Attention lapses correspond to the number of trials with a reaction time longer than 2000 ms [24]. Response accuracy was the proportion of correct responses. The state of mental fatigue was characterized by an increase in reaction time, an increase in lapses of attention, and/or a decrease in accuracy [24]. A comparison of cognitive task performance was conducted between the initial and concluding 5 min of each task.
Physiological and psychological measurements
Hydration status and body mass
Urine specific gravity (USG) was measured using a refractometer (PAL-09S, Atago, Japan) before and after exercise. Nude BM to the nearest 10 g was measured using a weighing scale (BC-316, Tanita, Japan) before and after the trial. Total sweating loss (TSL) was calculated using the following formula: TSL = BM before the trial – BM after the trial. Dehydration level was calculated using the following formula: Dehydration level = TSL/ BM before the trial × 100) [25].
Thermoregulatory responses
Throughout the two trials, Tre was obtained using a disposable rectal thermistor (ITP010-11, Nikkiso-Therm, Japan) that was self-inserted approximately 150 mm into the rectum. HR was monitored continuously via telemetry using an HR monitor (H10, Polar, USA) affixed to the chest. Four skin temperatures (chest, forearm, thigh, and calf) were also recorded via iButtons® (Thermocron SL type; KN Laboratory, Japan) affixed using hypoallergenic polyacrylate adhesive tape. The Tsk was calculated using the following formula from Ramanathan [26]: . The core-to-skin temperature gradient was calculated using the following formula: Core-to-skin temperature gradient = Tre – Tsk.
Salivary cortisol
Saliva samples were collected as described previously both before and after the 45-min task and after exercise [27]. Participants sat and rinsed their mouths with distilled water for 30 s three times and then rested for 5 min. The participants were asked to expectorate their saliva through a short plastic straw into a collection vial. Subsequently, the saliva samples were frozen at − 80 °C and stored until the end of the study period. Cortisol concentration was measured using enzyme immunoassay kits (Salimetrics, Carlsbad, CA) according to the manufacturer’s protocol.
Perceptual scales
Self-reported mental fatigue was measured using a visual analog scale (M-VAS) before and after the 45-min task. Participants were asked to indicate their perceived level of mental fatigue (from not all to completely exhausted) by placing a mark on a 100-mm line [28].
Subjective thermal sensation (TS; 9-point scale ranging from 1 = “very cold” to 9 = “very hot”) [29] and comfort (TC; 7-point scale ranging from 1 = “very uncomfortable” to 7 = “very comfortable”) [30] were recorded at before and after both the 45-min task and TTE. The RPE (15-point scale) [31] was recorded before and after the TTE.
Statistical analysis
Descriptive data were presented as means ± standard deviations. All statistical computations were performed using the IBM SPSS Statistics 28 software package (SPSS, Inc., USA). The normality of the data and homogeneity of variance between the trials were tested using Shapiro–Wilk’s and Levene’s tests, respectively. When the result of Shapiro–Wilk’s test or Levene’s test was less than the significant level, data were analyzed using non-parametric tests. Non-parametric data were analyzed using Friedman’s two-way analysis of variance (perceptual scales). When a significant difference was found, the pairwise comparisons were tested using Wilcoxon’s signed-rank test. In all other cases, a two-way (trial × time) repeated-measures analysis of variance was performed to compare the data for the different experimental conditions. Where appropriate, a paired sample t-test was then used to identify any changes. The TTE, TSL, and dehydration levels were compared using the dependent samples t-test. The level of significance was set at p < 0.05. Cohen’s d (d) was used as a measure of effect size for paired samples, with 0.2 to < 0.6, ≥ 0.6 to < 1.2, ≥ 1.2 to < 2.0, ≥ 2.0 to < 4.0, and ≥ 4.0 representing small, moderate, large, very large and extremely large treatment effects, respectively [32].
Results
Time to exhaustion
All participants ran for a shorter time in the HYP trial than in the SKIN trial (p = 0.028, d = 1.12). The mean exercise time to exhaustion was 538 ± 200 s for the HYP trial, and 757 ± 324 s for the SKIN trial, respectively (Fig. 3).
