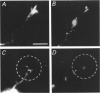
Abstract
1. The role of actin microfilaments in synaptic transmission was tested by monitoring spontaneous and evoked transmitter release from developing neuromuscular synapses in Xenopus nerve-muscle cultures, using whole-cell recording of synaptic currents in the absence and presence of microfilament-disrupting agents cytochalasins B and D. 2. Treatment with cytochalasins resulted in disruption of microfilament networks in the growth cone and the presynaptic nerve terminal of spinal neurons in Xenopus nerve-muscle cultures, as revealed by rhodamine-phalloidin staining. 3. The same cytochalasin treatment did not significantly affect the spontaneous or evoked synaptic currents during low-frequency stimulation at 0.05 Hz in these Xenopus cultures. Synaptic depression induced by high-frequency (5 Hz) stimulation, however, was reduced by this treatment. Paired-pulse facilitation for short interpulse intervals was also increased by the treatment. 4. These results indicate that disruption of microfilaments alters short-term changes in transmitter release induced by repetitive activity, without affecting normal synaptic transmission at low frequency. 5. Our results support the notion that actin microfilaments impose a barrier for mobilization of synaptic vesicles from the reserve pool, but do not affect the exocytosis of immediately available synaptic vesicles at the active zone.
Full text
PDF

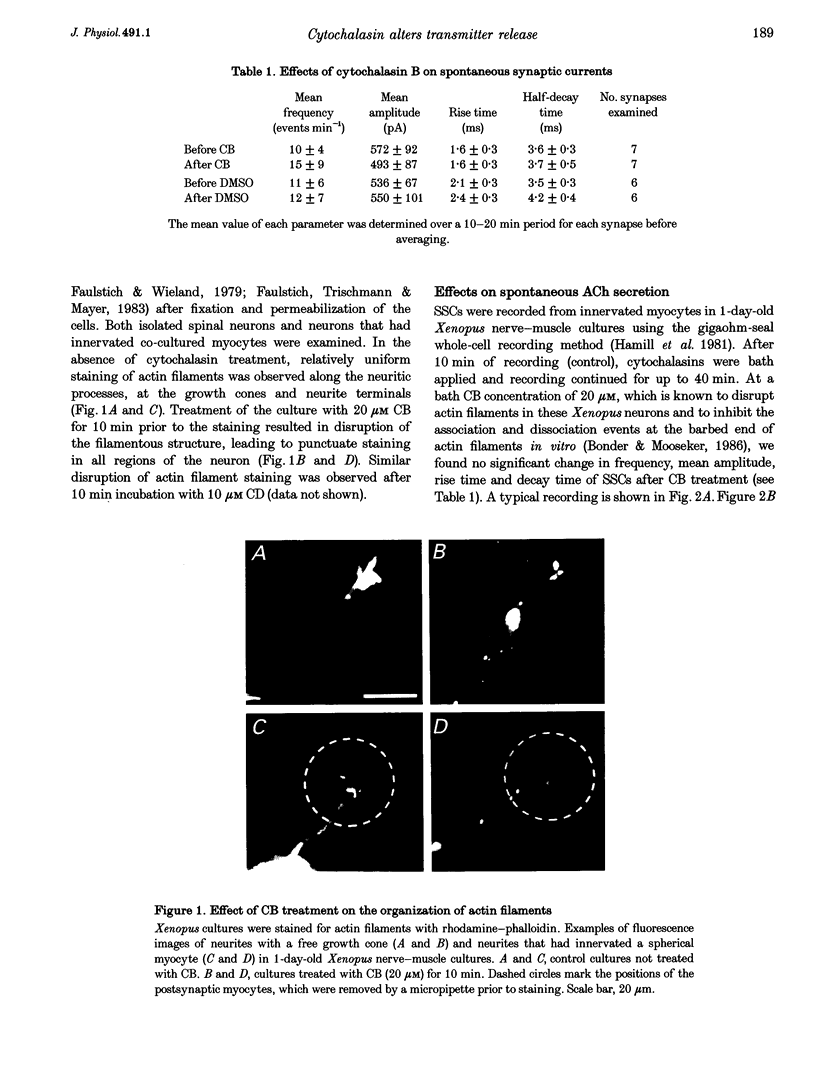
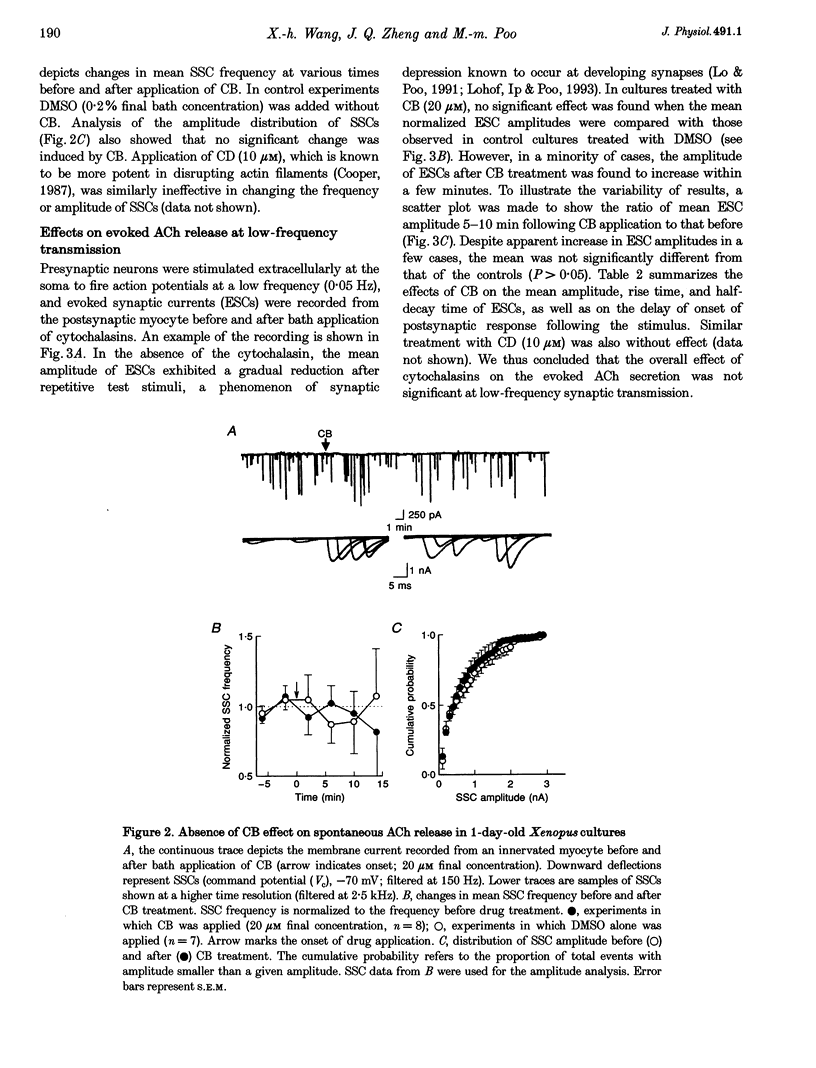
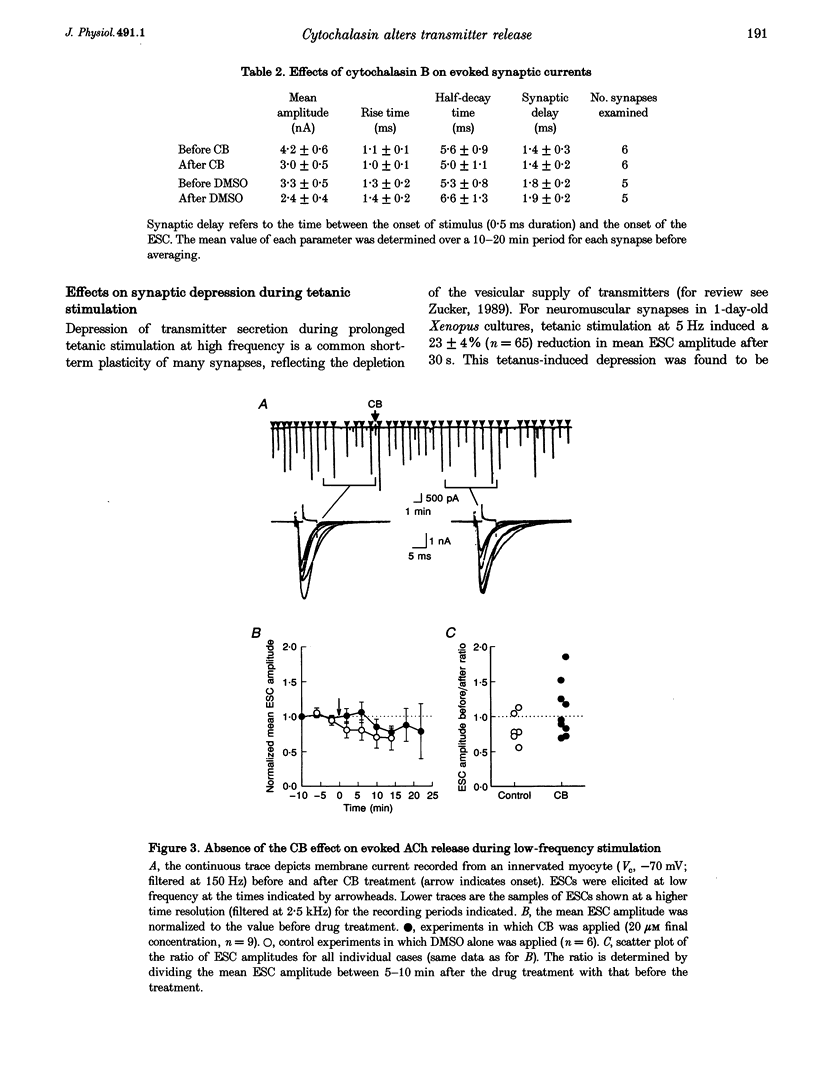
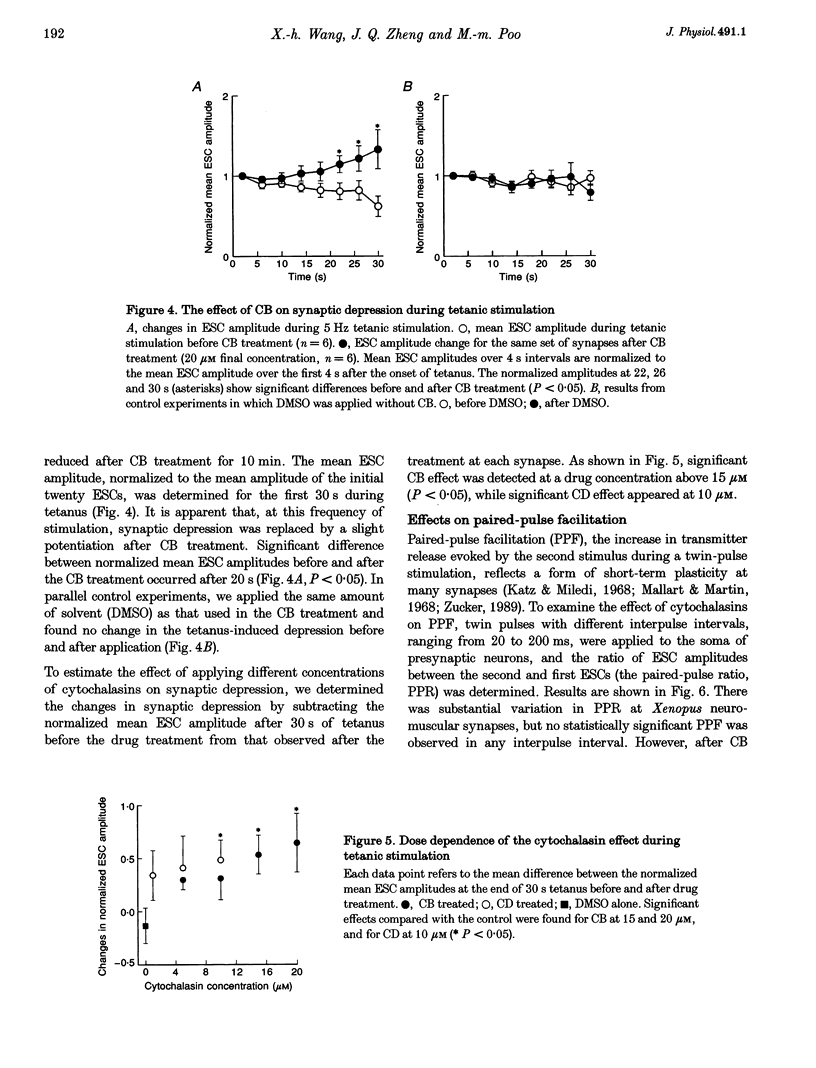
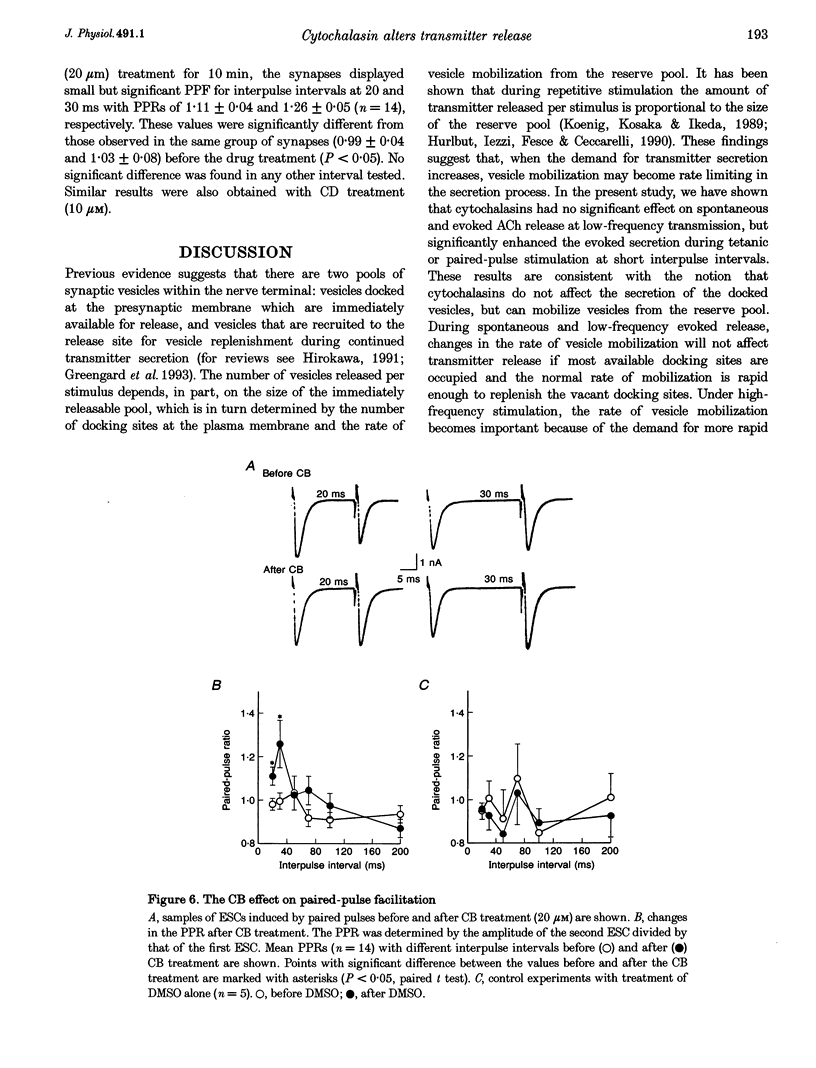


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D., Bader M. F. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988 Sep;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Benfenati F., Valtorta F., Chieregatti E., Greengard P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron. 1992 Feb;8(2):377–386. doi: 10.1016/0896-6273(92)90303-u. [DOI] [PubMed] [Google Scholar]
- Bernstein B. W., Bamburg J. R. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron. 1989 Aug;3(2):257–265. doi: 10.1016/0896-6273(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Henkel A. W. Okadaic acid disrupts clusters of synaptic vesicles in frog motor nerve terminals. J Cell Biol. 1994 Mar;124(5):843–854. doi: 10.1083/jcb.124.