Abstract
Background
In microbial cell factories, substrate accessibility to enzyme is a key factor affecting the biosynthesis of natural products. As a robust chassis cells for biofuels and bioproducts, Saccharomyces cerevisiae also encounters the challenge since different enzymes and precursors are typically compartmentalized in different organelles. Such spatial separation could largely limit the efficiency of enzymatic reactions. In this study, the production of the hydrophobic product (vitamin A) was highly improved by metabolic engineering combined with degrading lipid droplets (the primary organelle storing β-carotene) to achieve efficient contact between β-carotene and 15, 15’-β-carotene monooxygenases in Saccharomyces cerevisiae.
Results
To efficiently produce vitamin A in Saccharomyces cerevisiae, ten 15, 15’-β-carotene monooxygenases (BCMOs) were firstly evaluated. The strain carrying marine bacterium 66A03 (Mb. BCMO) achieved the highest vitamin A titer. Co-adding 10% dodecane and 1% dibutylhydroxytoluene increased vitamin A titer to 19.03 mg/L in two-phase fermentation. Since most β-carotene is stored in LDs while BCMO is located in the cytosol, we developed a strategy to release β-carotene from LDs to better contact with BCMO. By overexpressing TGL3 and TGL4 using an ion-responsive promoter after high accumulation of β-carotene in LDs, LDs were sequentially degraded, which dramatically improved vitamin A production. Finally, by overexpressing tHMG1, ERG20, and CrtI and introducing Vitreoscilla hemoglobin, vitamin A titer reached 219.27 mg/L, which was a 10.52-folds increase over the original strain in shake flasks, and finally reached 1100.83 mg/L in fed-batch fermentation. The effectiveness of LDs degradation on promoting the formation of β-carotene cleaved product has also been verified in β-ionone synthesis with 44.07% increased yield.
Conclusions
Overall, our results highlighted the significance of sequential degrading LDs on vitamin A overproduction in recombinant yeast, and verified that combining metabolic and LDs engineering is an efficient strategy to improve vitamin A production. This integrated strategy can be applied to the overproduction of other hydrophobic compounds with similar characteristics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12934-024-02596-7.
Keywords: Vitamin A, Saccharomyces cerevisiae, β-carotene, Metabolic engineering, Lipid droplets degradation
Background
Vitamin A (retinoids), including retinol, retinal, and retinoic acid, is a kind of fat-soluble isoprenoid compound [1, 2]. Vitamin A has been widely applied in foods, pharmaceuticals, and cosmetics owing to its vital functions in improving night blindness, dry eye, immune system function, and skeletal development [3, 4]. The current industrial processes to produce vitamin A rely on chemical synthesis using substances derived from petroleum, such as acetone and acetylene [5]. However, due to the high cost of reagents, complex purification steps, and the possibility of hazardous compound residues, these manufacturing processes may not be ideal for safe and sustainable production [6]. Alternatively, the rapid development of synthetic biology has enabled the industrial production of vitamin A using microbial cell factories a more cost-effective and environmentally friendly approach [7].
Vitamin A biosynthesis has been well characterized and established in Escherichia coli, Yarrowia lipolytica, and Saccharomyces cerevisiae [8–10]. Through introducing heterologous 15,15’-β-carotene monooxygenase (BCMO), β-carotene was cleaved by BCMO to synthesize retinal, which was then oxidized or reduced under the catalysis of retinal dehydrogenase or retinal reductase to produce retinoic acid or retinol, respectively [11, 12]. Until now, four major strategies have been adopted in the optimization of vitamin A production in microbial cell factories, including enhancing the precursor supply, screening or engineering key enzymes, cofactor engineering, and optimizing fermentation conditions. For example, retinal production was increased by introducing an exogenous mevalonate (MVA) pathway in E. coli [13]. Park et al. screened three sources of BCMOs and achieved high production of retinol in Y. lipolytica [9]. NADH oxidase noxE was co-expressed with RDH12 and Mb.BCMO in S. cerevisiae to achieve selective production of retinol [10]. Additionally, fermentation conditions optimization strategies such as choosing suitable carbon sources [6], adding extractants for two-phase extraction fermentation such as dodecane [14], Tween 80 [9], and olive oil [6], and adding antioxidants were also supplied to improve vitamin A biosynthesis [14].
Although microbial synthesis of vitamin A has shown promise in laboratory settings, to date, vitamin A titer in engineered microorganisms still cannot meet industrial production requirements. There are three challenges need to be addressed. Firstly, the catalytic activity of key enzymes needs to be improved [9]. Screening of enzymes and rational modification by protein engineering are proper approaches. Secondly, for lipophilic products like vitamin A, efficient separation and purification remain significant challenges in industrialization. The establishment of a two-phase fermentation system for in situ extraction of the product to the organic phase can efficiently alleviate the metabolic burden on cells and further promote its production [15]. Thirdly, the spatial separation of precursors and enzymes greatly limits catalytic efficiency [16]. Different enzymes, precursors, and cofactors are usually embedded in different organelles due to their unique physicochemical environments [17]. Thus, organelle compartmentalization has been developed through anchoring enzymes from metabolic pathways to sub-organelles to induce substrate channeling [18]. However, several substantial limitations might hinder its further application. Firstly, linking localization tags on enzymes may interfere with their structure and folding, resulting in a decrease in enzyme activity [19]. Secondly, targeting plenty of enzymes to organelles can increase metabolic burden and cause a negative impact on the normal function of organelles [20]. Finally, the targeted enzymes may only be relocated on the surfaces of organelles and still cannot achieve complete contact between substrates and enzymes [21]. Previous studies have shown that most β-carotene was accumulated in LDs [22], while heterologous expressed BCMO was located in cytosol in yeast [23]. Accordingly, the spatial separation between enzymes and substrates might be a bottleneck limiting vitamin A overproduction.
In this study, we developed a combined strategy that involves systematic metabolic and LDs engineering in S. cerevisiae, due to its safety (GRAS) and industrial robustness [24], to try to overproduce vitamin A. These included screening efficient BCMOs, co-adding dodecane and dibutylhydroxytoluene (BHT) for two-phase fermentation, sequential degrading LDs to release β-carotene to efficient contact with BCMO by overexpressing LDs degradation genes, and increasing β-carotene supply by pathway engineering and improve oxygen utilization by overexpressing Vitreoscilla hemoglobin (VHb). After 96 h of fermentation, vitamin A titer was increased to 219.27 mg/L in shake flask, which was 10.52-folds increment compared with original strain, and the value finally reached 1100.83 mg/L in a 7 L fermenter. The effectiveness of this LDs degradation strategy has also been verified in another β-carotene cleaved product, β-ionone synthesis. Our results demonstrated that combining metabolic engineering and LDs degradation with two-phase extraction fermentation is an effective way to improve vitamin A production using S. cerevisiae as cell factory.