Fig. 3.
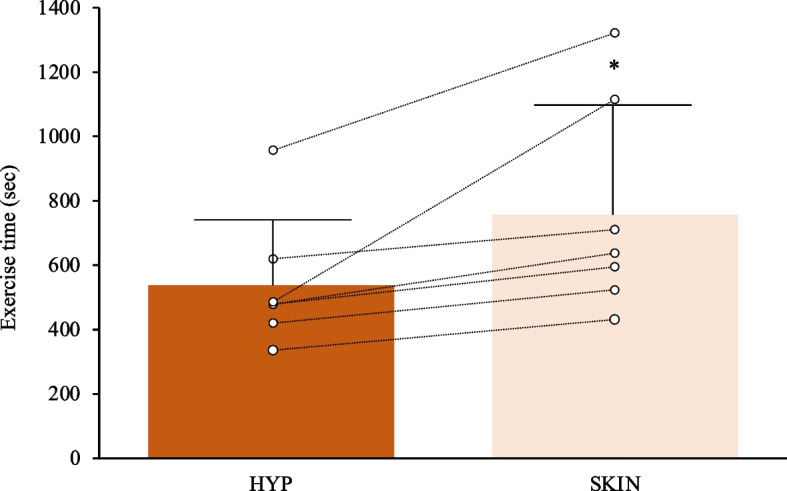
The time to exhaustion under the two experimental trials. The lines denote the raw data from individual participants (n = 7). *p < 0.05
Thermoregulatory responses
At rest, Tre was similar between trials. A HYP trial increased Tre by 0.86 ± 0.26℃ (p = 0.001, d = 4.34) and was significantly higher (37.69 ± 0.18℃) than that of the SKIN trial (36.96 ± 0.13℃; p = 0.001, d = 4.65) at the end of task. The Tre increased progressively in both trials during exercise (Fig. 4A). Tsk increased progressively in both trials during the 45-min task, albeit there were significant differences in Tsk at the end of the task between trials. The Tsk at exhaustion was significantly lower in the HYP trial (34.86 ± 0.50℃) than in the SKIN trial (35.62 ± 0.29℃; p = 0.020, d = 1.86) (Fig. 4B). There were no significant differences in core-to-skin temperature gradient before and after the 45-min task (p > 