Abstract
Trigeminal neuropathic pain (TNP), migraine, and cluster headache (CH) profoundly impact the quality of life and present significant clinical challenges due to their complex neurobiological underpinnings. This review delves into the pivotal role of the hypothalamus in the pathophysiology of these facial pain syndromes, highlighting its distinctive functions and potential as a primary target for research, diagnosis, and therapy. While the involvement of the hypothalamus in migraine and CH has been increasingly supported by imaging and clinical studies, the precise mechanisms of its role remain under active investigation. The role of the hypothalamus in TNP, in contrast, is less explored and represents a critical gap in our understanding. The hypothalamus’s involvement varies significantly across these conditions, orchestrating a unique interplay of neural circuits and neurotransmitter systems that underlie the distinct characteristics of each pain type. We have explored advanced neuromodulation techniques, such as deep brain stimulation (DBS) and optogenetics, which show promise in targeting hypothalamic dysfunction to alleviate pain symptoms. Furthermore, we discuss the neuroplastic changes within the hypothalamus that contribute to the chronicity of these pains and the implications of these findings for developing targeted therapies. By offering a comprehensive examination of the hypothalamus’s roles, this paper aims to bridge existing knowledge gaps and propel forward the understanding and management of facial neuralgias, underscoring the hypothalamus’s critical position in future neurological research.
Keywords: Trigeminal neuropathic pain, Hypothalamus, Neuroplasticity, Neuromodulation, Therapeutic potential of hypothalamus
Background
Trigeminal neuropathic pain (TNP) is a form of chronic pain that occurs in the distribution of the trigeminal nerve, which is responsible for sensation in the face. TNP is a debilitating condition resistant to conventional therapies [16, 65, 142]. The neural pathways involved in TNP processing encompass multiple brain regions, including the brainstem, thalamus, cortex, periaqueductal gray (PAG), and nucleus accumbens core (NAc) [11, 45, 61, 79–81, 96, 186, 190].
Traditionally known for regulating autonomic and homeostatic functions, the hypothalamus is recently being recognized as a key contributor to trigeminal pain modulation. This role is more acknowledged in migraine and cluster headache (CH), where hypothalamic activation coincides with pain episodes and is associated with autonomic symptoms [41, 123, 162]. In the context of TNP, preclinical studies suggest that the hypothalamus may influence trigeminal pain processing through its interactions with critical pain centers such as the trigeminal nucleus caudalis (TNC) and PAG. Specific hypothalamic regions, including the posterior and lateral hypothalamus, have shown potential in modulating pain perception by regulating neurotransmitters that impact both sensory and emotional aspects of TNP [63, 82]. These findings support the hypothesis that hypothalamic dysfunction could contribute to the heightened pain sensitivity observed in TNP, highlighting it as an intriguing area of interest for further exploration in the development of future therapeutic strategies.
Despite these promising preclinical insights, the hypothalamic role in TNP is still incompletely understood. While existing literature primarily examines the hypothalamus in migraine and CH, relatively few studies focus specifically on TNP. Moreover, the specific mechanisms through which the hypothalamus exerts its influence on TNP—such as interactions with other pain-processing regions and detailed pathway dynamics—remain to be fully elucidated. This review aims to bridge existing knowledge gaps by providing a comprehensive analysis of hypothalamic contributions to TNP, primarily based on findings from preclinical studies. Although much of the current understanding comes from animal models, these insights lay a foundational framework for clinical research exploring the hypothalamus’s role in TNP. By distinguishing both the unique and shared roles of the hypothalamus in TNP, migraine, and CH, we hope to deepen understanding of its function in trigeminal pain modulation and inspire targeted therapeutic approaches for TNP in clinical settings.
Methods
Procedures of literature search and study selection
We systematically searched the literature using PubMed and the Scopus Index to explore the hypothalamic contributions to TNP and compare these to migraine and CH. Our search strategy consisted of a computerized review of journal articles without restrictions on publication date, using the keywords “hypothalamus,” “hypothalamic stimulation,” “trigeminal neuropathic pain,” “trigeminal pain,” “orofacial pain,” “migraine,” “cluster headache,” and “pain modulation.”
To provide a thorough analysis, our review included both animal and human studies. Animal studies provided insight into fundamental biological mechanisms and potential experimental treatments, while human studies contributed valuable information on clinical manifestations, imaging results, and therapeutic outcomes. We selected 194 unique entries, applying stringent criteria focused on relevance to our topic and the novelty of findings concerning the hypothalamus’s role in TNP, migraine, and CH (Fig. 1). The first author conducted the screening of papers and identified studies that significantly contributed to our understanding of hypothalamic involvement in these conditions. We selected articles based on discussions of hypothalamic alterations in response to TNP and headache disorders, as well as various neuromodulation techniques targeting the hypothalamus. This review integrates findings on the hypothalamus in TNP, comparing and contrasting its role with that in migraine and CH while ensuring a rigorous selection of relevant literature.
Fig. 1.
Flowchart describing the study selection methods. n = number of papers
Structure and connectivity of the hypothalamus
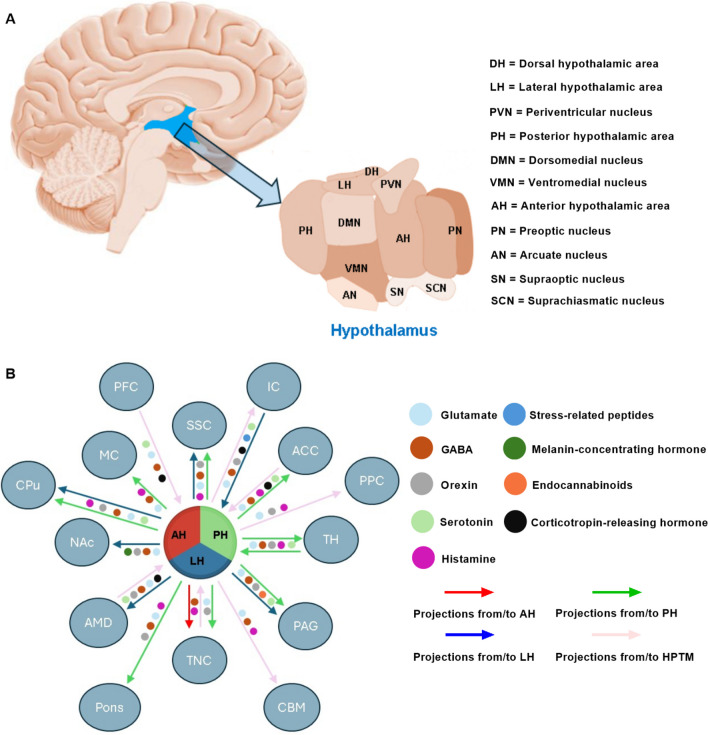
The hypothalamus, located beneath the thalamus as part of the diencephalon, is a highly conserved brain region involved in pain modulation across species, including humans, primates, and rodents [36, 159, 187]. Through complex networks with other brain regions, the hypothalamus plays a crucial role in the central nervous system (CNS) pathways responsible for pain processing. Within the hypothalamus, distinct regions—the anterior hypothalamus (AH), posterior hypothalamus (PH), and lateral hypothalamus (LH)—host specialized nuclei that contribute uniquely to pain processing (Fig. 2). Preclinical and clinical studies have shown that these hypothalamic nuclei project to various cortical and subcortical areas involved in pain modulation, including the prefrontal cortex (PFC), anterior cingulate cortex (ACC), periaqueductal gray (PAG), and TNC, with connections mediated by neurotransmitters and neuropeptides like glutamate, GABA, serotonin, histamine, orexin, and endocannabinoids [28, 51, 82, 123, 124, 144, 158, 175].
Fig. 2.
Anatomical Location and Connections of Hypothalamus. (A) Location of Hypothalamus (blue area) in the human brain and its subdivisions. (B) Hypothalamic connections with brain regions involved in CNS pain processing pathway and associated neurotransmitters. DH = dorsal hypothalamic area, LH = lateral hypothalamic area, PVN = periventricular nucleus, PH = posterior hypothalamic area, DMN = dorsomedial nucleus, VMN = ventromedial nucleus, AH = anterior hypothalamic area, PN = preoptic nucleus, AN = arcuate nucleus, SN = supraoptic nucleus, SCN = suprachiasmatic nucleus, HPTM = hypothalamus, IC = insular cortex, PFC = prefrontal cortex, MC = motor cortex, SSC = somatosensory cortex, NAc = nucleus accumbens core, TH = thalamus, AMD = amygdala, PAG = periaqueductal gray, PPC = posterior parietal cortex, TNC = trigeminal nucleus caudalis, CBM = cerebellum, ACC = anterior cingulate cortex
In the AH, the paraventricular nucleus (PVN) plays a central role in regulating stress responses and pain through the hypothalamic-pituitary-adrenal (HPA) axis. This interaction influences autonomic responses and cortisol release in the stress animal models, with PVN projections carrying stress-related peptides that facilitate pain-related stress responses [84, 170].
The PH modulates descending pain pathways and maintains essential reciprocal connections with trigeminal pain-processing brain areas. Clinical and preclinical studies indicate that the PH links the hypothalamus to higher-order pain processing and autonomic regulation through serotonin and melanin-concentrating hormone pathways [64, 68, 82, 123, 124, 160].
The LH, meanwhile, contains orexin-producing neurons traditionally associated with energy regulation but also implicated in pain modulation. Chemically-induced rat orofacial pain study has shown that LH’s orexinergic system connects extensively with pain-processing regions, fostering adaptive responses to both internal and external stressors [55, 63].