Materials and methods
Strains and cultivation
The starting chassis strain was β-carotene producing strain YBX-01 [25], and the related engineered strains are listed in Table 1. E. coli DH5α was used for plasmids construction and purification. The engineered yeast strains were cultured in Yeast Extract-Peptone-Dextrose (YPD) liquid medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose). For shake-flask fermentation, the recombinant yeast colonies were inoculated into 3 mL YPD medium and cultured at 30 °C on a rotary shaker (220 rpm) overnight. The seed cultures were inoculated into 250 mL flasks containing 50 mL YPD medium at an initial OD600 of 0.05 and cultured under the same conditions for 96 h. Dodecane with BHT (1%) was added to the culture at a volumetric ratio of 1:10 to form a hydrophobic phase above the culture phase. LB (Luria-Bertani broth) medium with antibiotics (50 µg/mL of kanamycin) was used for the cultivation of recombinant E. coli.
Table 1.
Strains used in this study
| Strains | Genotype/ Description | Source |
|---|---|---|
| YBX-01 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10 | Bu et al., [24] |
| YJL-01 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Hs.BCMO-TCYC1 | This study |
| YJL-02 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Gg.BCMO-TCYC1 | This study |
| YJL-03 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Mm.BCMO-TCYC1 | This study |
| YJL-04 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Rn.BCMO-TCYC1 | This study |
| YJL-05 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Cs.BCMO-TCYC1 | This study |
| YJL-06 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Sc.BCMO-TCYC1 | This study |
| YJL-07 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Ssp.BCMO-TCYC1 | This study |
| YJL-08 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Ff.BCMO-TCYC1 | This study |
| YJL-09 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Ss.BCMO-TCYC1 | This study |
| YJL-10 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- Mb.BCMO-TCYC1 | This study |
| YJL-11 | YJL-10, ΔHO::TADH1-BCMO-PGAL10-PGAL1-MCS2-TCYC1 | This study |
| YJL-12 | YJL-11, ΔTy4::TADH1-BCMO-PGAL10-PGAL1-MCS2-TCYC1 | This study |
| YJL-13 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1-PLN1-(G4S)2-BCMO-TCYC1 | This study |
| YJL-14 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1-oleosin-(G4S)2-BCMO-TCYC1 | This study |
| YJL-15 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1-DGA1-(G4S)2-BCMO-TCYC1 | This study |
| YJL-16 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- BCMO-(G4S)2-PLN1-TCYC1 | This study |
| YJL-17 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- BCMO-(G4S)2-oleosin-TCYC1 | This study |
| YJL-18 | FY1679-01B, Δgal1-10-7::TADH1-CrtI-PGAL10-PGAL1-CrtYB-TCYC1; Δgal80::TADH1-CrtE-PGAL10-PGAL1- BCMO-(G4S)2-DGA1-TCYC1 | This study |
| YJL-19 | YJL-10, ΔHO::TADH1-TGL3-PGAL10-PGAL1-TGL4-TCYC1 | This study |
| YJL-20 | YJL-10, ΔHO:: PARG1-TGL3-TCYC1; ΔTy4:: PARG1-TGL4-TCYC1 | This study |
| YJL-21 | YJL-20, Δdelta1:: PARG1-TGL3-TCYC1; Δdelta2:: PARG1-TGL4-TCYC1 | This study |
| YJL-22 | YJL-21, ΔDPP1::TADH1-tHMG1-PGAL10-PGAL1-ERG20-TCYC1 | This study |
| YJL-23 | YJL-22, ΔrDNA:: PGAL1-CrtI-TCYC1 | This study |
| YJL-24 | YJL-10, ΔrDNA:: PGAL1-CrtI-TCYC1 | This study |
| YJL-25 | YJL-23, ΔLPP1:: PGAL1-VHb-TCYC1 | This study |
| YJL-30 | YBX-01, ΔDPP1:: PGAL1-VvCCD1-TCYC1 | This study |
| YJL-31 | YJL-30, ΔHO:: PARG1-TGL3-TCYC1; ΔTy4:: PARG1-TGL4-TCYC1 | This study |
Plasmid construction
All plasmids used in this study were listed in Table S1. All primers (Table S2) were ordered from Sangon Biotech (Shanghai, China). Genomic DNA from S. cerevisiae FY1679-01B was used for PCR amplification of DNA fragments, promoters, and homologous arms. Plasmid pUMRI-21 was used as a template for DNA construction in the yeast genome, which was kindly provided by Prof. Hong-wei Yu. Plasmid DNA and PCR products were purified using TIANprep Rapid Mini Plasmid Kit (TIANGEN, Beijing, China) and HiPure PCR Pure Maxi Kit (Magen, Guangzhou, China). The ClonExpress Ultra One Step Cloning kits V2 were purchased from Vazyme (Nanjing, China).
Yeast transformation and strain screening
To integrate pUMRI derivative plasmids into the yeast genome, the plasmids were previously linearized from the junction of homologous arms using corresponding restriction enzymes. The integration was performed using the lithium acetate/polyethylene glycol/single-stranded carrier DNA transformation method [26]. G418 plates were used to select the recombinant strains. The correct colonies were passaged overnight at 30 °C, 220 rpm. Recombination between the duplicated loxp flanks resulted in 5-fluoroorotic acid (5-FOA) resistance due to URA3 excision [27]. 5-FOA resistant colonies were picked and checked for loss of the targeted marker by replica-plating on YPD and YPD-G418 plates.
Observation of yeast LDs by confocal microscopy
Confocal microscopy for LDs staining and image acquisition followed the method of Lv et al. [28]. with a slight modification. Yeast cells were harvested at an optical density of 5.0 (OD600), washed, and re-suspended with 500 µL PBS. 20 µL of Nile red staining solution (0.1% Nile red in DMSO) was added, and the solution was mixed thoroughly and incubated for 10 min in the dark at room temperature. Images were acquired using an Olympus AX70 Fluorescence Microscope (Olympus, Tokyo, Japan) with a fluorescence excitation at 488 nm.
Analytical methods
The intracellular β-carotene was extracted using hot HCl-acetone [29]. The analyses of β-carotene were performed on a HPLC system (Agilent 1200 LC) equipped with a C18 column (4.6 mm×150 mm) and the UV/VWD signals were detected at 450 nm. The mobile phase consisted of acetonitrile-methanol-isopropanol (50:30:20 v/v) with a flow rate of 1 mL/min at 40 °C.
The protocol for quantification of vitamin A in dodecane and intracellular was based on the method of Hu et al. [30] with a slight modification. For intracellular vitamin A analysis, cells were harvested by centrifugation and disrupted by RETSH Mixer Mill MM 400. The cell extracts were diluted using methanol and then analyzed on a HPLC system (Agilent 1200 LC) equipped with a C18 column (4.6 mm×150 mm) and the UV/VWD signals were detected at 352 nm. The mobile phase consisted of 92.5% acetonitrile and 7.5% acetic acid solution (2% v/v) at a flow rate of 0.6 mL/min at 40 °C. The standard curves of retinal, retinol and retinoic acid were prepared for quantification.