0.05). The core-to-skin temperature gradient at exhaustion was significantly wider in the HYP trial (4.13 ± 0.37℃) than in the SKIN trial (2.28 ± 0.32℃; p = 0.001, d = 4.49) (Fig. 4C).
Fig. 4.
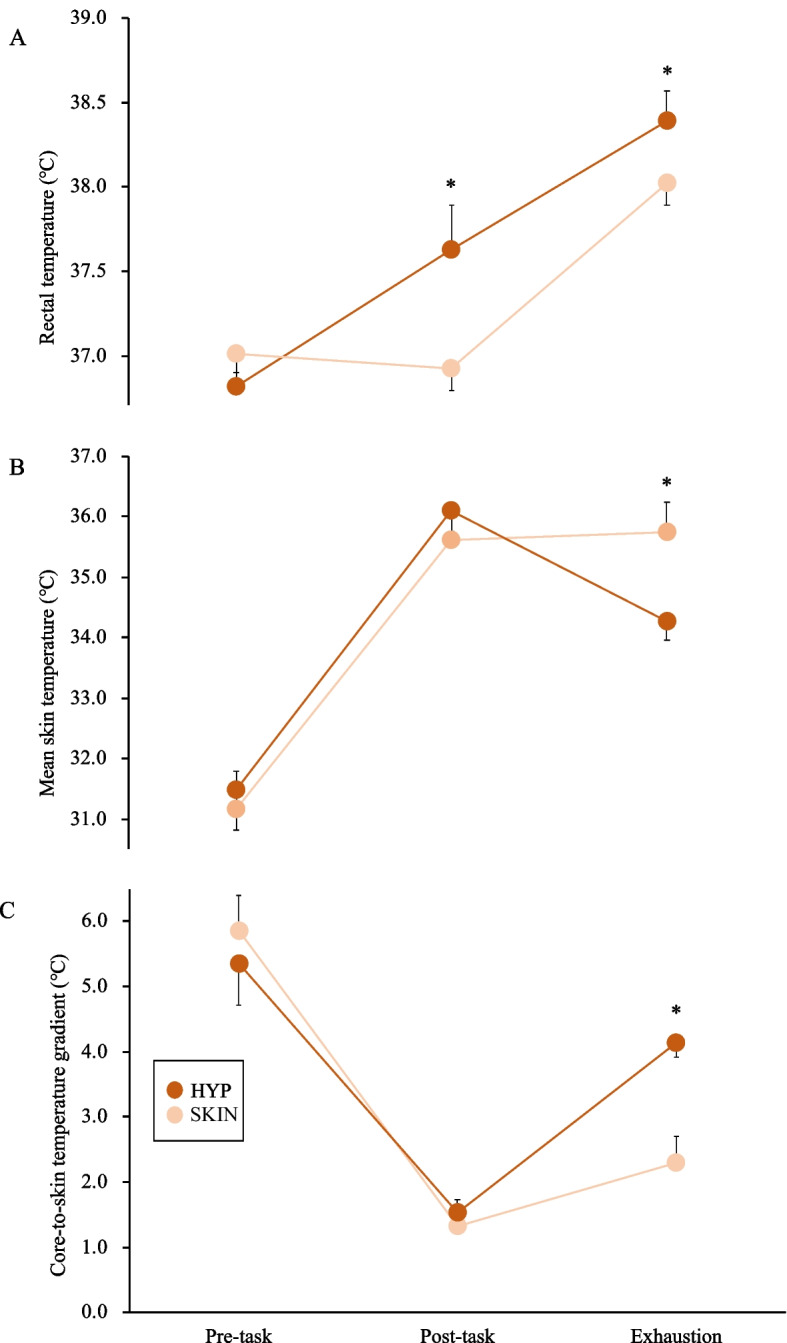
The rectal temperature (A), mean skin temperature (B), and core-skin temperatures gradient (C) under the two experimental trials. The mean values are expressed as mean ± SD. Time × intervention effect *p < 0.05, HYP vs. SKIN
Cognitive task performance, perceptual scales, and salivary cortisol
There were no significant differences in reaction time, lapses of attention, and accuracy for each task between trials (Table 1; p > 0.05). M-VAS increased progressively in both trials after the 45-min task, albeit no significant differences in M-VAS between trials (Figure 5; p = 0.349, d = 0.38). There was no significant difference in TS, TC, RPE, and salivary cortisol throughout the trials (Table 1).
Table 1.