Differences among TNP, migraine, and CH
TNP is typically caused by direct injury or dysfunction of the trigeminal nerve or its branches and primarily affects one side of the face, often corresponding to the trigeminal nerve distribution. The pain experienced is intensely severe and constant. Nature of pain is often described as burning, throbbing, or aching, often combined with a sensation of numbness or tingling in the affected area. The pain may be triggered by light touch or activities that involve touching or moving the face, such as shaving, eating, or even exposure to air drafts, though it generally lacks systemic symptoms. The sensitization involves activating satellite glial cells and altering sodium and potassium conductance, increasing neuronal excitability. This is accompanied by elevated release of pro-nociceptive neuropeptides like substance P and CGRP, amplifying pain signals relayed to the TNC and ascending through the trigeminothalamic pathway to key brain regions [5, 16, 44]. Standard treatments for TNP include anticonvulsants and nerve blocks, with surgery being an option in more severe cases to alleviate the pain [16, 192].
Migraine is a complex neurological disorder influenced by genetics and environmental factors, with neurovascular disruption, especially in the trigeminovascular system, playing a central role [23]. Migraine pain, typically unilateral and pulsating, may include aura, during which cortical spreading depression (CSD) activates trigeminal fibers and promotes the release of CGRP, substance P, and neurokinin A, causing vasodilation and neurogenic inflammation [32, 37, 157, 169]. Attacks last 4–72 h, often with nausea, vomiting, and sensitivity to light and sound [23, 167]. Sensitization within the TNC may lead to chronic migraine via central sensitization. Triggers include stress, hormonal shifts, diet, sensory stimuli, and environmental changes [145]. Neuroimaging shows altered connectivity between the thalamus, hypothalamus, and prefrontal cortex, indicating maladaptive top-down modulation. Elevated CGRP and TRP channels are key in pain processing and neurovascular responses [43, 59, 89]. Common treatments include triptans and NSAIDs [24, 118].
CH is marked by intense, circadian-patterned pain due to hypothalamic activation, which drives the trigeminal-autonomic reflex [107, 147]. This activation underlies CH’s rhythmic, episodic nature, with attacks often occurring at fixed times. CH is always unilateral, centering around the orbit and temple, with excruciating, piercing, or burning pain lasting 15–180 min, occurring up to eight times daily in bouts lasting weeks or months. Autonomic symptoms—tearing, nasal congestion, and ptosis—are prominent on the affected side [115, 122]. The pathophysiology involves the PH, which triggers parasympathetic outflow via the superior salivatory and trigeminal nuclei [6, 56]. Hypothalamic orexin and hypocretin systems are upregulated, and inflammatory mediators like histamine increase, correlating with CH’s autonomic symptoms. Elevated CGRP and PACAP levels highlight neuropeptide involvement in CH [27, 69, 73]. Imaging reveals altered hypothalamic connectivity with autonomic and pain-processing regions. Triggers include alcohol, strong odors, and histamine release, with attacks often aligning daily during bouts [119]. Treatments include oxygen therapy, triptans, and preventive medications to lessen attack frequency and severity [126, 171].
All three conditions—TNP, migraine, and CH—involve severe, recurrent pain in the head or facial regions. They share common features such as trigeminal system involvement and potential triggers. Despite these similarities, they differ significantly in their pathophysiology, pain characteristics, symptoms, duration, and treatment approaches. TNP typically arises from the peripheral nervous system (PNS), frequently attributed to damage or irritation of the trigeminal nerve. In contrast, some theories suggest that migraine and CHs may primarily involve dysregulations within specific CNS structures; however, this perspective remains under debate, and evidence on the origin of these conditions is not yet conclusive [16, 124, 131]. In cases of TNP, the initial activation occurs at the TNC, followed by subsequent engagement of CNS pathways. Conversely, one prevailing hypothesis suggests that headaches may originate from the activation of nociceptors associated with meningeal blood vessels, which could then trigger the activation of trigeminovascular neurons within the spinal TNC [16, 155].
Pathophysiological differences of hypothalamus in TNP in comparison to migraine and cluster headache
The hypothalamus is integral to the pathophysiology of TNP, migraine, and CH, with distinct activation patterns, neurochemical roles, and neural projections that differentiate its functions across these conditions.
Trigeminal neuropathic pain
In the TNP processing pathway, the hypothalamus is an integral part, with its activation often occurring in relation to or following activation in other pain-processing regions, such as the TNC, thalamus, and primary and secondary somatosensory cortices [38, 61, 190]. However, TNP may not always follow a distinct onset sequence, with pain processing pathways varying based on individual factors and injury characteristics. Chronic constriction injury of the infraorbital nerve (CCI-ION) rat model study has revealed that the hypothalamus could modulate sensory-discriminative and affective-motivational TNP dimensions through neurochemical pathways and its extensive connections with pain-related regions [82]. Central to this process are excitatory glutamatergic mechanisms involving NMDA and AMPA receptors, which contribute to synaptic plasticity and central sensitization, while GABAergic neurons provide inhibitory control over pain thresholds [53, 133]. Additionally, hypothalamic activation influences pain perception via the sympathetic nervous system through vasoconstriction and blood flow adjustment, hormonal modulation through the release of vasopressin, oxytocin, and autonomic as well as endocrine responses; with the HPA axis playing a significant role in regulating pain perception and stress responses in TNP [25, 86, 121, 158, 170].
The PH is central to descending pain modulation in TNP, primarily through GABAergic projections to the ventrolateral periaqueductal gray (vlPAG) and TNC [9, 16, 50, 82]. This modulation involves both pronociceptive and antinociceptive effects mediated by orexin-A and orexin-B peptides, which regulate pain sensitivity within the trigeminal system [71, 74]. Rodent studies have demonstrated that the A11 nucleus, which contains both GABAergic and dopaminergic neurons, exhibits strong bilateral projections to the TNC and becomes activated during facial nociception [1, 2]. In CCI-ION rats, PH activation also shows increased c-Fos, indicating heightened activity during TNP [2, 82, 87].
Chronic Constriction Injury of the Infraorbital Nerve (CCI-ION)
Definition: The CCI-ION model is a well-established preclinical model used to study trigeminal neuropathic pain (TNP). It involves creating a chronic constriction injury in the infraorbital branch of the trigeminal nerve, leading to persistent pain and hypersensitivity.
Procedure: A small skin incision, approximately 7 mm in length, was made along the curve of the frontal bone in an anterior-posterior orientation, positioned 2 mm above the eye of targeted side. The fascia and muscle were carefully separated from the bone using a periosteal elevator while moving laterally. Once the eye was retracted, the infraorbital nerve (ION) became visible on the surface of the maxillary bone. To prepare for ligature placement, about 8 mm of the ION was gently isolated from the surrounding connective tissue. The nerve was then slightly stretched with a blunt needle featuring a curved tip to facilitate ligature placement. Two ligatures were placed 3–4 mm apart, and each was gently tightened until the ION was minimally constricted. The incision was then closed using sutures.
Behavioral Manifestations: Animals exhibit behaviors such as face grooming, avoidance, anxiety and hypersensitivity to mechanical (von Frey test) and thermal stimuli.
Relevance to Humans: The model simulates key features of TNP in humans, including spontaneous pain and allodynia.
Application: CCI-ION model provides insights into TNP mechanisms and the role of specific pathways. CCI-ION model is also used to evaluate potential therapeutic interventions for TNP.
The LH is observed to modulate arousal and pain sensitivity through orexin signalling in clinical studies, with reciprocal projections to the TNC [35, 124]. Dysregulation of orexin and cholinergic signalling in the LH heightened pain sensitivity and altered modulation pathways, exacerbating trigeminal pain disorders in chemical-induced orofacial pain rat model [1, 102, 165, 168].
The PVN enhances stress response via corticotropin-releasing hormone (CRH), influencing the HPA axis. This response intensifies pain chronicity in TNP by releasing stress-induced glucocorticoids, which aggravate pain through neuroinflammatory mechanisms, including microglial activation [17, 28, 33, 179]. Through its ipsilaterally predominant pervicellular and magnocellular neuronal projections to the TNC, the PVN has been observed to impact both ascending and descending pain pathways in clinical and preclinical studies, distinguishing its role from migraine and CH, where it predominantly exacerbates autonomic symptoms [1, 12, 127–129, 155].
The SCN regulates circadian rhythms, indirectly modulating pain sensitivity across different times of the day in TNP. Clinical studies indicated that dysregulation in the SCN can lead to variations in pain perception and exacerbation of symptoms, aligning with circadian pain fluctuations often reported in TNP [21, 93, 154].
Dopamine release dysregulation within the ARC influences prolactin secretion and affects emotional responses related to TNP perception in patients with trigeminal neuropathy [5, 104, 120]. Additionally, the periventricular zone, containing opioid and cannabinoid receptors, contributes to endogenous pain control in TNP patient [112]. Alterations in these receptor activities can reduce pain thresholds, affecting endogenous inhibition pathways [66, 120].
These findings collectively highlight the multifaceted role of the hypothalamus in the neural network managing pain, positioning it as a critical target for neuromodulation therapies in severe TNP conditions (Table 1). In Table 1, nociceptive dural input has also been included as TNP because it involves the activation of trigeminal second-order neurons in the TNC. This input from the dura mater engages trigeminal nerve fibers, particularly Aδ and C fibers, which are responsible for transmitting pain sensations associated with irritation. Such stimulation of trigeminal pathways leads to central sensitization and modulation of pain, characteristics of TNP [8].
Table 1.