For β-ionone quantification, the culture was centrifuged at 8000 rpm for 3 min, and the dodecane layer was collected [31]. Samples were measured by gas chromatography–mass spectroscopy (GC − MS) using an Agilent 6890 gas chromatograph coupled with Agilent 5975 C mass spectrometer according to the methods of Meng et al. [32].
Quantitative real-time PCR (qRT-PCR) analysis
The protocol for qRT-PCR analysis was based on the method of Bu et al. [22] with a slight modification. Specific primers for the analysis of gene expression were designed and used in qRT-PCR (Table S3). The housekeeping gene ACT1 was used as the reference gene to normalize the different samples. The relative gene expression analysis was performed using the 2 − ΔΔCT method [33].
Yeast intracellular ATP determination
The intracellular ATP was extracted and measured from the harvested yeast cells by using the ENLITEN® ATP Assay System Bioluminescence Detection Kit (Promega, USA) according to the manufactures’ instructions [25].
Fed-batch fermentation
The protocol for fed-batch fermentation was based on the method of Xie et al. [24]. with a slight modification. Single colonies were picked into a 500 ml flask containing 125 ml YPD and culturing at 30 °C and 230 rpm for 12 h to an OD600 of 8–12. Four flasks of seed culture were inoculated into a 7-L bioreactor (Shanghai Baoxing Bio-Engineering Equipment Co., Ltd., China) containing 4.5 L of fermentation medium, which consisted of 25 g/L glucose, 15 g/L (NH4)2SO4, 8 g/L KH2PO4, 3 g/L MgSO4, 0.72 g/L ZnSO4.7H2O, 10 ml/L trace metal solution, and 12 ml/L vitamin solution. Fermentation was carried out at 30 °C with an agitation speed of 200 to 500 rpm and an airflow rate of 1 vvm to 2 vvm. pH was controlled at 5.5 by automatic addition of 28% ammonia hydroxide.
A two-stage fed-batch strategy with dodecane in situ extraction was employed in this study. In the first stage, feeding solution I containing 500 g/L glucose, 9 g/L KH2PO4, 2.5 g/L MgSO4, 3.5 g/L K2SO4, 0.28 g/L Na2SO4, 10 ml/L trace metal solution and 12 ml/L vitamin solution was used to achieve fast cell growth. In the second stage, feeding solution II contained the same mineral salts and trace elements as described above, but was supplemented with 250 g/L glucose and 250 g/L glycerol, along with 10 g/L yeast extract and 20 g/L peptone. This concentrated medium was used for inducing the accumulation of product. The glucose concentration in the fermentation broth was measured by a glucose assay kit (Beyotime, Shanghai, China). The ethanol concentration was analyzed using HPLC (Agilent 1200 LC) [10].
Results and discussion
Mining BCMOs for vitamin a biosynthesis in S. Cerevisiae
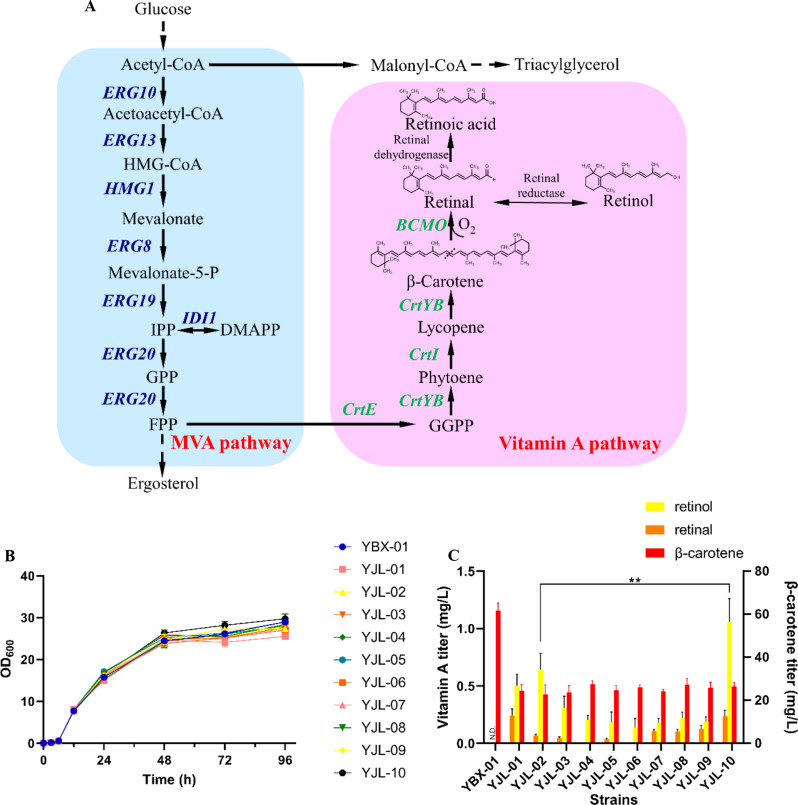
In our previous work, an engineered S. cerevisiae strain YBX-01 producing β-carotene was constructed by introducing CrtE, CrtYB, and CrtI from Xanthophyllomyces dendrorhous under Gal promoter, which uncoupled the cell growth and the product accumulation [25]. This strain was chosen as starting strain to produce vitamin A in this study. To efficiently produce vitamin A from β-carotene, we firstly assessed 10 BCMOs derived from various organisms in YBX-01 strain after codon optimization under the control of GAL promoter, respectively. These BCMOs included Homo sapiens (Hs.BCMO), Gallus gallus (Gg.BCMO), Mus musculus (Mm.BCMO), Rattus norvegicus (Rn.BCMO), Chlorella sorokiniana (Cs.BCMO), Schaalia cardiffensis F0333 (Sc.BCMO), Sphingomonas sp. PP-CC-3 A-396 (Ssp.BCMO), Fusarium flagelliforme (Ff.BCMO), Streptomyces stelliscabiei (Ss.BCMO) and Marine bacterium 66A03 (Mb. BCMO). Ten recombinant strains were thus generated (YJL-01 ~ 10). Among them, Cs. BCMO was truncated before introduction due to possessing a predicted signal peptide at the N-terminus (Fig. S1). As shown in Fig. 1A., in the fermentation process of 96 h, all recombinant strains showed a similar growth pattern to the parent strain YBX-01, indicating that the overexpression of these heterologous BCMOs did not interfere with cell growth.
Fig. 1.
Mining BCMOs for vitamin A biosynthesis in S. cerevisiae. (A) Biosynthetic pathways of vitamin A in engineered S. cerevisiae. (B) Cell growth of BCMOs carrying strains. (C) The vitamin A and β-carotene titer in BCMOs carrying strains. All data represent the mean±s.d. of biological triplicates. **P < 0.01
Among the tested strains, the strain YJL-10 carrying Mb. BCMO produced the highest vitamin A titer at 1.30 mg/L after 96 h fermentation, including 0.24 mg/L retinal and 1.06 mg/L retinol (Fig. 1B). This indicates Mb. BCMO is the most efficient BCMO for vitamin A production in S. cerevisiae. Compared with other sources of BCMO, it shows the highest activity toward β-carotene, followed by β-cryptoxanthin, α-carotene, and γ-carotene, leading to the highest vitamin A titer [34]. Mb. BCMO also showed the high production activity in Y. lipolytica and E. coli genetic strains [9, 13]. Therefore, Mb. BCMO was chosen for the subsequent work and renamed BCMO. It should be pointed out that no retinoic acid was detected in all strains. In addition, the titer of retinol was much higher than retinal, indicating that there are endogenous reductases in S. cerevisiae could be responsible for conversion of retinal to retinol, such as Adh6, Adh7 and Env9 [7, 14].
Enhancing vitamin A production via two-phase fermentation
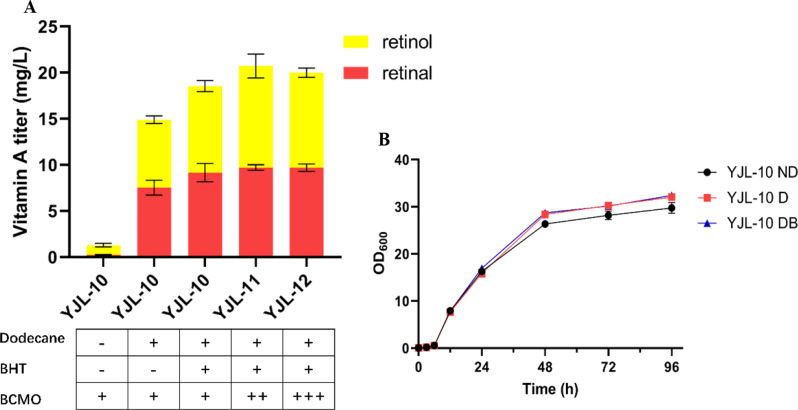
Enhancing the production of lipophilic compounds through metabolic engineering often faces the bottleneck of limited storage capacity within cells [10]. Using synthetic or natural oils as extractive agents for two-phase fermentation can partially solve this problem [9]. Additionally, vitamin A is chemically unstable and easy to be decomposed when exposed to lights or oxygen [11]. The addition of antioxidants, such as BHT and butyl hydroxyanisole (BHA) can efficiently prevent the decomposition of vitamin A [14]. Herein, we co-added 10% dodecane and 1% antioxidant BHT after 12 h cultivation of strain YJL-10 for two-phase fermentation. The results of 96 h fermentation showed that the vitamin A titer was significantly increased to 19.03 mg/L (9.16 mg/L retinal, 9.37 mg/L retinol and 0.50 mg/L retinoic acid) with 13.64-folds enhancement compared to that without organic phase (Fig. 2A), and no vitamin A was detected in cells. This indicated the high extraction efficiency of dodecane on vitamin A, and also proved combined adding extractant with protective agent is greatly beneficial for vitamin A synthesis and accumulation. Due to the secretion of lipophilic product, the metabolic burden was alleviated, as indicated by a slight improvement of cell growth (Fig. 2B) [9]. Compared with the addition of dodecane alone, simultaneous addition of dodecane and BHT increased the titer of vitamin A by 24.14%, indicating that the extraction by dodecane has the major contribution to the great enhancement of vitamin A production. For lipophilic products such as retinal and retinol, adding specific organic solvents at a proper concentration for in situ extraction of the product can effectively promote the targeted product synthesis by overcoming the limitation of intracellular storage capacity and reducing the possible feedback inhibition of an end-product. However, it should be emphasized that the specific added solvent should possess high extraction selectivity, which can only efficiently extract the end-product without extracting its substrate. In this study, only very small amounts of β-carotene (1.82 mg/L) was detected in the dodecane phase compared to 46.83 mg/L β-carotene remained in cells. Due to the largely lower level of retinoic acid compared to retinal and retinol, the titer of retinoic acid was not shown in the following study.
Fig. 2.
Enhancing vitamin A production via two-phase fermentation. (A) The vitamin A titer via addition of dodecane, BHT, and increasing BCMO copy numbers. (B) Cell growth of YJL-10 in different fermentation systems. ND: no dodecane; D: with dodecane; DB: with dodecane and BHT. All data represent the mean±s.d. of biological triplicates
It was noted that there was still partial β-carotene (4.88 mg/g DCW) remaining in YJL-10 strain. To further improve the cleavage of β-carotene to overproduce vitamin A, the expression of BCMO was increased by enhancing the gene copy number, generating two recombinant strains YJL-11 and YJL-12. Unfortunately, the titer of vitamin A was not significantly improved (Fig. 2A), implied that only increasing the expression level of BCMO cannot efficiently improve vitamin A, and there are other bottlenecks limiting vitamin A overproduction.
Targeting BCMO to lipid droplets by fusion proteins for improving vitamin A production
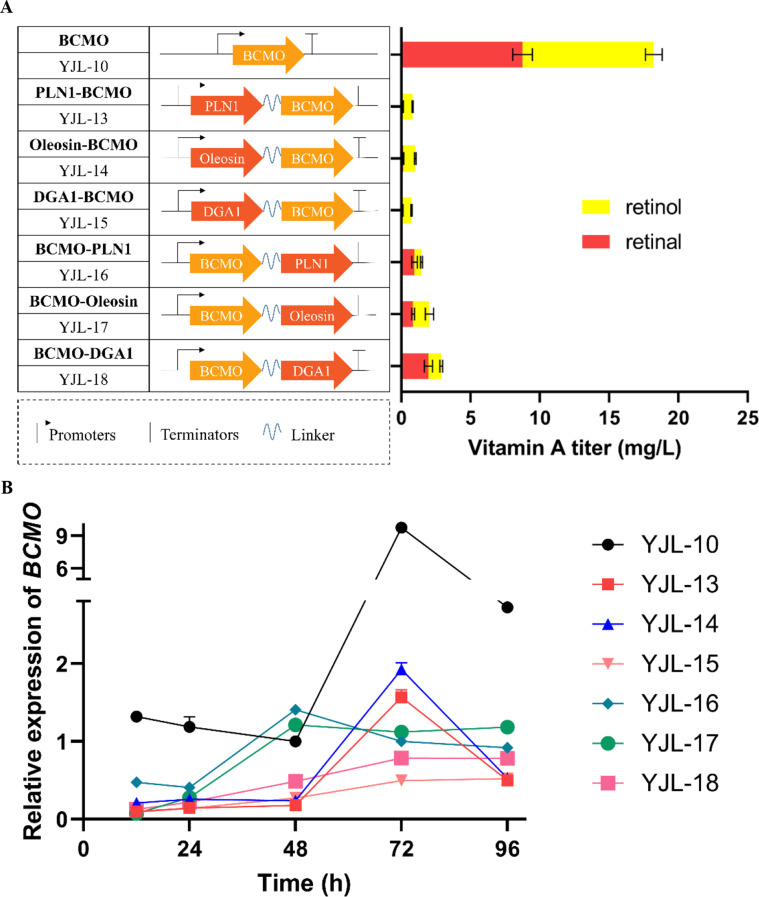
Our previous studies have shown that in S. cerevisiae, above 80% β-carotene is stored in LDs [22], the main organelles storing hydrophobic compounds in vivo [35], while according to previous literature [23], exogenously expressed BCMO was located in cytosol. Therefore, we speculated that the spatial separation of substrate (β-carotene) and enzyme (BCMO) is the key factor limiting the efficient production of vitamin A. To verify this hypothesis, we applied subcellular compartmentalization to minimize the substrate-enzyme distance, namely targeting BCMO to the LDs through linking BCMO with anchoring proteins. According to previous literatures, PLN1, oleosin and DGA1 can locate on the surface of LDs in S. cerevisiae [16, 36, 37]. Thus, we fused BCMO with the three anchoring proteins at C-terminus with a flexible linker spacer (GGGGSGGGGS), respectively. The flexible linker spacer can well keep the two parts of the fusion protein in close proximity and allow the interaction between domains [38]. The obtained fusion proteins (anchor protein-GGGGSGGGGS-BCMO) were introduced into strain YBX-01, and generated three engineered strains YJL-13, 14 and 15, respectively. Likewise, BCMO was also fused at N-terminus respectively (BCMO-GGGGSGGGGS-anchor protein) to construct the strains YJL-16, 17 and 18. Unexpectedly, the fermentation data showed that vitamin A titer in these recombinant strains all decreased drastically compared to the reference strain YJL-10 (Fig. 3A). We speculated that there might be two reasons accounting for the negative results. Firstly, the fusion of anchor proteins with BCMO might negatively affected the expression and (or) structure of BCMO, which highly decreased its catalytic activity, as reported by Stanisławska-Sachadyn et al. [39]. Secondly, the fusion proteins can only relocate BCMO on the surfaces of LDs, while β-carotene was stored inside the cores of LDs, and thus targeted BCMO on the surface of LDs cannot efficiently contact with β-carotene. We determined the expression level of BCMO by RT-PCR and found that the expression of fused BCMO was significantly decreased (Fig. 3B), which verified the negative effect of linking BCMO with a guided protein on the expression of BCMO. Thus, in subsequent work, we focused to verify the second assumption by developing an alternative strategy that breakdown LDs by genetic modulating LDs degradation genes to disperse β-carotene within the cells, which achieve the efficient contact between β-carotene and BCMO. The correctness of the hypothesis could be verified by the largely improved production of vitamin A.
Fig. 3.
The vitamin A titer (A), and relative expression of BCMO (B) in different engineered strains harboring functional fusion enzymes. Linker sequence, GGGGSGGGGS. All data represent the mean ± s.d. of biological triplicates
Degradation of LDs for improved vitamin A production
LDs are small endoplasmic reticulum-derived organelles for the storage of hydrophobic products [20]. Unlike mitochondria and peroxisomes, degradation of LDs has minimal negative effects on cells [40]. In S. cerevisiae, TGL3 and TGL4 are primarily responsible for the degradation of cellular LDs [41–43]. To achieve this purpose, TGL3 and TGL4 were co-overexpressed by increasing gene copy number under GAL promoter in YJL-10 strain, which achieved the LDs degradation synchronized with vitamin A synthesis, and YJL-19 strain was generated. However, the fermentation data showed a simultaneous sharp decrease in the production of β-carotene and vitamin A in the YJL-19 strain, to 15.35 mg/L and 3.91 mg/L, respectively. This could be attributed to the fact that degrading LDs during β-carotene synthesis is unfavorable for β-carotene accumulation, which definitely resulted in a significant reduction of vitamin A production.
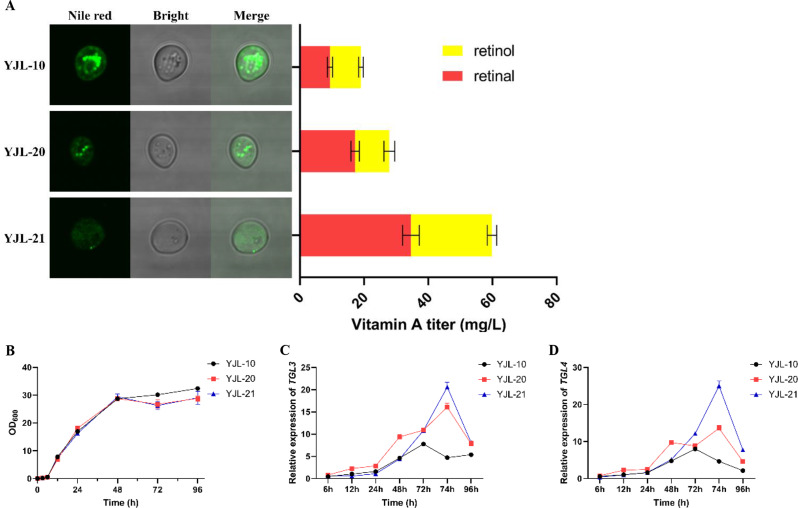
To solve this problem, we implemented a sequential degradation of LDs strategy, in which β-carotene was allowed to be accumulated in LDs before 72 h fermentation, after which the degradation of LDs was initiated to release β-carotene. To achieve this purpose, we employed promoter engineering to activate the expression of TGL3 and TGL4 by adding manganese ion after 72 h fermentation to deliver an induction signal to cells, according to previous work that ARG1 is highly induced by exogenous manganese ions [44, 45]. Thus, the manganese ion responsive inducible promoter PARG1 was cloned and linked with an additional TGL3 and TGL4 in YJL-10 and constructed YJL-20 strain. The RT-PCR data indicated that adding 1.5 mM MnCl2 after 72 h fermentation boosted the expression of TGL3 and TGL4 by 3.42 and 2.91 folds respectively relative to those in strain YJL-10 after 2 h of addition (Fig. 4C and D). As fermentation progressed, both genes showed a significant increased pattern. Visualization of LDs in the YJL-20 strain using confocal microscopy showed a significant decrease in the number of LDs compared to the YJL-10 strain, confirming the effectiveness of overexpressing TGL3 and TGL4 on LDs breakdown. As expected, the sequential engineering LDs resulted in a 37.83% increase in vitamin A titer, reaching 26.23 mg/L after 96 h of fermentation, composed of 15.50 mg/L of retinal and10.70 mg/L of retinol (Fig. 4A). The biomass of YJL-20 showed small decrement compared with YJL-10 (Fig. 4B), suggesting that LDs degradation only slightly affected cell growth. The results verified the effectiveness of sequential degrade LDs on improving vitamin A production (Fig. 4A).
Fig. 4.
Effect of degrading lipid droplets on intracellular LDs formation observed by laser confocal images and vitamin A titer (A), cell growth (B), and relative expression of TGL3 (C) and TGL4 (D). All data represent the mean ± s.d. of biological triplicates
Due to the presence of residual LDs in the cells, the expression levels of TGL3 and TGL4 were further increased by randomly integrating them at δ-locus in YJL-20 strain, and constructed strain YJL-21 strain. RT-PCR experiment indicated that the expression levels of TGL3 and TGL4 were increased by 1.28-folds and 1.84-folds compared to YJL-20, respectively (Fig. 4C and D). Correspondingly, the number of LDs in YJL-21 further decreased and no formed LDs were observed after 96 h fermentation (Fig. 4A). The genetic modulation led to vitamin A titer further increased by 1.28-folds compared with YJL-20 and reached 59.83 mg/L (including 34.57 mg/L retinal, 25.26 mg/L retinol) (Fig. 4A). It should be noted that there was still a small amount of β-carotene (21.32 mg/L) remaining in the cell, which could be ascribed to the fact that the partial β-carotene is stored in the cell membrane and LDs fragments [22, 46]. To overproduce vitamin A, previous works tried to strengthen the metabolic flux of MVA pathway and β-carotene synthesis module for providing sufficient β-carotene [7, 9]. However, due to the compartmentalization within lipid droplets, a considerable proportion of the β-carotene remained undegraded. In the present study, due to the degradation of LDs, the intracellular levels of β-carotene decreased from 46.83 mg/L (strain YJL-10) to 21.32 mg/L (strain YJL-21), which correspondingly led to vitamin A level increase from 19.03 mg/L to 59.83 mg/L. These results indicated that the application of LDs delay degradation strategy enhancing vitamin A yield is mainly caused by increasing the conversion ratio of β-carotene to vitamin A. Therefore, it is believed that combining LDs delay degradation with strengthening β-carotene production is a more efficient way to enhance vitamin A production.
Enhancing vitamin A production via improving precursor β-carotene and oxygen supply by genetic modulation
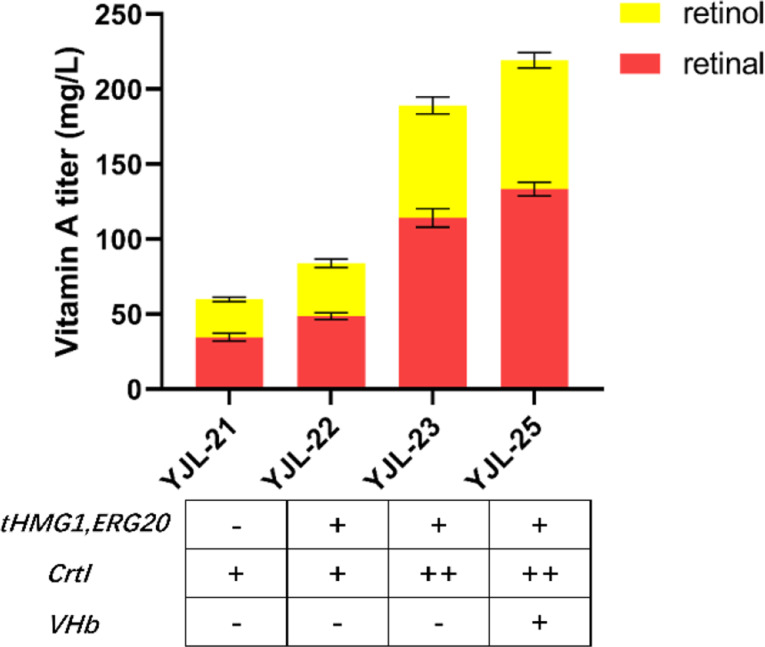
To further validate the above results, we overexpressed tHMG1 and ERG20, the key enzymes in the MVA pathway, in strain YJL-21 to improve the metabolic flux of MVA pathway for further enhancing vitamin A production, and generated strain YJL-22. After 96 h of fermentation, the vitamin A titer raised to 83.79 mg/L with 40.05% increment compared to YJL-21 strain. In the β-carotene synthesis module, upregulation of CrtI can greatly promote β-carotene synthesis [47]. Thus, we increased the expression of CrtI through randomly integrating additional CrtI at the rDNA sites in YJL-22, and generated strain YJL-23. As expected, vitamin A titer increased to 189.05 mg/L, which was 125.62% higher than that of the YJL-22 strain (Fig. 5), confirming that increasing β-carotene synthesis is favorable for improvement of vitamin A production.
Fig. 5.
Enhancing metabolic flux and improving oxygen supply for vitamin A production. All data represent the mean ± s.d. of biological triplicates
The cleavage of β-carotene by BCMO to form vitamin A requires oxygen. The limitation of oxygen thus might become another bottleneck for efficient production of vitamin A in S. cerevisiae. It has been reported that overexpression of Vitreoscilla hemoglobin (VHb) can improve oxygen supply under oxygen-limited conditions [48–50]. The heterologous VHb was overexpressed in YJL-23 under the control of GAL promoter and generated strain YJL-25. The resulting strain produced 219.27 mg/L vitamin A (including 133.35 mg/L retinal and 85.92 mg/L retinol), which showed a 15.99% increment relative to YJL-23 strain. We further analyzed the cell growth and intracellular ATP content in YJL-23 and YJL-25, and found that the highest biomass and ATP content in strain YJL-25 with VHb overexpression were increased by 14.52% and 26.86%, respectively (Fig. S4). These results indicated that providing extra oxygen is critical for the improved production of vitamin A in recombinant yeast cells. It should be pointed out that in order to avoid oxidation of vitamin A, the oxygen concentration needs to be carefully optimized. To achieve this purpose, the application of multi-stage oxygen supply strategy according to the cell growth and the stages of product synthesis might be a suitable choice.
Fed-batch fermentation of vitamin A in 7 L fermenter
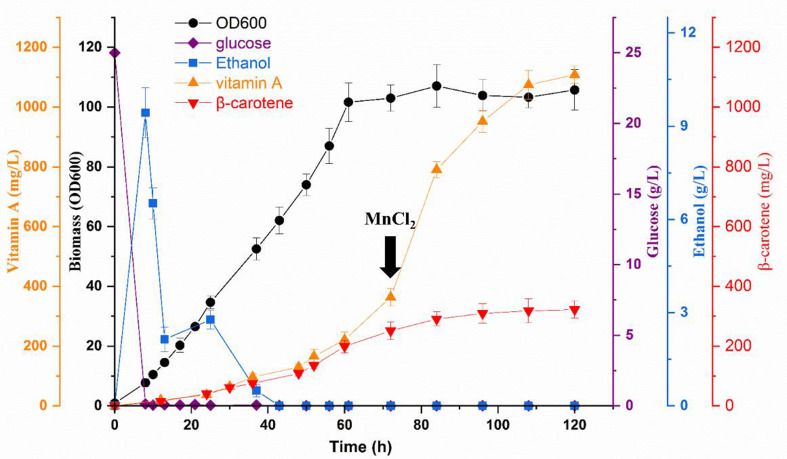
Through a series of genetic modifications, the titer of vitamin A in strain YJL-25 reached 219.27 mg/L after 96 h of fermentation in shaking flask, which was 10.52-folds increase compared with that of original strain YJL-10 (Fig. 5). To maximize vitamin A production, fed-batch fermentation was conducted in a 7 L fermenter at pH 5.5 using YJL-25, in which the auxotroph marker URA3 had been complemented during construction. A two-stage fed-batch strategy with dodecane in situ extraction was adopted in which dodecane with BHT (1%) was added at a volumetric ratio of 1:10 at 12 h. In the first stage, feeding solution I was supplied to allow the biomass accumulation as rapidly as possible, and in the second stage, gene expression was induced to accumulate vitamin A at the highest level possible. When the glucose in the initial fermentation medium was consumed and ethanol content was less than 3 g/L, the feeding solution I was supplied. When the cells entered the mid-log phase that the biomass (OD600) reached around 70, the solution II containing a mixture of glucose and glycerol was fed to induce the high accumulation of vitamin A. During fermentation, 1.5 mM MnCl2 was added at 72 h to initiate the degradation of LDs. The rapid accumulation of vitamin A was observed at the second feeding stage, especially after the addition of MnCl2 at 72 h (Fig. 6). At the end of the fed-batch fermentation, 1100.83 mg/L of vitamin A was obtained, which consisted of 595.38 mg/L retinal and 505.45 mg/L retinol (Fig. 6). It should be noted that there was 322.18 mg/L of β-carotene remaining in the cell membrane and LDs fragments. To further enhance vitamin A production, specific genetic strategies could be applied, such as altering the composition of cell membrane to release β-carotene into cytoplasm or anchoring BCMO on the cell membrane.
Fig. 6.
High-density fermentation of YJL-25 with dodecane in situ extraction for vitamin A production. Profile of biomass, glucose, ethanol, β-carotene and vitamin A accumulation during fed-batch fermentation. Error bars represent standard deviations from three detections of each sample
In the present work, due to the truth that the separation of substrates and enzymes limits vitamin A formation, the strategy of delayed degradation of LDs was developed, which successfully greatly improved the yield of vitamin A. In order to verify the universality of this strategy, we also construct the β-ionone production strain YJL-30 by introducing the carotenoid cleavage dioxygenases from Vitis vinifera L. cv. Cabernet Sauvignon (VvCCD1) [32] in YBX-01, which can cleavage β-carotene at the 9, 10 (9’, 10’) position to produce β-ionone. LDs degradation module was further integrated in YJL-30 and generated YJL-31 strain. The fermentation results showed that the yield of β-ionone in YJL-31 was increased by 44.07% compared to YJL-30 with 13.58% decreased of β-carotene content (Fig. S5). It well proved that this strategy could be applied to overproduce other β-carotene cleaved products. The results of the present study also confirmed that obtaining high-efficiency BCMO and increasing precursor supply are the key to improving vitamin A synthesis. To further promote vitamin A synthesis, protein engineering and metabolic engineering could be applied to rationally modify BCMO and increase the synthesis of β-carotene, respectively. Until now, vitamin A biosynthesis has been established in E. coli, Y. lipolytica, and S. cerevisiae [8–10]. Due to the risk of endotoxin and phage infection, the application of E. coli in natural products biosynthesis is limited. Yeasts thus become promising chassis for microbial production [24]. It should be pointed out that the vitamin A level in this study is still lower than that of Y. lipolytica. This is mainly because Y. lipolytica has a natural advantage in the biosynthesis of lipid-soluble terpenoids due to its rich content of acetyl CoA, and the strong ability to form LDs compared to S. cerevisiae [51]. Therefore, it is expected that the adoption of LDs delayed degradation strategy in Yarrowia lipolytica cell factory could achieve more significant increase of vitamin A level.
Conclusion
In this study, we attempted to integrate multiple metabolic engineering to enhance vitamin A biosynthesis in S. cerevisiae. By screening efficient BCMOs, co-adding extracts and antioxidant agents, sequential degrading LDs, and overexpressing key genes and heterologous VHb, the vitamin A yield was increased to 219.27 mg/L with 10.52-folds enhancement compared to the original strain in shake flasks, and finally reached 1100.83 mg/L in fed-batch fermentation. The effectiveness of this delayed LDs degradation strategy has also been verified in another β-carotene cleaved product, β-ionone synthesis. In summary, our results specifically highlighted the key effect of degrading LDs on overproducing vitamin A in yeast chassis. This integrated strategy can be applied to the overproduction of other hydrophobic derivatives compounds such as astaxanthin, and other yeast chassis cells, such as Yarrowia lipolytica.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
GLY designed the study. JYL carried out the experimental work, analyzed the data, prepared all charts and tables, and drafted the manuscript. XB, YBL, CQD, and GLY edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD2101401) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJB360002).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66(7):606–30. [DOI] [PubMed] [Google Scholar]
- 2.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3(4):385–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong SH, Kim KR, Oh DK. Biochemical properties of retinoid-converting enzymes and biotechnological production of retinoids. Appl Microbiol Biotechnol. 2015;99(19):7813–26. [DOI] [PubMed] [Google Scholar]
- 4.Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol. 2005;23(4):482–7. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan K, Buys EM. Insights into the role of bacteria in vitamin a biosynthesis: future research opportunities. Crit Rev Food Sci Nutr. 2019;59(19):3211–26. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Kwak S, Jin YS. Vitamin A production by engineered Saccharomyces cerevisiae from xylose via two-phase in situ extraction. ACS Synth Biol. 2019;8(9):2131–40. [DOI] [PubMed] [Google Scholar]
- 7.Mo Q, Song W, Xue Z, Yuan J. Multi-level engineering of Saccharomyces cerevisiae for the synthesis and accumulation of retinal. Green Chem. 2022;24(21):8259–63. [Google Scholar]
- 8.Kim YS, Kim NH, Kim HJ, Lee JK, Kim SW, Oh DK. Effective production of retinal from beta-carotene using recombinant mouse beta-carotene 15,15’-monooxygenase. Appl Microbiol Biotechnol. 2007;76(6):1339–45. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Lee D, Kim JE, Park S, Park JH, Ha CW, et al. Efficient production of retinol in Yarrowia Lipolytica by increasing stability using antioxidant and detergent extraction. Metab Eng. 2022;73:26–37. [DOI] [PubMed] [Google Scholar]
- 10.Lee YG, Kim C, Sun L, Lee TH, Jin YS. Selective production of retinol by engineered Saccharomyces cerevisiae through the expression of retinol dehydrogenase. Biotechnol Bioeng. 2022;119(2):399–410. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez R, Vaz B, Gronemeyer H, de Lera AR. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev. 2014;114:1–125. [DOI] [PubMed] [Google Scholar]
- 12.dela Seña C, Riedl KM, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH. The human enzyme that converts dietary provitamin a carotenoids to vitamin A is a dioxygenase. J Biol Chem. 2014;289(19):13661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang HJ, Yoon SH, Ryu HK, Kim JH, Wang CL, Kim JY, et al. Retinoid production using metabolically engineered Escherichia coli with a two-phase culture system. Microb Cell Fact. 2011;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Q, Zhang T, Yu H, Ye L. Selective biosynthesis of retinol in S. Cerevisiae. Bioresour. Bioprocess. 2022;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Yu H, Ye L. From β-carotene to retinoids: a review of microbial production of vitamin A. J Agric Food Chem. 2024;72(38):20752–62. [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Qiao Y, Li F, Xu Y, Yan Y, Madzak C, et al. Subcellular engineering of lipase dependent pathways directed towards lipid related organelles for highly effectively compartmentalized biosynthesis of triacylglycerol derived products in Yarrowia Lipolytica. Metab Eng. 2019;55:231–8. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Wang D, Li R, Huang L, Dai Z, Zhang X. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab Eng. 2021;67:104–11. [DOI] [PubMed] [Google Scholar]
- 18.Hammer SK, Avalos JL. Harnessing yeast organelles for metabolic engineering. Nat Chem Biol. 2017;13(8):823–32. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui MS, Thodey K, Trenchard I, Smolke CD. Advancing secondary metabolite biosynthesis in yeast with synthetic biology tools. FEMS Yeast Res. 2012;12(2):144–70. [DOI] [PubMed] [Google Scholar]
- 20.Cao X, Yang S, Cao C, Zhou YJ. Harnessing sub-organelle metabolism for biosynthesis of isoprenoids in yeast. Synth Syst Biotechnol. 2020;5(3):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttanus HM, Feng X. Compartmentalized metabolic engineering for biochemical and biofuel production. Biotechnol J. 2017;12(6):101002. [DOI] [PubMed] [Google Scholar]
- 22.Bu X, Lin JY, Duan CQ, Koffas MAG, Yan GL. Dual regulation of lipid droplet-triacylglycerol metabolism and ERG9 expression for improved β-carotene production in Saccharomyces cerevisiae. Microb Cell Fact. 2022;21(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Wang Y, Yao M, Xiao W. Research progress of vitamin a biosynthesis. CIESC J. 2022;73(10):4311–23. [Google Scholar]
- 24.Xie W, Lv X, Ye L, Zhou P, Yu H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng. 2015;30:69–78. [DOI] [PubMed] [Google Scholar]
- 25.Bu X, Lin JY, Cheng J, Yang D, Duan CQ, Koffas M, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels. 2020;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gietz RD, Schiestl RH. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2(1):35–7. [DOI] [PubMed] [Google Scholar]
- 27.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14(2):115–32. [DOI] [PubMed] [Google Scholar]
- 28.Lv X, Liu J, Qin Y, Liu Y, Jin M, Dai J, et al. Identification of gene products that control lipid droplet size in yeast using a high-throughput quantitative image analysis. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(2):113–27. [DOI] [PubMed] [Google Scholar]
- 29.Xie W, Liu M, Lv X, Lu W, Gu J, Yu H. Construction of a controllable β-carotene biosynthetic pathway by decentralized assembly strategy in Saccharomyces cerevisiae. Biotechnol Bioeng. 2014;111(1):125–33. [DOI] [PubMed] [Google Scholar]
- 30.Hu Q, Yu H, Ye L. Production of retinoic acid by engineered Saccharomyces cerevisiae using an endogenous aldehyde dehydrogenase. Biotechnol Bioeng. 2022;119(11):3241–51. [DOI] [PubMed] [Google Scholar]
- 31.Shi JT, Wu YY, Sun RZ, Hua Q, Wei LJ. Synthesis of β-ionone from xylose and lignocellulosic hydrolysate in genetically engineered oleaginous yeast Yarrowia Lipolytica. Biotechnol Lett Published Online Oct. 2024;8. 10.1007/s10529-024-03534-8. [DOI] [PubMed]
- 32.Meng N, Yan GL, Zhang D, Li XY, Duan CQ, Pan QH. Characterization of two Vitis vinifera carotenoid cleavage dioxygenases by heterologous expression in Saccharomyces cerevisiae. Mol Biol Rep. 2019;46:6311–23. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 34.Kim YS, Kim NH, Yeom SJ, Kim SW, Oh DK. In vitro characterization of a recombinant blh protein from an uncultured marine bacterium as a beta-carotene 15,15’-dioxygenase. J Biol Chem. 2009;284(23):15781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Huang M, Chen H, Lu X, Tian Y, Hu P, et al. Metabolic engineering of Yarrowia Lipolytica for high-level production of squalene. Bioresour Technol. 2024;394:130233. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, Li J, Huang S, Stephanopoulos G. Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia Lipolytica. Metab Eng. 2021;68:152–61. [DOI] [PubMed] [Google Scholar]
- 37.Walther TC, Farese RV Jr. The life of lipid droplets. Biochim Biophys Acta. 2009;1791(6):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanisławska-Sachadyn A, Sachadyn P, Ihle K, Sydorczuk C, Wiejacha K, Kur J. The construction of bifunctional fusion proteins consisting of MutS and GFP. J Biotechnol. 2006;121(2):134–43. [DOI] [PubMed] [Google Scholar]
- 40.Bureau JA, Oliva ME, Dong Y, Ignea C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat Prod Rep. 2023;40(12):1822–48. [DOI] [PubMed] [Google Scholar]
- 41.Ma Y, Liu N, Greisen P, Li J, Qiao K, Huang S, et al. Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia Lipolytica. Nat Commun. 2022;13(1):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Athenstaedt K, Daum G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J Biol Chem. 2003;278:23317–23. [DOI] [PubMed] [Google Scholar]
- 43.Athenstaedt K, Daum G. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J Biol Chem. 2005;280:37301–9. [DOI] [PubMed] [Google Scholar]
- 44.Xiao C, Pan Y, Huang M. Advances in the dynamic control of metabolic pathways in Saccharomyces cerevisiae. Eng Microbiol. 2023;3:100103. [Google Scholar]
- 45.Hosiner D, Gerber S, Lichtenberg- Fraté H, Glaser W, Schüller C, Klipp E. Impact of acute metal stress in Saccharomyces cerevisiae. PLoS ONE. 2014;9(1):e83330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner N, Ramirez-Sarmiento CA, Agosin E. Protein engineering of carotenoid cleavage dioxygenases to optimize β-ionone biosynthesis in yeast cell factories. Food Chem. 2019;299:125089. [DOI] [PubMed] [Google Scholar]
- 47.Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, et al. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces Dendrorhous. Appl Environ Microbiol. 2007;73(13):4342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Hughes DE, Bailey JE. Intracellular expression of Vitreoscilla hemoglobin alters the aerobic metabolism of Saccharomyces cerevisiae. Biotechnol Prog. 1994;10(3):308–13. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Tan T. Enhanced S-adenosylmethionine production by increasing ATP levels in Baker’s yeast (Saccharomyces cerevisiae). J Agric Food Chem. 2018;66(20):5200–9. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Feng Y, Cui Q, Song X. Expression of Vitreoscilla hemoglobin enhances production of arachidonic acid and lipids in Mortierella Alpina. BMC Biotechnol. 2017;17(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren X, Liu M, Yue M, Zeng W, Zhou S, Zhou J, et al. Metabolic pathway coupled with fermentation process optimization for high-level production of retinol in Yarrowia Lipolytica. J Agric Food Chem. 2024;72(15):8664–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.