Cognitive task performance, perceptual scales, salivary cortisol, and heart rate in the experimental trial. Values are expressed as means ± SD (n = 7)
| Trial | Pre-task | First 5 min of AX-CPT | Last 5 min of AX-CPT | First 5 min of Stroop task | Last 5 min of Stroop task | Post-task | Exhaustion | |
|---|---|---|---|---|---|---|---|---|
| Reaction time (ms) | HYP | - | 0.456 ± 0.072 | 0.421 ± 0.090 | 0.607 ± 0.088 | 0.602 ± 0.078 | - | - |
| SKIN | - | 0.440 ± 0.098 | 0.450 ± 0.115 | 0.639 ± 0.133 | 0.709 ± 0.151 | - | - | |
| Lapses of attention | HYP | - | 3 ± 2 | 1 ± 1 | 1 ± 1 | 2 ± 2 | - | - |
| SKIN | - | 2 ± 1 | 1 ± 1 | 1 ± 2 | 3 ± 5 | - | - | |
| Accuracy (%) | HYP | - | 96 ± 5 | 97 ± 3 | 96 ± 5 | 97 ± 3 | - | - |
| SKIN | - | 98 ± 2 | 95 ± 5 | 98 ± 3 | 95 ± 9 | - | - | |
| Thermal sensation | HYP | 4.1 ± 1.5 | - | - | - | - | 6.0 ± 0.8 | 8.3 ± 0.8 |
| SKIN | 3.6 ± 0.8 | - | - | - | - | 6.7 ± 0.8 | 8.4 ± 0.5 | |
| Rating of perceive exertion | HYP | - | - | - | - | - | 10.0 ± 3.0 | 18.7 ± 1.6 |
| SKIN | - | - | - | - | - | 9.6 ± 3.0 | 19.2 ± 0.9 | |
| △Salivary cortisol concentration (%) | HYP | 100 ± 0 | - | - | - | - | 74.2 ± 49.6 | 148.2 ± 82.5 |
| SKIN | 100 ± 0 | - | - | - | - | 89.0 ± 61.3 | 122.0 ± 45.4 | |
| Heart rate (bpm) | HYP | 64 ± 5 | - | - | - | - | 98 ± 18* | 192 ± 8 |
| SKIN | 69 ± 11 | - | - | - | - | 76 ± 11 | 189 ± 14 |
*p < 0.05, HYP vs. SKIN
Fig. 5.
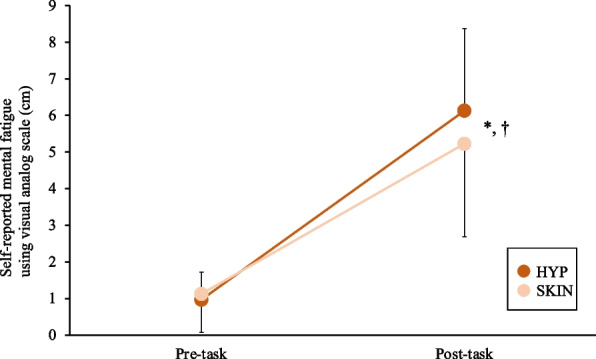
The self-reported mental fatigue using visual analog scale under the two experimental trials. The mean values are expressed as mean ± SD. *p < 0.05, vs. rest in the HYP trial. †p < 0.05 vs. rest in the SKIN trial
Heart rate
The HR was higher in the HYP trial than in the SKIN trial after the 45-min task (p = 0.021, d = 1.48), albeit there were no significant differences in HR before and after exercise between trials (Table 1; p > 0.05).
Body water composition
There were no significant differences in USG both before (HYP: 1.019 ± 0.005, SKIN: 1.022 ± 0.009; p = 0.292, d = 0.41) and after (HYP: 1.016 ± 0.008, SKIN: 1.022 ± 0.009; p = 0.127, d = 0.71) trials. The TSL was higher in the HYP trial (1600 ± 306 g) than in the SKIN trial (770 ± 387 g; p = 0.001 d = 2.38). The dehydration level was higher in the HYP trial (2.37 ± 0.37%) than in the SKIN trial (1.14 ± 0.52%; p = 0.001, d = 2.73).
Discussion
This study compared the effects of exposure to heat stress (high core and skin temperatures: HYP vs. high skin temperature alone: SKIN) in addition to a mental fatigue on aerobic exercise capacity in the heat. In accordance with the proposed hypothesis, the aerobic exercise capacity was impaired when combined with high core and skin temperatures (HYP) compared with high skin temperature only (SKIN).