Overview of preclinical and clinical studies on the involvement of the Hypothalamus in the TNP processing pathway
| Subject condition | Stimulation method | Analyzing procedure | Analyzed brain region | Findings of hypothalamus | Reference |
|---|---|---|---|---|---|
| Formalin- induced orofacial pain in rats | Intra-LH microinjection of Carbachol, formalin injection into the upper lip | Histological verification | LH | Neural activation | [165] |
| Nociceptive dural input in rats | Hypothalamic microinjections of Orexin A and B | Extracellular recordings | PH | Increased activity | [9] |
| TNP in mice | CCI-ION | Behavioral tests, optogenetic and chemogenetic manipulation, IHC | Hypothalamic A11 nucleus | Increased activity | [114] |
| Trigeminal pain processing in rats | Microinjections of Fluorogold (FG) into TNC | IHC | PVN, LH, A11. | Increased activity | [1] |
| Trigeminal pain processing in rats | Facial formalin test, microinjections of Muscimol and 6-OHDA | Behavioral tests, IHC, Fos expression | Hypothalamic A11 nucleus | Increased activity in GABAergic neurons. | [2] |
| Nociceptive dural input in rats | Microinjections (Bicuculline, Somatostatin, Cyclo-somatostatin) into PH | Extracellular recordings | PH | Increased activity in GABAergic neurons. | [10] |
| Light-evoked trigeminal neural activity in rats | Bright light stimulation, microinjections of BMI into PH | Electrophysiological recordings, reflex lacrimation, MAP | PH | Increased activity | [94] |
| TNP in rats | CCI-ION | In vivo extracellular recordings, behavioral tests, IFC | PH | Increased PH activity in response to trigeminal pain. | [82] |
| Orofacial pain in rats | Capsaicin injection | Behavior tests, microinjections and pharmacological manipulations, histological analysis | PH | Increased orexinergic activity | [101] |
| TNP | DBS | Neuroimaging (PET, fMRI), clinical assessments, electrophysiology | PH | Increased activity in the PH | [153] |
| Trigeminal autonomic reflex in healthy participants | Kinetic oscillation stimulation | fMRI | AH | Increased activity | [134] |
| Orofacial pain in rats | Chemical stimulation using carbachol | Behavior tests, histological analysis | LH | Neural activation | [63] |
| TNP | CCI-ION | Behavior tests, microinjections and pharmacological manipulations, histological analysis | Posterolateral hypothalamus | Loss of noradrenergic terminals | [95] |
| Orofacial pain in rats | Capsaicin injection | Behavior tests, histological analysis | PH | Increased orexinergic activity | [102] |
| TNP trigeminal neuropathic pain, fMRI functional magnetic resonance imaging, DBS deep brain stimulation, LH lateral hypothalamus, PET positron emission tomography, PH posterior hypothalamus, PVN paraventricular nucleus, AH anterior hypothalamus, CCI-ION chronic constriction injury of infraorbital nerve | |||||
Migraine
In migraine, the hypothalamus is involved in both the prodromal and headache phases and influences pain and autonomic dysregulation [123, 124, 130, 162]. Functional imaging studies have shown that hypothalamic activation often coincides with migraine symptoms, supporting its role in modulating migraine episodes [124, 139].
In migraine, the hypothalamus interacts with multiple brain regions—including the thalamus, periaqueductal gray (PAG), and TNC—primarily to regulate trigeminovascular responses and autonomic disturbances such as nausea, photophobia, and phonophobia [23, 40, 124, 132]. However, these sensory disturbances are less pronounced in TNP, where hypothalamic activation focuses more on descending pain modulation and central sensitization [82].
Increased activation of AH has been observed during migraine attacks [134]. The SCN within the AH regulates circadian rhythms and has been closely linked to the periodicity of migraine attacks. Dysregulation in the SCN, often related to disturbances in sleep-wake cycles, is a known trigger for migraine, as altered SCN activity can exacerbate circadian imbalances and increase susceptibility to attacks [4, 175]. In contrast, in TNP, SCN dysregulation primarily affects circadian fluctuations in pain sensitivity without contributing to episodic patterns [21, 93, 154].
The PH houses the tuberomammillary nucleus (TMN) and increased activity of TMN has been found to be associated with enhanced alertness and sensory responsiveness in transgenic mice [52]. Modulating TMN activity has been shown to affect migraine duration and intensity, underscoring its influence on migraine pathophysiology [72]. While the PH modulates both sensory and vascular responses in migraines, its role in TNP predominantly involves influencing nociceptive processing through descending pain pathways [16, 82].
The LH modulates autonomic symptoms such as nausea and vomiting during migraine through its projections to the TNC. Both clinical research and mouse migraine model revealed that dysregulated LH activity can amplify both pain and autonomic responses, contributing to the sensory and autonomic disturbances characteristic of migraine [140, 183]. Furthermore, brain imaging investigation showed reduced regional activity within the LH prior to the onset of migraines in migraineurs [131]. In contrast, LH activation in TNP primarily regulates pain sensitivity through orexin signalling [35, 124].
The PVN in migraine regulates the stress response through corticotropin-releasing hormone (CRH), activating the HPA axis and increasing cortisol levels. This amplifies headache severity by engaging autonomic centers and stress responses [57, 67], , a feature less emphasized in TNP, where PVN activity is more directly linked to neuroinflammatory pathways [17, 28, 33].
Cluster headache
The hypothalamus’s involvement in circadian regulation and autonomic responses aligns with CH’s characteristic daily or seasonal attack patterns [149, 161, 188]. Neuroimaging studies, including PET and fMRI, consistently demonstrate increased PH activity in CH, implicating the PH in triggering severe headache episodes and influencing attack frequency and intensity. While PH activation is also prominent in TNP, in CH, it is more directly associated with episodic patterns and vascular modulation, which are not primary features in TNP [49, 115, 123, 124, 172, 185].
The SCN within the AH plays a crucial role in regulating circadian rhythms, a prominent feature in CH given its tendency to follow specific daily and seasonal patterns. Dysregulation within the SCN can lead to disruptions in these rhythms, potentially triggering or exacerbating CH episodes [4, 134]. While the SCN also influences circadian rhythms in TNP [21, 93], its role in CH is more pronounced, with increased SCN activity during attacks leading to severe disruptions in circadian rhythms and heightened attack susceptibility [149, 188].
The PVN in CH plays a key role in autonomic symptoms such as ptosis, lacrimation, and nasal congestion through CRH release and HPA axis activation [155]. This contrasts with its role in TNP, where PVN activity contributes to neuroinflammation and pain chronicity rather than autonomic symptoms [17, 28, 33]. Moreover, the PVN’s projections to brainstem autonomic centers further enhance the severity of CH attacks, highlighting its distinct contributions compared to TNP.
Hypothalamus and its neural interactions in TNP and their role in migraine and CH
The hypothalamus is a key player in modulating pain and stress responses, with widespread connections to various brain regions involved in the TNP processing pathway. These connections and activity changes illustrate the complex network through which the hypothalamus may modulate the TNP processing pathway. They also highlight the neurochemical alterations that intensify pain perception during TNP. Figure 3 illustrates the hypothalamus-centered neural pathways of migraine and CH pathophysiology, based on findings from both clinical research and animal model studies. In contrast, TNP pathophysiology is depicted based on findings from preclinical rodent studies, primarily using the CCI-ION model.
Fig. 3.
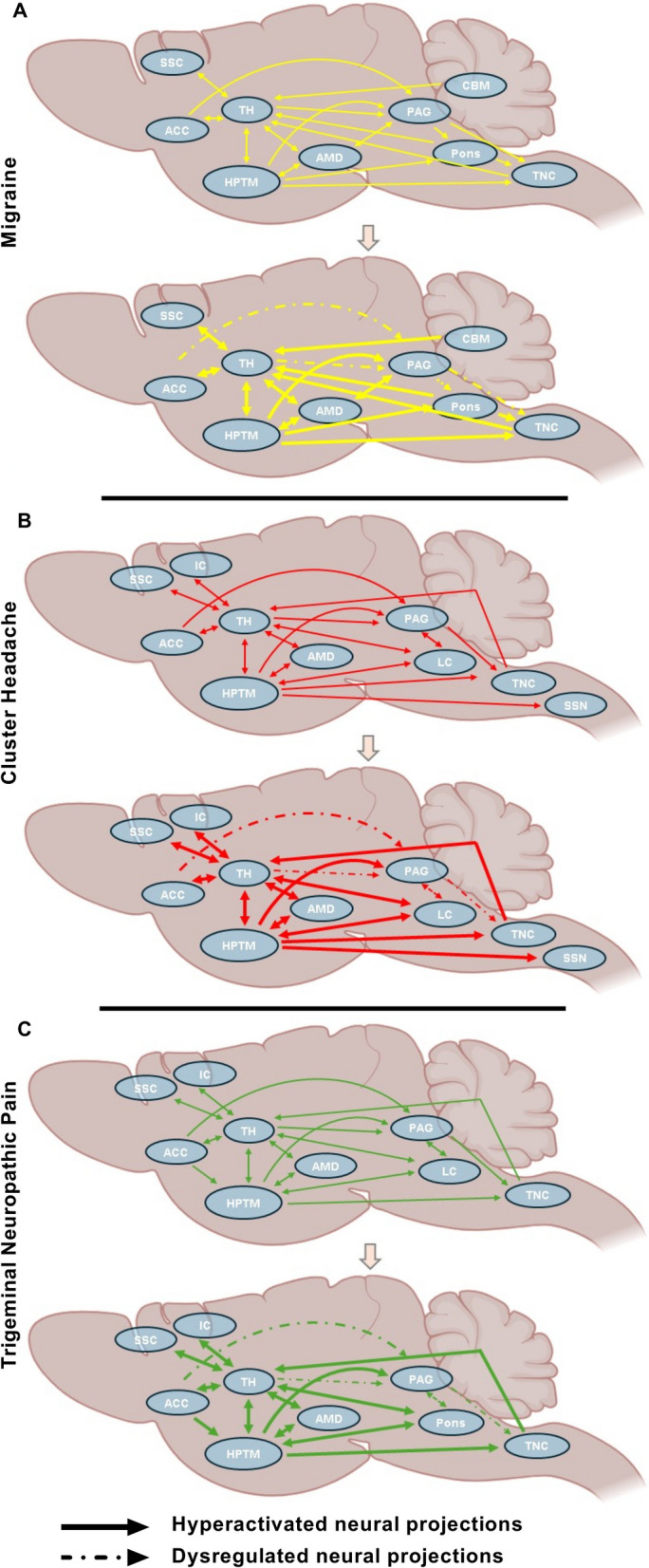
Hypothalamus-Centric Neural Circuitry Alterations in Migraine, CH, and TNP. (A) Alterations of neural projections during migraine pathophysiology. (B) Alterations of neural projections during CH pathophysiology. (C) Alterations of neural projections during TNP pathophysiology. HPTM = hypothalamus, IC = insular cortex, SSC = somatosensory cortex, TH = thalamus, AMD = amygdala, PAG = periaqueductal gray, TNC = trigeminal nucleus caudalis, CBM = cerebellum, ACC = anterior cingulate cortex, LC = locus coeruleus, SSN = Superior Salivatory Nucleus
Trigeminal nucleus caudalis
Studies in rat models have shown that several hypothalamic nuclei, including the PVN, LH, A11, perifornical area (PFX), and retrochiasmatic area, project to the TNC, with a significant number of neurons from the trigeminal brainstem nuclear complex (TNBC) also directly projecting to various hypothalamic regions [1, 54, 84, 114]. Through its reciprocal trigeminal-hypothalamic connections, the PH can both suppress and amplify pain responses in the CCI-ION rat model, positioning it as a physiological regulator of TNC activity [9, 82, 94]. In the PVN of naïve rats, there are direct descending GABAergic projections that interact and influence the activity of laminae I and II Aδ and C nociceptors within the TNC neurons [155]. Studies have shown increased activity in these nuclei due to elevated glutamate and diminished GABAergic signalling, which facilitate pain transmission to the thalamus and cortex, thus integrating the hypothalamus into the broader neural network that governs pain perception [60, 82, 153]. However, while the connection between the hypothalamus and the TNC primarily modulates direct pain post-injury in TNP, in migraine, the hypothalamus receives increased input from the TNC which may amplify pain and autonomic responses during migraine attacks [124, 155], and in CH, it is involved in circadian timing and the modulation of cyclical attacks [135].
Pons
PH has direct interaction with the A7 catecholamine cell group in the pons [87]. On the other hand, the parabrachial nucleus (PBN) receives trigeminal nociceptive signals from the TNC and after integrating nociception and visceral sensory information, relays to the hypothalamus. The hypothalamus also has significant connections with the reticular formation. GABAergic projections from the hypothalamus to the locus coeruleus in CCI-ION rats get dysregulated in TNP condition [95]. Increased activity in the reticular formation during TNP, mediated by heightened norepinephrine and dopamine release, enhances the overall sensory responsiveness and pain thresholds [60, 153]. During migraine, this connection regulates brainstem centers that are involved in headache onset and maintenance [132, 163]. In CH, the hypothalamus-pons link is significant for its role in circadian rhythm regulation, which is crucial in triggering headache attacks [149].
Cerebellum
The hypothalamus indirectly interacts with the cerebellum via brainstem nuclei, impacting coordination and motor responses related to sensory inputs. Clinical findings suggest that during TNP, changes in GABA and glutamate levels alter cerebellar processing, impacting motor control and the coordination of pain response [7, 65]. In migraine, this pathway may modulate coordination and autonomic responses [194], while in CH, its role remains unclear but could involve autonomic dysregulation [188].
Periaqueductal Gray (PAG)-Rostral Ventromedial Medulla (RVM) system
Both PH and LH of naïve rat were found to send direct projections to the PAG-RVM system [58, 94]. Additionally, a stress rat model study revealed that the dorsomedial hypothalamus sends cholecystokinin projections to the RVM [184]. In TNP, there is an increased release of neurotransmitters such as glutamate, endorphins, and enkephalins from the PAG, which typically act to inhibit pain. However, in CCI-ION rats, the hypothalamic inhibitory projections to the PAG are enhanced, paradoxically reducing the effectiveness of the descending pain processing pathway and leading to heightened pain perception [13, 82, 97]. During a migraine and in the period immediately preceding it, altered hypothalamus-PAG connectivity impairs pain inhibition [20, 132, 182]. In CH, it contributes to both the modulation of pain and the onset of headache attacks [48].
Ventral Posteromedial (VPM) nucleus of the thalamus
The PH engages in a bidirectional interaction with the VPM thalamus. This interaction involves the PH receiving nociceptive information from the VPM thalamus through trigeminovascular inputs, and optogenetic inhibition of the PH in CCI-ION rats has been shown to have a modulatory effect on the thalamus [64, 82]. In TNP, altered thalamic function, marked by increased glutamate release, enhances pain signal transmission to the cortex, highlighting the critical role of hypothalamic-thalamic interactions in pain modulation and perception [177, 180]. In migraine, altered hypothalamic-thalamic connectivity affects pain processing and transmission, contributing to migraine’s intense headache and sensory disturbances [75, 92]. During CH, this pathway serves as a key relay for pain signals during attacks [125].
Amygdala
In TNP, increased amygdala activity, driven by elevated hypothalamic CRH, exacerbates pain perception and emotional distress. This heightened activity is supported by reciprocal sensory projections between the amygdaloid complex and the hypothalamus [64, 84]. Additionally, melanin-concentrating hormone (MCH) projections from the LH directly innervate the amygdala, reinforcing this interconnection that influences both chronic pain processing and emotional regulation in response to nociceptive stimuli in mice model of inflammation [85]. For migraine sufferers, it influences the emotional aspects of headache pain [22], while in CH, it modulates both pain perception and the associated emotional response [151].
Nucleus Accumbens core (NAc) and Ventral Tegmental Area (VTA)
The carbachol-injected rat model showed that the LH-VTA-NAc circuit plays a critical role in modulating pain by regulating dopamine release in the NAc [137]. LH sends direct MCHergic projections to NAc. In addition, LH stimulation activates orexinergic neurons that project to the VTA. These projections lead to dopamine release in the NAc, which helps reduce pain perception by increasing NAc activity. In contrast, blocking dopamine receptors in the NAc diminishes the pain-relieving effects of LH stimulation in formalin-injected orofacial rat pain model [165]. Stimulating NAc activity has also been observed to improve TNP condition in CCI-ION rats [80]. This connection may influence pain perception in migraine and cluster headache (CH) through its role in reward pathways, potentially affecting the overall headache experience. However, direct evidence for this mechanism is yet to be confirmed by research.
Caudate Putamen (CPu)
The PH and the premammillary region send both histaminergic and non-histaminergic projections to the CPu complex, while the LH projects MCH neurotransmission [85, 139, 174]. Histaminergic projections could modulate pain through inflammatory responses, potentially altering pain thresholds, and thus affecting TNP. Non-histaminergic projections, possibly involving neurotransmitters like GABA or glutamate, could play crucial roles in either suppressing or enhancing pain perception, respectively. The MCH neurotransmission from the LH may impact sensory processing and emotional responses to TNP, due to its roles in sleep and energy balance. Therefore, while these neural interactions could hypothetically suggest alterations in the hypothalamus-CPu pathway in response to chronic pain, further investigations are required to directly establish this implication. In migraine, it could be involved in modulating motor aspects of the condition and the pain experience [110]. In CH pathophysiology, its involvement is yet to be elucidated.
Anterior Cingulate Cortex (ACC)
The hypothalamus possesses a reciprocal affective and motivational connection related to pain with the ACC [77]. Optogenetic stimulation in the CCI-ION rat model revealed that ACC stimulation alters TNP, driven by changes in serotonin and norepinephrine levels, leading to increased pain-related distress and cognitive focus on pain [136]. In migraine, the ACC plays a significant role in the perception of pain and the emotional response to headaches [99]. During CH, this connection is involved in processing the emotional aspects of pain and modulating the headache experience [125].
Insular cortex (IC)
The hypothalamus modulates the IC through autonomic pathways, affecting visceral, sensory, emotional, and autonomic aspects of TNP processing. Altered GABAergic and glutamatergic signalling in TNP increases IC activity, enhancing pain perception. Hypothalamic stimulation can deactivate the insula, which may modify TNP progression. Studies on rodent trigeminal pain models depicted that both anterior and dorsal IC have strong connections with the LH, primarily via neurons expressing the 5-HT1A receptor, projecting to both rostral and caudal LH sections [79, 82, 83, 90, 100, 101, 178]. During both migraine and CH, it might regulate the autonomic responses that occur during attacks, such as tearing and nasal congestion [135].
Motor cortex
The hypothalamus exerts an influence on the motor cortex through its roles in autonomic and endocrine regulation, affecting how motor responses are generated in response to sensory stimuli [153]. Studies have shown that PH stimulation results in increased spiking activity in the motor cortex and heightened motor cortex activity in CCI-ION rats has been observed to alter the TNP processing pathway [81, 189]. For CH, this connection could influence motor responses related to headache attacks [34]. In migraine, the role of the hypothalamus-motor cortex connection remains to be fully elucidated.