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonder E. M., Mooseker M. S. Cytochalasin B slows but does not prevent monomer addition at the barbed end of the actin filament. J Cell Biol. 1986 Jan;102(1):282–288. doi: 10.1083/jcb.102.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Benfenati F., Valtorta F., Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- Evers J., Laser M., Sun Y. A., Xie Z. P., Poo M. M. Studies of nerve-muscle interactions in Xenopus cell culture: analysis of early synaptic currents. J Neurosci. 1989 May;9(5):1523–1539. doi: 10.1523/JNEUROSCI.09-05-01523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Trischmann H., Mayer D. Preparation of tetramethylrhodaminyl-phalloidin and uptake of the toxin into short-term cultured hepatocytes by endocytosis. Exp Cell Res. 1983 Mar;144(1):73–82. doi: 10.1016/0014-4827(83)90443-3. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hackett J. T., Cochran S. L., Greenfield L. J., Jr, Brosius D. C., Ueda T. Synapsin I injected presynaptically into goldfish mauthner axons reduces quantal synaptic transmission. J Neurophysiol. 1990 Apr;63(4):701–706. doi: 10.1152/jn.1990.63.4.701. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Sobue K., Kanda K., Harada A., Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989 Jan;108(1):111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Iezzi N., Fesce R., Ceccarelli B. Correlation between quantal secretion and vesicle loss at the frog neuromuscular junction. J Physiol. 1990 Jun;425:501–526. doi: 10.1113/jphysiol.1990.sp018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Koenig J. H., Kosaka T., Ikeda K. The relationship between the number of synaptic vesicles and the amount of transmitter released. J Neurosci. 1989 Jun;9(6):1937–1942. doi: 10.1523/JNEUROSCI.09-06-01937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. W., Sugimori M., Llinás R. R., McGuinness T. L., Greengard P. Effects of synapsin I and calcium/calmodulin-dependent protein kinase II on spontaneous neurotransmitter release in the squid giant synapse. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8257–8261. doi: 10.1073/pnas.87.21.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. J., Poo M. M. Activity-dependent synaptic competition in vitro: heterosynaptic suppression of developing synapses. Science. 1991 Nov 15;254(5034):1019–1022. doi: 10.1126/science.1658939. [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Ip N. Y., Poo M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Dreyer F., Aktories K. Actin involvement in exocytosis from PC12 cells: studies on the influence of botulinum C2 toxin on stimulated noradrenaline release. J Neurochem. 1989 Feb;52(2):370–376. doi: 10.1111/j.1471-4159.1989.tb09131.x. [DOI] [PubMed] [Google Scholar]
- Nishida E., Maekawa S., Sakai H. Characterization of the action of porcine brain profilin on actin polymerization. J Biochem. 1984 Feb;95(2):399–404. doi: 10.1093/oxfordjournals.jbchem.a134620. [DOI] [PubMed] [Google Scholar]
- Rodriguez Del Castillo A., Lemaire S., Tchakarov L., Jeyapragasan M., Doucet J. P., Vitale M. L., Trifaró J. M. Chromaffin cell scinderin, a novel calcium-dependent actin filament-severing protein. EMBO J. 1990 Jan;9(1):43–52. doi: 10.1002/j.1460-2075.1990.tb08078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Vitale M. L. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993 Nov;16(11):466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Benfenati F., Greengard P. Structure and function of the synapsins. J Biol Chem. 1992 Apr 15;267(11):7195–7198. [PubMed] [Google Scholar]
- Vitale M. L., Seward E. P., Trifaró J. M. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995 Feb;14(2):353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]