Previous studies have investigated the combined effects of mental fatigue from a prolonged cognitive task and exposure to heat stress using protocol induced to the elevation of both Tc and Tsk [15, 16]. However, some exposures to heat stress (35–36℃ and 60%RH) at rest for approximately 90–120 min increase Tsk [33] but not Tc [34]. Therefore, this study employed protocol with (hyperthermia: HYP) and without (SKIN) Tc increase in addition to elevation of Tsk and mental fatigue. The results of this study are consistent with previous studies showing that reaching a high Tc impaired aerobic exercise capacity [3, 4], although mental fatigue is added. Therefore, when considering heat stress exposure in addition to mental fatigue, it is suggested that hyperthermia, which is accompanied by an increase in Tc more than an increase in Tsk alone, is additively induced by an impairment of aerobic exercise capacity. On the other hand, this observation suggested interaction effects between heat stress and mental fatigue [15], which followed the recently proposed “most severe strain takes precedence” principle [35], whereby the more severe strain, “heat stress”, takes precedence over the less severe strain “mental fatigue” [36]. Moreover, Van Cutsem et al. [16] suggested that stressing the brain (e.g., heat stress and mental fatigue) will impair endurance performance; however, at some point, further stressing the brain (e.g., combining heat stress and mental fatigue) will not result in a further decrease in endurance and cognitive performance, and a floor effect is observed. It is possible that a higher Tc was a more severe stressor than other stressors (i.e. mental fatigue) in this study, which, in turn, affected aerobic exercise capacity.
In the HYP trial, Tsk increased progressively during the 45-min task but decreased during exercise, which in turn greater the core-to-skin temperature gradient. In this study, the water temperature used in the HYP trial was 40℃, the same as in the previous study [15] and hotter than the chamber temperature. A possible explanation is that this phenomenon may have been caused by the drop in ambient temperature associated with entering the chamber after warm water immersion. In addition, the decrease in Tsk after immersion may be the result of heat transfer via evaporation because sweat is not able to evaporate during water immersion, and this result seems to be related to TS as well. It is known that a greater core-to-skin temperature gradient elicited by reducing Tsk improves cardiovascular load and prevents deterioration of oxygen supply to exercising muscles and brain, which in turn has a positive impact on exercise performance [37, 38]. However, even though the core-to-skin temperature gradient increased during exercise after warm water immersion, it did not affect the HR response and exercise time was shorter compared with exposure to the heat chamber. Therefore, this study suggested that the attainment of a high Tc appears to have been a limiting factor inhibiting aerobic exercise capacity rather than the magnitude of the core-to-skin temperature gradient, although there is no single Tc value associated with fatigue [39].
The state of mental fatigue induced by prolonged cognitive tasks could increase the perception of effort (RPE) [8, 10] and, therefore, reduce exercise capacity, since RPE has been deemed a key variable for stopping exercise [9]. However, changes in cortisol and the magnitude of mental fatigue caused by the 45-min task were similar levels between trials. Research on the difference between environmental conditions to load cognitive tasks is limited, but Valenza et al. [36] and Otani et al. [15] demonstrated that there were no significant differences in the magnitude of mental fatigue when the effects of cognitive tasks were examined under thermoneutral and hot environments. These results and the findings of this study indicate that mental fatigue may not be exacerbated by ambient temperature or an increase in Tc due to heat exposure. In addition, in hot environments, the limiting factor appears to be a higher Tc, and it has been suggested that an elevated Tc reduces the drive to exercise, which is reflected in higher RPE [40]. Conversely, RPE in this study was inconsistent with these above studies, and similar in value between the trials despite hyperthermia via warm water immersion before exercise. Schlader et al. [41, 42] reported that a change in thermal sensation and discomfort, and degree of Tsk affected voluntary exercise intensity, and, thus, RPE. The same value of perceptual discomfort and Tsk may have affected RPE in both trials in this study. The increase in RPE due to perceptual discomfort and decrease in Tsk can be improved by body cooling [43, 44], and further study is warranted to examine the effects of body cooling for combined mental load and heat exposure.
Limitations
In the HYP trial, the participants were immersed in warm water, in contrast to the SKIN trial. It is established that hydrostatic pressure induces bradycardia and increases stroke volume during water immersion [45]. The HR in the HYP trial was observed to be higher than that in the SKIN trial following the completion of the 45-min task. Given the shallow water depth employed in this study and the elevated HR observed, it can be reasonably inferred that the effects of thermal stress and mental tasks due to water temperature are indeed significant.