Somatosensory cortex (SSC)
The hypothalamus influences the primary and secondary somatosensory cortices (SSC1 and SSC2) through thalamic relay modulation. In SSC1, increased glutamatergic activity and reduced inhibition heighten pain perception, while in SSC2, altered neurotransmitter release, such as substance P and calcitonin gene-related peptide (CGRP), enhances pain signal integration. Hypothalamic stimulation in both preclinical and clinical studies activates SSC1, directly impacting cortical processing, and the LH projects MCH-expressing neurons to the SSC [60, 85, 125, 189]. In migraine and CH, this connection plays a role in processing sensory aspects of headache pain, contributing to the intense pain experienced during attacks [42, 60, 125].
Posterior parietal cortex
The hypothalamus, through its connections with the thalamus and other cortical areas, impacts the posterior parietal cortex, which integrates sensory information with motor responses. Altered neurotransmitter release, including increased norepinephrine and reduced GABA, affects the integration of sensory inputs and motor planning, leading to maladaptive pain responses [98]. In migraine, it might be involved in spatial awareness and sensory integration, although its role is not fully understood [150]. For CH, the involvement of this pathway is unclear but may relate to sensory processing [125].
Prefrontal cortex (PFC)
Specific regions such as the dorsolateral PFC (dlPFC), and the ventromedial PFC (vmPFC) have been implicated in modulating the affective and cognitive dimensions of pain [76, 116]. The PFC exerts a top-down modulatory influence on the hypothalamus, affecting pain perception through the regulation of descending inhibitory pathways and stress-related responses. In TNP, the PFC-hypothalamic pathway modulates not only cognitive aspects of pain but also the chronicity of pain by influencing how the hypothalamus interacts with other pain-related regions such as the brainstem and thalamus. Top-down modulation from the PFC, particularly through the dorsolateral PFC, can suppress or amplify pain responses via hypothalamic regulation of autonomic and neuroendocrine systems. Dysfunction in this top-down control may contribute to the persistence and exacerbation of pain in TNP [19, 143].
During migraine, the PFC’s involvement includes influencing both the cognitive and emotional responses to headache pain. The vmPFC, in particular, plays a role in emotional regulation, while the dlPFC is involved in pain modulation and decision-making regarding pain management [88]. Decreased dlPFC-hypothalamic functional connectivity has been observed in migraineurs, immediately prior to a migraine event [141]. In CH, disruptions in PFC-hypothalamic connectivity may affect executive functions and emotional regulation during attacks, potentially influencing the frequency and severity of headache episodes by altering stress and autonomic responses [181].
Hypothalamus (Internal Interactions)
Different hypothalamic nuclei, such as the PVN, LH, and A11, collaborate to regulate functions like stress responses, autonomic regulation, and pain modulation. Alterations in the balance of neurotransmitters including CRH, orexins, and endorphins within the hypothalamus can lead to dysregulated pain and stress responses, which may exacerbate the symptoms of TNP [26, 84, 103, 117]. Additionally, the PH is subject to strong GABAergic inhibitory control; when this inhibition is reduced, it prompts significant increases in sympathetic activity. This interaction between neurotransmitter balance and GABAergic control observed in both naïve and CCI-ION rats highlights the complex neural mechanisms by which the hypothalamus influences autonomic and pain systems [82, 94]. However, in migraine, the hypothalamus is involved in regulating circadian rhythms and homeostatic functions, which are crucial in headache management [72, 78]. For CH, internal hypothalamic interactions are central in generating circadian-related triggers and modulating the pain response during attacks, contributing to the characteristic periodicity of these headaches [73].
Other trigeminal sensory and motor nuclei in TNP
In addition to the TNC, other trigeminal sensory nuclei, such as the principal sensory nucleus (PSN) and the spinal trigeminal nucleus oralis (Sp5O), are responsible for transmitting sensory information such as fine touch and proprioception [146, 148]. While these nuclei are involved in general sensory processing, their direct role in TNP is less understood. Changes in their activity may contribute to altered sensory experiences in TNP, but further research is needed to clarify their specific involvement in pain modulation within the trigeminal system.
Similarly, the motor nucleus of the trigeminal nerve (Mo5), which controls the muscles of mastication [148], may be implicated in the motor responses associated with orofacial pain in TNP. Although there is limited evidence directly linking Mo5 to TNP, motor dysfunctions, such as abnormal jaw reflexes or changes in muscle tone, may result from maladaptive pain-related motor responses. This highlights the potential for motor nuclei to play a role in TNP, though more focused studies are required.
Targeting the hypothalamus for the alteration of the TNP Processing Pathway
Although relatively few, several preclinical studies with neuromodulation approaches targeting the hypothalamus have shown promise in managing TNP by altering the release of specific neurotransmitters involved in the modulation of descending pain processing pathways (Fig. 4). These findings suggest the need to conduct extensive studies in both clinical and preclinical domains to understand the individual variability in the structure and function of the hypothalamus in the aspect of TNP. Table 2 provides a comparative analysis of various neuromodulation approaches across TNP, migraine, and CH, to highlight the current state of technology application.
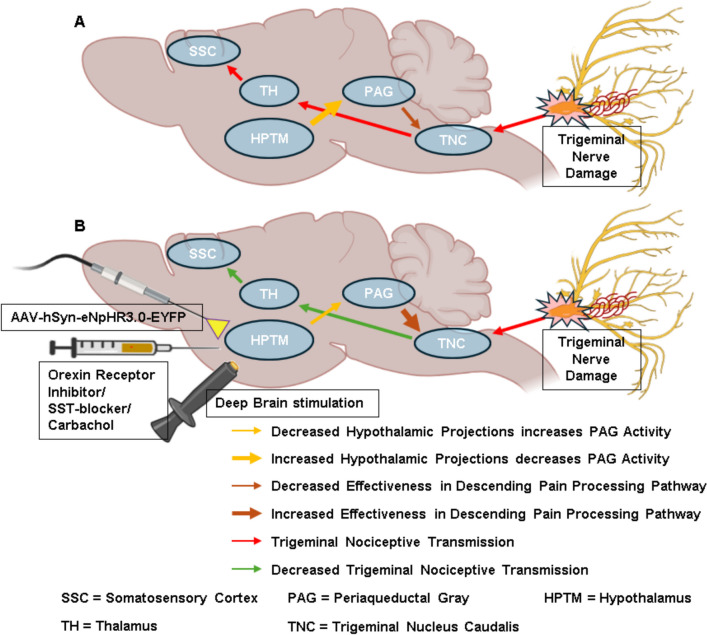
Fig. 4.
Hypothalamic activity modulation alters the efficacy of descending pain processing pathway to inhibit trigeminal nociceptive transmission. (A) Enhanced hypothalamic activity leads to decreased effectiveness in descending pain processing pathways. (B) Altered hypothalamic activity by neuromodulation leads to increased effectiveness in descending pain processing pathways. SST = Somatostatin, AAV-hSyn-eNpHR3.0-EYFP = optogenetic virus
Table 2.
Comparison of neuromodulation approaches targeting hypothalamus in TNP, migraine, and CH
| Technology/Approach | Condition | Laboratory-Level Research | Clinical Use |
|---|---|---|---|
| Pharmacological Approaches | TNP | Tricyclic antidepressants, SNRIs, SST-antagonists, orexin receptor inhibitors, carbachol microinjection, GABAergic receptor inhibitors, α−2 adrenoceptor interactions [2, 10, 87, 165]. | None specified, but pharmacological agents like TCAs and SNRIs are widely used in clinical settings for TNP management [39, 156]. |
| Migraine | Research on specific pharmacological agents targeting hypothalamic pathways such as Suvorexant (orexin receptor antagonist), glyceryltrinitrate, SST-receptor antagonist [10, 46, 70]. | Ergotamine and dihydroergotamine (DHE), beta-blockers, antiepileptic drugs, NSAIDs, SNRIs, Triptan, Erenumab, Galcanezumab, pituitary adenylate cyclase-activating peptide 1–38 (PACAP1-38) and casein kinase 1 delta (CKIδ) [138, 152, 176]. | |
| CH | Investigations into hypothalamic involvement in CH pathogenesis using muscimol, gabazine, PACAP38, naratriptan [155]. | Medications like Verapamil, Topiramate, sumatriptan and corticosteroids are used [106]. | |
| Optogenetic Stimulation | TNP | Optogenetic inhibition of the PH, stimulation of dopamine D2 receptors in A11 nucleus [82, 114]. | Currently limited to experimental models like CCI-ION rat model; not yet in clinical use. |
| Migraine | There is a significant gap in optogenetic research targeting the hypothalamus in migraine pain models. Future research covering this gap might potentially reveal new therapeutic management. | No current clinical application; optogenetics is primarily research-focused at this stage. | |
| CH | There is a significant gap in optogenetic research targeting the hypothalamus in CH pain models. Future research covering this gap might potentially reveal new therapeutic management. | Not in clinical use, remains a laboratory research tool. | |
| Electrical Stimulation | TNP | DBS focusing hypothalamus in the TNP animal model is yet to be done. However, this could open a new strategic door for TNP management. | DBS has been used in patients with TNP [50] |
| Migraine | Research on hypothalamic involvement in migraine modulation via DBS, rTMS [14]. | rTMS and DBS could be emerging treatments for refractory migraine; however, still under study. | |
| CH | DBS of the hypothalamus was explored in animal models for understanding pain and autonomic pathophysiology [3]. | Hypothalamic DBS is a recognized treatment for refractory CH [31, 49, 91, 108, 173]. |
Pharmacologic approaches
Pharmacological interventions, including tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), impact pain modulation through multiple neural pathways, including those influencing the hypothalamic-brainstem connection. These drugs act on serotonergic and noradrenergic systems distributed throughout the brain, altering pain perception by modulating descending pain pathways that interact with hypothalamic nuclei as well as broader brainstem regions. Clinical studies have shown that these drugs can modulate neurotransmitter activity in pathways associated with trigeminal pain processing, helping to reduce pain perception [18, 62]. Additionally, nociceptive dural input rat model using intra-hypothalamic injections of specific receptor antagonists and agonists has highlighted the role of the hypothalamus in pain control; however, it is important to note the ethical and practical limitations of translating these direct hypothalamic interventions to human clinical practice. Blocking somatostatin (SST) receptors in the PH using SST antagonists has been shown to produce antinociceptive effects in animal models of trigeminal pain [9, 10]. Similarly, the administration of carbachol, a cholinergic receptor agonist, in the LH of formalin-induced orofacial rat model increased orexinergic neuron firing, which correlated with reduced pain-related behaviors in rats, indicating a decrease in orofacial pain perception [165]. Carbachol microinjection into the PH of rats also interacts with α−2 adrenoceptors in the spinal dorsal horn and the A7 catecholamine cell group in the pons, further modulating nociception and the descending pain processing pathway [87].
Optogenetic stimulation
Optogenetic techniques have demonstrated efficacy in modulating pain responses in preclinical animal models of TNP by targeting specific neural pathways. In CCI-ION rats, inhibiting the PH using optogenetic approach reduced pain-related behaviors by enhancing activity in the ventrolateral periaqueductal gray (vlPAG). This modulation facilitated descending inhibitory pathways projecting to the TNC, leading to decreased trigeminal pain transmission and perception [82]. Additionally, optogenetic stimulation of dopamine D2 receptors in the A11 nucleus of CCI-ION mice attenuated TNP, underscoring the importance of the dopaminergic pathway from the A11 nucleus to the TNC in pain modulation [114]. However, optogenetic stimulation remains a research-focused technique limited to animal models and has yet to be applied in clinical practice for human patients, given the ethical and practical limitations of such direct interventions in humans.
Electrical stimulation
Hypothalamic stimulation, particularly in the medial preoptic nucleus (MPO) and the PVN, inhibits spinal cord neurons’ response to noxious stimuli, underscoring the hypothalamus’s role in antinociception. DBS in the PH enhances inhibitory projections to the brainstem and spinal cord by modulating neurotransmitter release, including serotonin, norepinephrine, and GABA, effectively reducing pain transmission and perception in patients with neuropathy involving the first trigeminal branch, and orofacial pain [50, 105]. Acute PH stimulation in naïve rats also contributes to pain management by interrupting autonomic control circuits, inhibiting evoked trigeminal nerve activity [94].
Potential prospects for hypothalamus modulation in clinical studies
Recent neuromodulation advances emphasize the hypothalamus’s crucial role in pain processing, presenting new therapeutic options. This suggests potential applicability in directly modulating hypothalamic pathways to alleviate TNP symptoms. Optogenetic stimulation, providing precise neuronal control, has also shown promise in preclinical TNP models, where targeted hypothalamic modulation reduced pain behaviors [82, 114]. Therefore, although still in the experimental stage, optogenetic modulation of the hypothalamus holds significant promise for translating research findings into human TNP management.
Deep transcranial magnetic stimulation (dTMS) and near-infrared (NIR) stimulation are innovative, non-invasive neuromodulation techniques that offer promising approaches for managing TNP. dTMS, unlike conventional TMS, uses specialized coils like the H-coil to reach deeper brain regions, such as the hypothalamus, which is critical in pain processing. This makes dTMS more suitable for conditions involving deep brain structures, though its deeper penetration requires careful clinical application [105, 113, 166, 193]. Nanoparticle-based NIR stimulation, including NIR optogenetic stimulation, also enables precise modulation of deep brain areas. Nanoparticles convert NIR light into localized heat or electrical signals, but its use in humans remains experimental due to challenges like biocompatibility, nanoparticle delivery control, and immune response management [29, 191]. Current efforts focus on animal models, and more research is necessary to address these challenges and validate the technique for human clinical applications. Despite these hurdles, the potential for this approach in treating complex pain disorders remains promising.
Both techniques reduce surgical risks and improve patient acceptability due to their non-invasive nature. Key factors for clinical use include accurate targeting with advanced imaging, optimizing stimulation parameters, and selecting appropriate patients. Additionally, understanding the long-term effects on brain function is essential. Therefore, continued research and clinical trials are essential to establish their efficacy and safety, potentially transforming TNP treatment in the future.
Discussion
The hypothalamus has been extensively recognized for its role in regulating pain perception and autonomic functions in humans. However, preclinical studies suggest that the hypothalamus’s involvement in TNP is multifaceted and complex, warranting specific consideration due to its distinct anatomical connections and functional roles in pain processing.
While hypothalamic DBS has been shown to increase pain thresholds in the trigeminal dermatome [91, 109], this effect primarily pertains to general nociceptive modulation rather than TNP-specific mechanisms. Preclinical studies have demonstrated promising results, suggesting that the hypothalamus could serve as a modulator in TNP by influencing descending pathways involved in trigeminal pain processing [1, 2, 82, 114]. However, these findings have not yet been translated into clinical studies, highlighting a significant gap between preclinical evidence and clinical application. It is essential to distinguish between the hypothalamus’s general pain modulation effects and its potential etiological role in TNP pathogenesis.
Presently, studies suggest that hypothalamus-induced changes in trigeminal neural activity include multiple descending pathways. While general neurotransmitter systems have been identified, specific interactions and regulatory mechanisms are still unclear. Post-injury changes in neurotransmitter levels within the hypothalamus, including glutamate, GABA, serotonin, and endogenous opioids, influence pain processing and modulation [15, 162]. Elevated c-Fos expression in rat hypothalamic neurons post-CCI-ION surgery indicates increased neuronal activity and involvement in TNP, highlighting the role of neurochemical imbalances in central sensitization [82]. Therefore, Investigating the molecular mechanisms of hypothalamic pain modulation—particularly neurotransmitter interactions like serotonin, norepinephrine, and endogenous opioids, and receptor dynamics of NMDA and AMPA receptors—is crucial for advancing this field.
Comparative studies on hypothalamic involvement in TNP, migraine, and CH are limited, yet essential for identifying unique pathophysiological features and developing targeted treatments. To address this gap, it is necessary to conduct studies that combine clinical observations, neuroimaging, and experimental research to highlight the distinct and overlapping roles of hypothalamic nuclei in these conditions.
Imaging small brain regions like the hypothalamus and brainstem presents significant challenges due to their size, location, and complex vascular networks [111]. Techniques such as fMRI, PET, and voxel-based morphometry offer valuable insights into their roles in pain processing. However, their small size and proximity to structures like ventricles and large blood vessels make achieving precise imaging results difficult, especially in human studies [30]. Artifacts caused by respiratory and cardiac motion, along with the inherent spatial resolution limits, can obscure activation patterns in these regions [47]. This is particularly critical for the hypothalamus, which is central to autonomic and pain regulation. Recent advances, such as ultra-high field MRI (7T MRI), have improved spatial resolution, allowing for better differentiation of these areas [164]. However, capturing dynamic changes in activity remains challenging. Continued development of imaging technologies is crucial for accurately mapping the involvement of the hypothalamus and brainstem in conditions like TNP, migraine, and CH.
Most current studies on TNP are cross-sectional, capturing only a single stage of disease progression, while longitudinal research on hypothalamic involvement from acute to chronic phases is notably limited. Conducting such studies could provide essential insights into the evolution of TNP, hypothalamic changes over time, and potential biomarkers for disease progression. To clarify the mechanisms by which hypothalamic nuclei influence TNP, future research should employ functional mapping and mechanistic studies using advanced neuroimaging and animal models. Development of targeted interventions that modulate hypothalamic function—integrating pharmacology with neuromodulation techniques like TMS, DBS, and optogenetic or chemogenetic approaches may reveal new therapeutic pathways. In addition, cross-disciplinary collaboration will be crucial to improve disease recognition and advance treatment options, ultimately enhancing TNP patient outcomes.
Conclusion
This review highlights the pivotal role of the hypothalamus in TNP, as observed in preclinical studies, and contrasts its functions with those in migraine and CH. Distinct hypothalamic nuclei are implicated in each condition, each with varying involvement in pain modulation and autonomic regulation. However, laboratory studies have yet to specifically target these nuclei in TNP, highlighting the need for focused mechanistic research. Clinically, neuromodulation techniques such as DBS and emerging pharmacological approaches should be also tailored to account for these hypothalamic distinctions. Understanding the unique roles of the hypothalamus and trigeminovascular pathways across TNP, migraine, and CH could facilitate the development of targeted therapies. Advances in mechanistic insights may lead to specific interventions that modulate TNP pathways, offering more effective and personalized treatment options for patients with this debilitating condition. Therefore, future research should prioritize a detailed exploration of these areas, supported by cross-disciplinary collaborations, to deepen understanding and improve therapeutic outcomes for TNP patients.
Acknowledgements
Not applicable.
Abbreviations
- AAV
Adeno-associated Virus
- ACC
Anterior Cingulate Cortex
- AH
Anterior Hypothalamus
- AMD
Amygdala
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
- ARC
Arcuate Nucleus
- CBM
Cerebellum
- CC
Corpus Callosum
- CCI
ION-Chronic Constriction Injury of Infraorbital Nerve
- CGRP
Calcitonin Gene-Related Peptide
- CH
Cluster Headache
- CNS
Central Nervous System
- CRH
Corticotropin-Releasing Hormone
- DBS
Deep Brain Stimulation
- EYFP
Enhanced Yellow Fluorescent Protein
- GABA
Gamma-Aminobutyric Acid
- GFAP
Glial Fibrillary Acidic Protein
- HPA
Hypothalamic-Pituitary-Adrenal
- HPTM
Hypothalamus
- IC
Insular Cortex
- ION
Infraorbital Nerve
- LH
Lateral Hypothalamus
- LTP
Long-Term Potentiation
- MC
Motor Cortex
- MCH
Melanin-Concentrating Hormone
- MD
Mediodorsal Thalamic Nucleus
- MPO
Medial Preoptic Area
- MRI
Magnetic Resonance Imaging
- NMDA
N-Methyl-D-Aspartate
- PACAP
Pituitary Adenylate Cyclase-Activating Polypeptide
- PAG
Periaqueductal Gray
- PBN
Parabrachial Nucleus
- PET
Positron Emission Tomography
- PFC
Prefrontal Cortex
- PFX
Preoptic Area Fiber Crossing
- PH
Posterior Hypothalamus
- PNS
Peripheral Nervous System
- PPC
Posterior Parietal Cortex
- PVN
Paraventricular Nucleus
- RVM
Rostral Ventromedial Medulla
- SCN
Suprachiasmatic Nucleus
- SSC
Somatosensory Cortex
- SSN
Superior Salivatory Nucleus
- SST
Somatostatin
- SUNA
Short-lasting Unilateral Neuralgiform headache attacks with Autonomic symptoms
- SUNCT
Short-lasting Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing
- TAC
Trigeminal Autonomic Cephalalgia
- TG
Trigeminal Ganglion
- TH
Thyroid Hormone
- TIC
Trigeminal Intercostal Nerve Complex
- TMN
Tuberomammillary Nucleus
- TMS
Transcranial Magnetic Stimulation
- TNBC
Trigeminal Brainstem Nuclear Complex
- TNC
Trigeminal Nucleus Caudalis
- TNP
Trigeminal Neuropathic Pain
- VMN
Ventromedial Nucleus
- VPM
Ventral Posteromedial Nucleus
- VTA
Ventral Tegmental Area
Authors’ contributions
JI and YSP contributed to the manuscript’s conceptualization and design. JI drafted the initial manuscript and prepared the figures. All authors contributed to the critical review of this manuscript and approved the final version.
Funding
This work was supported by the National Research Foundation of Korea (NRF2023R1A2C1008079), and the Brain Korea 21 FOUR of the National Research Foundation of Korea funded by the Ministry of Education (No. 5199990614277).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdallah K, Artola A, Monconduit L, Dallel R, Luccarini P (2013) Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS ONE 8(8):e73022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdallah K, Monconduit L, Artola A, Luccarini P, Dallel R (2015) GABAAergic inhibition or dopamine denervation of the A11 hypothalamic nucleus induces trigeminal analgesia. Pain 156(4):644–655 [DOI] [PubMed] [Google Scholar]
- 3.Akerman S, Tassorelli C (2020) Animals models for trigeminal autonomic cephalalgias. In: Leone M, May A (eds) Cluster Headache and other Trigeminal Autonomic Cephalgias. Headache. Springer, Cham, Switzerland, p 103–115
- 4.Alstadhaug K (2009) Migraine and the hypothalamus. Cephalalgia 29(8):809–817 [DOI] [PubMed] [Google Scholar]
- 5.Andreou AP, Edvinsson L (2020) Trigeminal Mechanisms of Nociception. In: Lambru G, Lanteri-Minet M (eds) Neuromodulation in Headache and Facial Pain Management: Principles, Rationale and Clinical Data. Springer Nature, Cham, Switzerland, p 3–31
- 6.Ashkenazi A, Silberstein S (2004) Periodic autonomic dysfunction without pain in a patient with cluster headache. Cephalalgia 24(11):1005–1006 [DOI] [PubMed] [Google Scholar]
- 7.Ayoub LJ, Seminowicz DA, Moayedi M (2018) A meta-analytic study of experimental and chronic orofacial pain excluding headache disorders. NeuroImage: Clin 20:901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartsch T, Goadsby P (2003) Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 126(8):1801–1813 [DOI] [PubMed] [Google Scholar]
- 9.Bartsch T, Levy M, Knight Y, Goadsby P (2004) Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain 109(3):367–378 [DOI] [PubMed] [Google Scholar]
- 10.Bartsch T, Levy M, Knight Y, Goadsby P (2005) Inhibition of nociceptive dural input in the trigeminal nucleus caudalis by somatostatin receptor blockade in the posterior hypothalamus. Pain 117(1–2):30–39 [DOI] [PubMed] [Google Scholar]
- 11.Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D (2006) Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 26(42):10646–10657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benarroch E (2006) Pain-autonomic interactions. Neurol Sci 27:s130–s133 [DOI] [PubMed] [Google Scholar]
- 13.Benarroch EE (2012) Periaqueductal gray: an interface for behavioral control. Neurology 78(3):210–217 [DOI] [PubMed] [Google Scholar]
- 14.Benjamin L, Levy MJ, Lasalandra MP, Knight YE, Akerman S, Classey JD, Goadsby PJ (2004) Hypothalamic activation after stimulation of the superior sagittal sinus in the cat: a Fos study. Neurobiol Dis 16(3):500–505 [DOI] [PubMed] [Google Scholar]
- 15.Bernstein C, Burstein R (2012) Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 8(2):89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bista P, Imlach WL (2019) Pathological mechanisms and therapeutic targets for trigeminal neuropathic pain. Medicines 6(3):91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn-Munro G (2004) Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep 8:116–124 [DOI] [PubMed] [Google Scholar]
- 18.Bonilla-Jaime H, Sánchez-Salcedo JA, Estevez-Cabrera MM, Molina-Jiménez T, Cortes-Altamirano JL, Alfaro-Rodríguez A (2022) Depression and pain: use of antidepressants. Curr Neuropharmacol 20(2):384–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bräscher A-K, Becker S, Hoeppli M-E, Schweinhardt P (2016) Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci 36(18):5013–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burch R, Wells R (2013) Pathophysiology of migraine. Headache 53(2):420 [DOI] [PubMed] [Google Scholar]
- 21.Burish MJ, Chen Z, Yoo SH (2019) Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol 225(1):e13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein R, Jakubowski M (2009) Neural substrate of depression during migraine. Neurol Sci 30:27–31 [DOI] [PubMed] [Google Scholar]
- 23.Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35(17):6619–6629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB (2013) Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe H eadache: results of the A merican migraine prevalence and prevention (AMPP) study. Headache: J Head Face Pain 53(8):1278–1299 [DOI] [PubMed] [Google Scholar]
- 25.Caldwell H, Young W (2006) Oxytocin and vasopressin: genetics and behavioral implications. Handb Neurochemistry Mol Neurobiol 3:573–607 [Google Scholar]
- 26.Campbell RE, Grove KL, Smith MS (2003) Distribution of corticotropin releasing hormone receptor immunoreactivity in the rat hypothalamus: coexpression in neuropeptide Y and dopamine neurons in the arcuate nucleus. Brain Res 973(2):223–232 [DOI] [PubMed] [Google Scholar]
- 27.Cevoli S, Pizza F, Grimaldi D, Nicodemo M, Favoni V, Pierangeli G, Valko PO, Baumann CR, Montagna P, Bassetti CL (2011) Cerebrospinal fluid hypocretin-1 levels during the active period of cluster headache. Cephalalgia 31(8):973–976 [DOI] [PubMed] [Google Scholar]
- 28.Chang S, Deussing JM (2022) Corticotropin-Releasing Hormone in the Paraventricular Nucleus of the Hypothalamus—Beyond Hypothalamic–Pituitary–Adrenal Axis Control. In: Neuroanatomy of Neuroendocrine Systems. Springer, Cham, Switzerland, p 231–250
- 29.Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, Huang AJ, Hashimotodani Y, Kano M, Iwasaki H (2018) Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 359(6376):679–684 [DOI] [PubMed] [Google Scholar]
- 30.Chuang KH, Chen JH (2001) IMPACT: image-based physiological artifacts estimation and correction technique for functional MRI. Magn Reson Med: Off J Int Soc Magn Reson Med 46(2):344–353 [DOI] [PubMed] [Google Scholar]
- 31.Clelland CD, Zheng Z, Kim W, Bari A, Pouratian N (2014) Common cerebral networks associated with distinct deep brain stimulation targets for cluster headache. Cephalalgia 34(3):224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Close LN, Eftekhari S, Wang M, Charles AC, Russo AF (2019) Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 39(3):428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condés-Lara M, Martínez-Lorenzana G, de Los Monteros-Zúñiga AE, López-Córdoba G, Córdova-Quiroga A, Flores-Bojórquez SA, González-Hernández A (2024) Hypothalamic paraventricular stimulation inhibits nociceptive wide dynamic range Trigeminocervical complex cells via Oxytocinergic Transmission. J Neurosci 44:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosentino G, Brighina F, Brancato S, Valentino F, Indovino S, Fierro B (2015) Transcranial magnetic stimulation reveals cortical hyperexcitability in episodic cluster headache. J Pain 16(1):53–59 [DOI] [PubMed] [Google Scholar]
- 35.Couvineau A, Voisin T, Nicole P, Gratio V, Abad C, Tan Y-V (2019) Orexins as novel therapeutic targets in inflammatory and neurodegenerative diseases. Front Endocrinol 10:709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crivii CB, Clichici SV, Filip AG (2021) Anatomy and Topography of the Hypothalamus. In: Uwaifo, G.I. (eds) The Human Hypothalamus. Contemporary Endocrinology. Humana, Cham, Switzerland, p 7–14
- 37.Da Silva AN, Tepper SJ, Evans RW (2012) Side-locked and side shifting primary headaches. Headache: J Head Face Pain 52:7 [DOI] [PubMed] [Google Scholar]
- 38.DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D (2002) Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22(18):8183–8192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis MP (2007) What is new in neuropathic pain? Support Care Cancer 15:363–372 [DOI] [PubMed] [Google Scholar]
- 40.De Tommaso M, Vecchio E (2013) Primary headaches and trigeminal neuralgia: neuropathic pain yes or not? Evidences from neurophysiological procedures. Expert Rev Neurother 13(9):1031–1039 [DOI] [PubMed] [Google Scholar]
- 41.Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G (2007) Hypothalamic activation in spontaneous migraine attacks. Headache: J Head Face Pain 47(10):1418–1426 [DOI] [PubMed] [Google Scholar]
- 42.Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ (2019) Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: a resting-state magnetic resonance imaging study. Cephalalgia 39(11):1366–1381 [DOI] [PubMed] [Google Scholar]
- 43.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F (2014) Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci 5(11):1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eide PK, Rabben T (1998) Trigeminal neuropathic pain: pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery 43(5):1103–1109 [DOI] [PubMed] [Google Scholar]
- 45.Elina K, Oh BH, Islam J, Kim S, Park YS (2021) Activation of CamKIIα expressing neurons on ventrolateral periaqueductal gray improves behavioral hypersensitivity and thalamic discharge in a trigeminal neuralgia rat model. J Headache Pain 22(1):47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdener S, Dalkara T (2014) Modelling headache and migraine and its pharmacological manipulation. Br J Pharmacol 171(20):4575–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fair DA, Miranda-Dominguez O, Snyder AZ, Perrone A, Earl EA, Van AN, Koller JM, Feczko E, Tisdall MD, van der Kouwe A (2020) Correction of respiratory artifacts in MRI head motion estimates. NeuroImage 208:116400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferraro S, Nigri A, Bruzzone MG, Brivio L, Proietti Cecchini A, Verri M, Chiapparini L, Leone M (2018) Defective functional connectivity between posterior hypothalamus and regions of the diencephalic-mesencephalic junction in chronic cluster headache. Cephalalgia 38(13):1910–1918 [DOI] [PubMed] [Google Scholar]
- 49.Franzini A, Ferroli P, Leone M, Broggi G (2003) Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery 52(5):1095–1101 [PubMed] [Google Scholar]
- 50.Franzini A, Messina G, Cordella R, Marras C, Broggi G (2010) Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. NeuroSurg Focus 29(2):E13 [DOI] [PubMed] [Google Scholar]
- 51.Freitas RL, Uribe-Mariño A, Castiblanco-Urbina MA, Elias-Filho DH, Coimbra NC (2009) GABAA receptor blockade in dorsomedial and ventromedial nuclei of the hypothalamus evokes panic-like elaborated defensive behaviour followed by innate fear-induced antinociception. Brain Res 1305:118–131 [DOI] [PubMed] [Google Scholar]
- 52.Fujita A, Bonnavion P, Wilson MH, Mickelsen LE, Bloit J, De Lecea L, Jackson AC (2017) Hypothalamic tuberomammillary nucleus neurons: electrophysiological diversity and essential role in arousal stability. J Neurosci 37(39):9574–9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y-J, Ji R-R (2010) Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol Ther 126(1):56–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauriau C, Bernard JF (2004) A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468(1):24–56 [DOI] [PubMed] [Google Scholar]
- 55.Gerashchenko D, Horvath TL, Xie XS (2011) Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociceptin/orphanin FQ blocks stress-induced analgesia in rats. Neuropharmacology 60(4):543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goadsby PJ (2018) Cluster headache and the trigeminal-autonomic reflex: driving or being driven? vol 38. Sage Publications Sage UK, London, England [DOI] [PubMed] [Google Scholar]
- 57.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S (2017) Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 97(2):553–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto M, Swanson LW (2004) Axonal projections from the parasubthalamic nucleus. J Comp Neurol 469(4):581–607 [DOI] [PubMed] [Google Scholar]
- 59.Greco R, Demartini C, Francavilla M, Zanaboni AM, Tassorelli C (2022) Antagonism of CGRP receptor: central and peripheral mechanisms and mediators in an animal model of chronic migraine. Cells 11(19):3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guerrero C (2019) The role of purinergic, 5-hydroxytryptaminergic and glutamatergic receptors in rat peripheral trigeminal nociception: Implications for migraine pain. Diss, Itä-Suomen yliopisto, Finland
- 61.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA (2011) Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci 31(16):5956–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hache G, Coudore F, Gardier AM, Guiard BP (2011) Monoaminergic antidepressants in the relief of pain: potential therapeutic utility of triple reuptake inhibitors (TRIs). Pharmaceuticals 4(2):285–342 [Google Scholar]
- 63.Haghparast A, Matini T, Rezaee L, Rahban M, Tehranchi A, Haghparast A (2020) Involvement of orexinergic system within the nucleus accumbens in pain modulatory role of the lateral hypothalamus in orofacial pain model. Neurochem Res 45(4):851–859 [DOI] [PubMed] [Google Scholar]
- 64.Halász B (2004) Hypothalamus, Anatomy of. Encyclopedia of Endocrine Diseases, 2:707–15
- 65.Henssen D, Dijk J, Knepflé R, Sieffers M, Winter A, Vissers K (2019) Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: a systematic review and meta-analysis. NeuroImage: Clin 24:102039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci 87(5):1932–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herman JP, Tasker JG (2016) Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol 7:137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann J, Baca SM, Akerman S (2019) Neurovascular mechanisms of migraine and cluster headache. J Cereb Blood Flow Metabolism 39(4):573–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann J, May A (2018) Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol 17(1):75–83 [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann J, Supronsinchai W, Akerman S, Andreou AP, Winrow CJ, Renger J, Hargreaves R, Goadsby PJ (2015) Evidence for orexinergic mechanisms in migraine. Neurobiol Dis 74:137–143 [DOI] [PubMed] [Google Scholar]
- 71.Holland P, Goadsby PJ (2007) The hypothalamic orexinergic system: pain and primary headaches: CME. Headache 47(6):951–962 [DOI] [PubMed] [Google Scholar]
- 72.Holland PR, Barloese M, Fahrenkrug J (2018) PACAP in hypothalamic regulation of sleep and circadian rhythm: importance for headache. J Headache Pain 19:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holland PR, Goadsby PJ (2009) Cluster headache, hypothalamus, and orexin. Curr Pain Headache Rep 13:147–154 [DOI] [PubMed] [Google Scholar]
- 74.Holle D, Katsarava Z, Obermann M (2011) The hypothalamus: specific or nonspecific role in the pathophysiology of trigeminal autonomic cephalalgias? Curr Pain Headache Rep 15:101–107 [DOI] [PubMed] [Google Scholar]
- 75.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA (2014) Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. Eneuro 1(1):e20.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hutcherson CA, Plassmann H, Gross JJ, Rangel A (2012) Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci 32(39):13543–13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO (1999) Pain-related neurons in the human cingulate cortex. Nat Neurosci 2(5):403–405 [DOI] [PubMed] [Google Scholar]
- 78.Imai N (2023) Molecular and cellular neurobiology of circadian and circannual rhythms in migraine: a narrative review. Int J Mol Sci 24(12):10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Islam J, Kc E, Kim S, Chung MY, Park KS, Kim HK, Park YS (2023) Optogenetic Inhibition of Glutamatergic Neurons in the dysgranular posterior insular cortex modulates Trigeminal Neuropathic Pain in CCI-ION rat. Neuromol Med 25(4):516–532 [DOI] [PubMed] [Google Scholar]
- 80.Islam J, Kc E, Kim S, Kim HK, Park YS (2021) Stimulating gabaergic neurons in the nucleus accumbens core alters the trigeminal neuropathic pain responses in a rat model of infraorbital nerve injury. Int J Mol Sci 22(16):8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Islam J, Kc E, Oh BH, Kim S, Hyun S-h, Park YS (2020) Optogenetic stimulation of the motor cortex alleviates neuropathic pain in rats of infraorbital nerve injury with/without CGRP knock-down. J Headache Pain 21:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Islam J, Kc E, So KH, Kim S, Kim HK, Park YY, Park YS (2023) Modulation of trigeminal neuropathic pain by optogenetic inhibition of posterior hypothalamus in CCI-ION rat. Sci Rep 13(1):489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Islam J, Rahman MT, Kc E, Park YS (2024) Deciphering the functional role of insular cortex stratification in trigeminal neuropathic pain. J Headache Pain 25(1):76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwata K, Takeda M, Oh SB, Shinoda M (2017) Neurophysiology of orofacial pain. In Contemporary Oral Medicine: A Comprehensive Approach to Clinical Practice. Springer International Publishing, Cham, Switzerland, p 1–23
- 85.Jang J-H, Park J-Y, Oh J-Y, Bae S-J, Jang H, Jeon S, Kim J, Park H-J (2018) Novel analgesic effects of melanin-concentrating hormone on persistent neuropathic and inflammatory pain in mice. Sci Rep 8(1):707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janig W (1990) The sympathetic nervous system in pain: physiology and pathophysiology. In: Stanton-Hicks M (ed), Pain and the Sympathetic Nervous System, Kluwer, Boston, MA, p 17–99
- 87.Jeong Y, Moes JR, Wagner M, Holden JE (2012) The posterior hypothalamus exerts opposing effects on nociception via the A7 catecholamine cell group in rats. Neuroscience 227:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J (2013) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26(1):58–64 [DOI] [PubMed] [Google Scholar]
- 89.Jing F, Zou Q, Wang Y, Cai Z, Tang Y (2021) Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J Headache Pain 22:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ju A, Fernandez-Arroyo B, Wu Y, Jacky D, Beyeler A (2020) Expression of serotonin 1A and 2A receptors in molecular-and projection-defined neurons of the mouse insular cortex. Mol Brain 13:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jürgens TP, