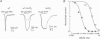Abstract
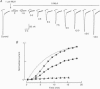
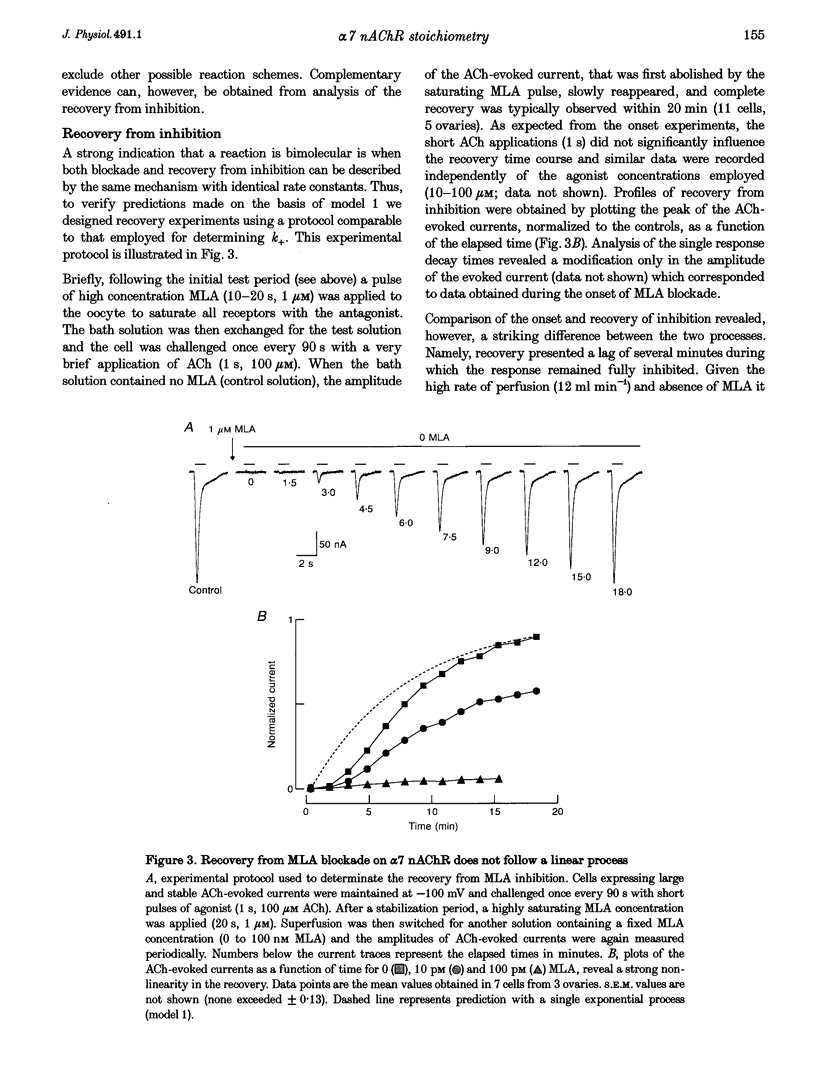
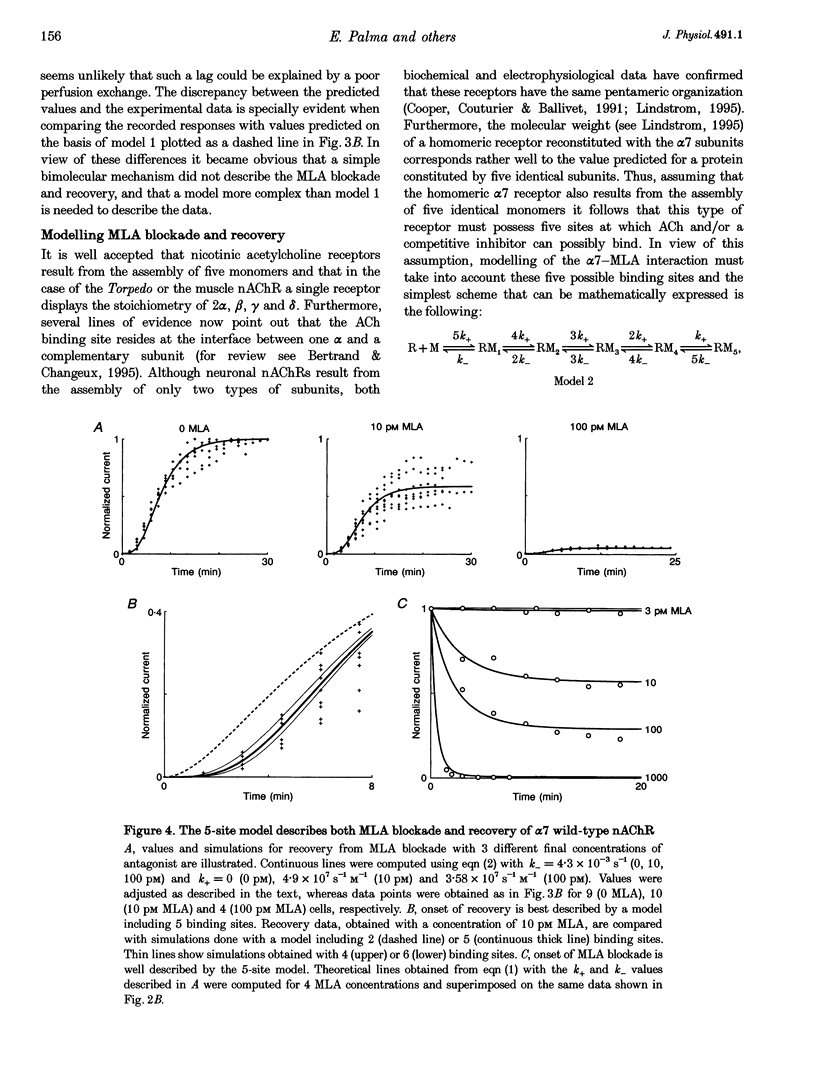
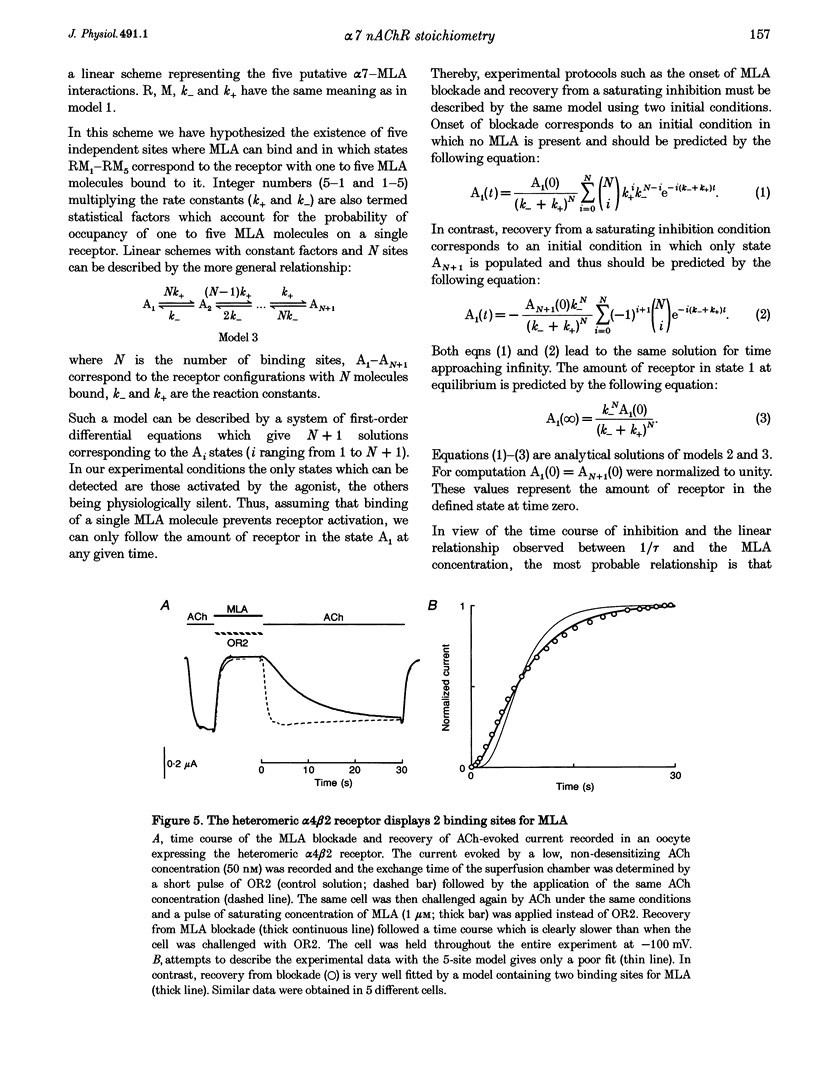
1. The recently isolated compound methyllycaconitine (MLA) is a plant toxin which is a competitive inhibitor of nicotinic acetylcholine receptors (nAChRs). We found that homomeric alpha 7 receptors display a very high sensitivity to MLA with an IC50 in the picomolar range. 2. The competitive nature of the alpha 7 MLA blockade was reinforced by the observation that this compound has no action on wild-type serotoninergic receptors (5-HT3), whereas it is a powerful antagonist of chimaeric receptors alpha 7-5-HT3. 3. The time course of MLA inhibition of the wild-type (WT) alpha 7 follows a monotonic exponential decay whose time constant is proportional to the MLA concentration and could be described by a bimolecular mechanism with a forward rate constant (k+) of 2.7 x 10(7) S-1 M-1. In contrast, recovery from MLA inhibition displays an S-shaped time course that is incompatible with a simple bimolecular reaction. 4. Given the pentameric nature of the neuronal nicotinic receptors, a linear chain model, including five putative MLA binding sites corresponding to the homomeric nature of alpha 7, is proposed. 