In this study, it is possible that dehydration caused by warm water immersion affected aerobic exercise capacity in the HYP trial. Dehydration > 2% BM degrades aerobic exercise and cognitive/mental performance in hot environments [25]. In contrast, previous systematic reviews reported that trained athletes demonstrated a lower in decrease effects on performance than untrained individuals [46]. Trangmar & Gonzalez-Alonso [47] additionally indicated that dehydration amounting to 3–4%BM augments the development of physiological strain to the body, in turn reducing endurance exercise capacity. In the HYP trial, the dehydration level was 2.37 ± 0.37%, but since they did not exceed 3% and were highly trained athletes, we believe that dehydration did not have that much of an impact on their performance.
In previous studies [8, 16], the participants were given incentives to ensure high motivation levels during the experimental trials. However, the present study did not provide any motivational intervention to address heat-related illnesses resulting from excessive heat strain caused by overexertion. It was observed that one of the participants experienced heat-related illnesses during the warm water immersion in the HYP trial. To improve future exercise performance and reduce the impact of heat strain, it would be beneficial for upcoming research to consider motivational intervention, even when exercising in a hot environment.
Conclusions
An elevated core body temperature, combined with increased skin temperature and mental fatigue, limits the aerobic exercise capacity of highly trained athletes in hot environments compared to heat exposure without an elevation of core body temperature. This suggests that athletes who are mentally fatigued should avoid conditions that increase their core body temperature to levels equivalent to hyperthermia before exercising to attenuate impairment in their endurance exercise performance in hot environments.
Acknowledgements
The authors would like to thank all the participants for participation in this study.
Abbreviations
- AX-CPT
AX-Continuous Performance Task
- BM
Body mass
- HR
Heart rate
- HYP
Hyperthermia
- V(●)O2max
Maximal oxygen uptake
- M-VAS
Mental fatigue visual analog scale
- RH
Relative humidity
- RPE
Rating of perceived exertion
- SKIN
Elevation in skin temperature
- Tc
Body core temperature
- TC
Thermal comfort
- Tre
Rectal temperature
- TS
Thermal sensation
- TSL
Total sweating loss
- Tsk
Mean skin temperature
- TTE
Time to exhaustion
- USG
Urine specific gravity
Authors’ contributions
TN designed and performed the research. TN, TS, and HM contributed to data acquisition. TN, and NE conducted to data analysis. TN contributed to data interpretation and all authors approved the final version of the manuscript.
Funding
This study was supported by JSPS KAKENHI Grant-in-Aid for Young Scientists (22K17713).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of the National Institute of Fitness and Sports in Kanoya (approval no.22–1-14). Each participant in the study was given detailed information about the research objectives, data collection procedures, benefits, risk, and confidentiality before written informed consent was obtained. The study complied with the latest version of the Declaration of Helsinki and was conducted according to international standards.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest were reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maughan R, Shirreffs S. Exercise in the heat: challenges and opportunities. J Sports Sci. 2004;22(10):917–27. [DOI] [PubMed] [Google Scholar]
- 2.Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29(9):1240–9. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat (1985). J Appl Physiol. 1999;86(3):1032–9. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MM, Cheung SS, Elder GC, Sleivert GG. Voluntary muscle activation is impaired by core temperature rather than local muscle temperature (1985). J Appl Physiol. 2006;100(4):1361–9. [DOI] [PubMed] [Google Scholar]
- 5.Sawka MN, Cheuvront SN, Kenefick RW. High skin temperature and hypohydration impair aerobic performance. Exp Physiol. 2012;97(3):327–32. [DOI] [PubMed] [Google Scholar]
- 6.Naito T, Saito T, Muraishi K, Takahashi H. Comparison of the effects of high and low levels of solar radiations on exercise capacity in hot outdoor environments. J Sports Med Phys Fitness. 2023;63(1):42–52. [DOI] [PubMed] [Google Scholar]
- 7.Otani H, Kaya M, Tamaki A, Watson P, Maughan RJ. Air velocity influences thermoregulation and endurance exercise capacity in the heat. Appl Physiol Nutr Metab. 2018;43(2):131–8. [DOI] [PubMed] [Google Scholar]
- 8.Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans (1985). J Appl Physiol. 2009;106(3):857–64. [DOI] [PubMed] [Google Scholar]
- 9.Pageaux B, Marcora SM, Rozand V, Lepers R. Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front Hum Neurosci. 2015;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MR, Coutts AJ, Merlini M, Deprez D, Lenoir M, Marcora SM. Mental Fatigue Impairs Soccer-Specific Physical and Technical Performance. Med Sci Sports Exerc. 2016;48(2):267–76. [DOI] [PubMed] [Google Scholar]
- 11.Barzegarpoor H, Amoozi H, Rajabi H, Button D, Fayazmilani R. The Effects of Performing Mental Exertion during Cycling Exercise on Fatigue Indices. Int J Sports Med. 2020;41(12):846–57. [DOI] [PubMed] [Google Scholar]
- 12.Meeusen R, Van Cutsem J, Roelands B. Endurance exercise-induced and mental fatigue and the brain. Exp Physiol. 2021;106(12):2294–8. [DOI] [PubMed] [Google Scholar]
- 13.Pageaux B, Lepers R. The effects of mental fatigue on sport-related performance. Prog Brain Res. 2018;240:291–315. [DOI] [PubMed] [Google Scholar]
- 14.Tucker R, Marle T, Lambert EV, Noakes TD. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J Physiol. 2006;574(Pt 3):905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otani H, Kaya M, Tamaki A, Watson P. Separate and combined effects of exposure to heat stress and mental fatigue on endurance exercise capacity in the heat. Eur J Appl Physiol. 2017;117(1):119–29. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem J, De Pauw K, Buyse L, Marcora S, Meeusen R, Roelands B. Effects of Mental Fatigue on Endurance Performance in the Heat. Med Sci Sports Exerc. 2017;49(8):1677–87. [DOI] [PubMed] [Google Scholar]
- 17.McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining Training and Performance Caliber: A Participant Classification Framework. Int J Sports Physiol Perform. 2022;17(2):317–31. [DOI] [PubMed] [Google Scholar]
- 18.Kalapotharakos VI, Ziogas G, Tokmakidis SP. Seasonal aerobic performance variations in elite soccer players. J Strength Cond Res. 2011;25(6):1502–7. [DOI] [PubMed] [Google Scholar]
- 19.ASHRAE. Fundamentals volume of the ASHRAE handbook. ASHRAE. 2005.
- 20.Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, et al. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin. 2013;2:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pageaux B, Lepers R, Dietz KC, Marcora SM. Response inhibition impairs subsequent self-paced endurance performance. Eur J Appl Physiol. 2014;114(5):1095–105. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Chai R, Nguyen HT, Marcora SM, Coutts AJ. Comparing the Effects of Three Cognitive Tasks on Indicators of Mental Fatigue. J Psychol. 2019;153(8):759–83. [DOI] [PubMed] [Google Scholar]
- 23.Dallaway N, Lucas SJE, Ring C. Cognitive tasks elicit mental fatigue and impair subsequent physical task endurance: Effects of task duration and type. Psychophysiology. 2022;59(12): e14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes TR, Oliveira DM, Simurro PB, Akiba HT, Nakamura FY, Okano AH, et al. No Sex Difference in Mental Fatigue Effect on High-Level Runners’ Aerobic Performance. Med Sci Sports Exerc. 2020;52(10):2207–16. [DOI] [PubMed] [Google Scholar]
- 25.American College of Sports M, Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, et al. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–90. [DOI] [PubMed]
- 26.Ramanathan NL. A New Weighting System for Mean Surface Temperature of the Human Body. J Appl Physiol. 1964;19:531–3. [DOI] [PubMed] [Google Scholar]
- 27.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–90. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem J, Marcora S, De Pauw K, Bailey S, Meeusen R, Roelands B. The Effects of Mental Fatigue on Physical Performance: A Systematic Review. Sports Med. 2017;47(8):1569–88. [DOI] [PubMed] [Google Scholar]
- 29.ISO. Ergonomics of the physical environment — Subjective judgement scales for assessing physical environments. ISO 10551. 2019:28.
- 30.Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1(1):1–20. [DOI] [PubMed] [Google Scholar]
- 31.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 32.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. [DOI] [PubMed] [Google Scholar]
- 33.Naito T, Saito T, Morito A, Yamada S, Shimomasuda M, Nakamura M. Pre-cooling with ingesting a high-carbohydrate ice slurry on thermoregulatory responses and subcutaneous interstitial fluid glucose during heat exposure. J Physiol Anthropol. 2022;41(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naito T, Ogaki T. Pre-cooling with intermittent ice ingestion lowers the core temperature in a hot environment as compared with the ingestion of a single bolus. J Therm Biol. 2016;59:13–7. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd A, Havenith G. Interactions in human performance: An individual and combined stressors approach. Temperature (Austin). 2016;3(4):514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenza A, Charlier H, Bianco A, Filingeri D. Independent and interactive effects of thermal stress and mental fatigue on manual dexterity. Am J Physiol Regul Integr Comp Physiol. 2020;319(6):R703–11. [DOI] [PubMed] [Google Scholar]
- 37.Cuddy JS, Hailes WS, Ruby BC. A reduced core to skin temperature gradient, not a critical core temperature, affects aerobic capacity in the heat. J Therm Biol. 2014;43:7–12. [DOI] [PubMed] [Google Scholar]
- 38.Choo HC, Peiffer JJ, Lopes-Silva JP, Mesquita RNO, Amano T, Kondo N, et al. Effect of ice slushy ingestion and cold water immersion on thermoregulatory behavior. PLoS ONE. 2019;14(2): e0212966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nybo L, Gonzalez-Alonso J. Critical core temperature: a hypothesis too simplistic to explain hyperthermia-induced fatigue. Scand J Med Sci Sports. 2015;25(Suppl 1):4–5. [DOI] [PubMed] [Google Scholar]
- 40.Simmons SE, Mundel T, Jones DA. The effects of passive heating and head-cooling on perception of exercise in the heat. Eur J Appl Physiol. 2008;104(2):281–8. [DOI] [PubMed] [Google Scholar]
- 41.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav. 2011;103(2):217–24. [DOI] [PubMed] [Google Scholar]
- 42.Schlader ZJ, Simmons SE, Stannard SR, Mundel T. Skin temperature as a thermal controller of exercise intensity. Eur J Appl Physiol. 2011;111(8):1631–9. [DOI] [PubMed] [Google Scholar]
- 43.Naito T, Iribe Y, Ogaki T. Ice ingestion with a long rest interval increases the endurance exercise capacity and reduces the core temperature in the heat. J Physiol Anthropol. 2017;36(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naito T, Haramura M, Muraishi K, Yamazaki M, Takahashi H. Impact of Ice Slurry Ingestion During Break-Times on Repeated-Sprint Exercise in the Heat. Sports Med Int Open. 2020;4(2):E45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen AS, Johansen LB, Stadeager C, Warberg J, Christensen NJ, Norsk P. Volume-homeostatic mechanisms in humans during graded water immersion (1985). J Appl Physiol. 1994;77(6):2832–9. [DOI] [PubMed] [Google Scholar]
- 46.Savoie FA, Kenefick RW, Ely BR, Cheuvront SN, Goulet ED. Effect of Hypohydration on Muscle Endurance, Strength, Anaerobic Power and Capacity and Vertical Jumping Ability: A Meta-Analysis. Sports Med. 2015;45(8):1207–27. [DOI] [PubMed] [Google Scholar]
- 47.Trangmar SJ, Gonzalez-Alonso J. Heat, Hydration and the Human Brain, Heart and Skeletal Muscles. Sports Med. 2019;49(Suppl 1):69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.