5. Both onset and recovery data obtained on the alpha 7 wild-type receptor are adequately described by this model assuming that a single MLA molecule is sufficient to block receptor function. 6. Analysis of MLA blockade and recovery of reconstituted heteromeric alpha 4 beta 2 receptors reveals, as expected, a time course compatible with only two binding sites for the toxin and, thus, further supports the validity of our model.
Full text
PDF

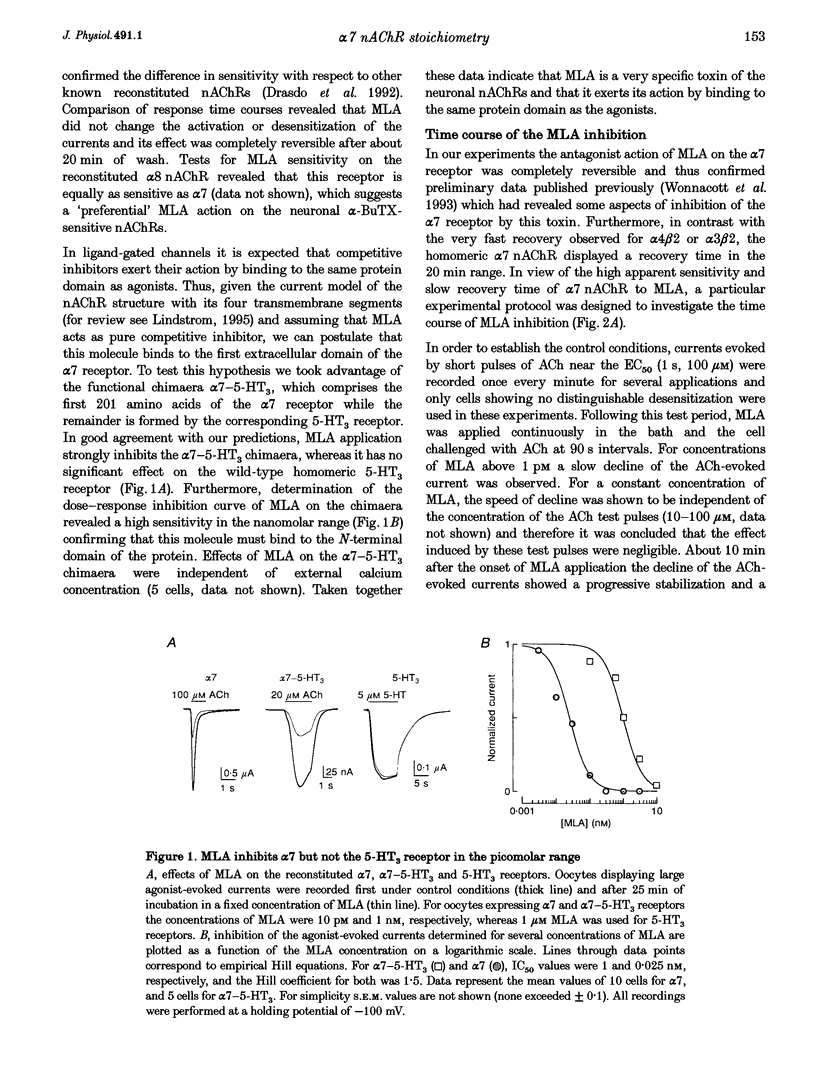
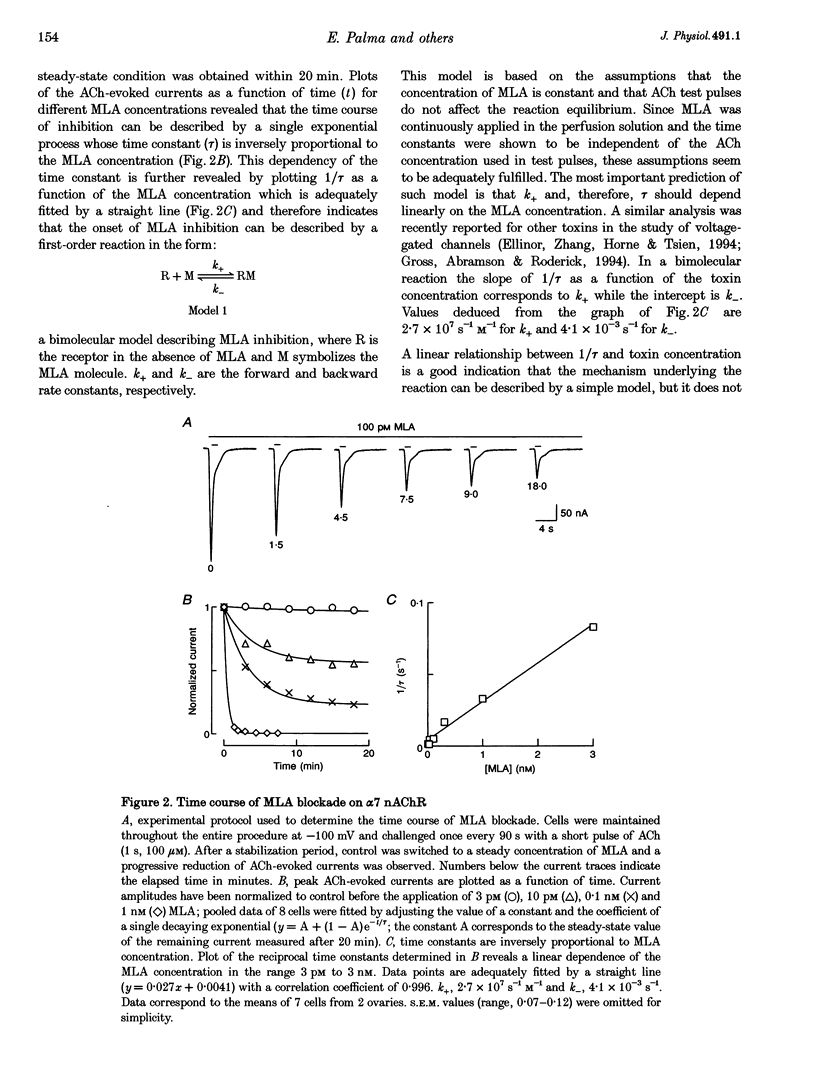




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkondon M., Pereira E. F., Wonnacott S., Albuquerque E. X. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992 Apr;41(4):802–808. [PubMed] [Google Scholar]
- Anand R., Peng X., Ballesta J. J., Lindstrom J. Pharmacological characterization of alpha-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of alpha 7 and alpha 8 subunit-containing subtypes. Mol Pharmacol. 1993 Nov;44(5):1046–1050. [PubMed] [Google Scholar]
- Balice-Gordon R. J., Lichtman J. W. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994 Dec 8;372(6506):519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Ballivet M., Gomez M., Bertrand S., Phannavong B., Gundelfinger E. D. Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate beta 2 subunit and Drosophila alpha subunits. Eur J Neurosci. 1994 May 1;6(5):869–875. doi: 10.1111/j.1460-9568.1994.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V. A. Actions of snake venom toxins on neuronal nicotinic receptors and other neuronal receptors. Pharmacol Ther. 1985;31(1-2):1–32. doi: 10.1016/0163-7258(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V. A., Hue B., Mony L., Sattelle D. B. Kappa-bungarotoxin blocks nicotinic transmission at an identified invertebrate central synapse. J Exp Biol. 1989 Jan;141:61–71. doi: 10.1242/jeb.141.1.61. [DOI] [PubMed] [Google Scholar]
- Clarke P. B. Nicotinic receptors in mammalian brain: localization and relation to cholinergic innervation. Prog Brain Res. 1993;98:77–83. doi: 10.1016/s0079-6123(08)62383-3. [DOI] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990 Dec;5(6):847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Eiselé J. L., Bertrand S., Galzi J. L., Devillers-Thiéry A., Changeux J. P., Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993 Dec 2;366(6454):479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Elgoyhen A. B., Johnson D. S., Boulter J., Vetter D. E., Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994 Nov 18;79(4):705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Horne W. A., Tsien R. W. Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature. 1994 Nov 17;372(6503):272–275. doi: 10.1038/372272a0. [DOI] [PubMed] [Google Scholar]
- Groebe D. R., Dumm J. M., Abramson S. N. Irreversible inhibition of nicotinic acetylcholine receptors by the bipinnatins. Toxin activation and kinetics of receptor inhibition. J Biol Chem. 1994 Mar 25;269(12):8885–8891. [PubMed] [Google Scholar]
- Gross A., Abramson T., MacKinnon R. Transfer of the scorpion toxin receptor to an insensitive potassium channel. Neuron. 1994 Oct;13(4):961–966. doi: 10.1016/0896-6273(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Kreienkamp H. J., Sine S. M., Maeda R. K., Taylor P. Glycosylation sites selectively interfere with alpha-toxin binding to the nicotinic acetylcholine receptor. J Biol Chem. 1994 Mar 18;269(11):8108–8114. [PubMed] [Google Scholar]
- Kukel C. F., Jennings K. R. Delphinium alkaloids as inhibitors of alpha-bungarotoxin binding to rat and insect neural membranes. Can J Physiol Pharmacol. 1994 Jan;72(1):104–107. doi: 10.1139/y94-016. [DOI] [PubMed] [Google Scholar]
- Loring R. H., Aizenman E., Lipton S. A., Zigmond R. E. Characterization of nicotinic receptors in chick retina using a snake venom neurotoxin that blocks neuronal nicotinic receptor function. J Neurosci. 1989 Jul;9(7):2423–2431. doi: 10.1523/JNEUROSCI.09-07-02423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchacz E., Buisson B., Bertrand D., Lukas R. J. Functional expression of nicotinic acetylcholine receptors containing rat alpha 7 subunits in human SH-SY5Y neuroblastoma cells. FEBS Lett. 1994 Nov 7;354(2):155–159. doi: 10.1016/0014-5793(94)01108-7. [DOI] [PubMed] [Google Scholar]
- Role L. W. Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol. 1992 Jun;2(3):254–262. doi: 10.1016/0959-4388(92)90112-x. [DOI] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Cockcroft V. B., Lunt G. G., Smillie F. S., Wonnacott S. Methyllycaconitine: a selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990 Sep 17;270(1-2):45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. 3. Effects of local anaesthetics on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):35–40. [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. I. Binding of the tritiated alpha-neurotoxin in the absence of effector. Mol Pharmacol. 1974 Jan;10(1):1–14. [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. II. Effect of cholinergic agonists and antagonists on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):15–34. [PubMed] [Google Scholar]
- de la Garza R., Freedman R., Hoffer B. J. Kappa-bungarotoxin blockade of nicotine electrophysiological actions in cerebellar Purkinje neurons. Neurosci Lett. 1989 Apr 24;99(1-2):95–100. doi: 10.1016/0304-3940(89)90271-1. [DOI] [PubMed] [Google Scholar]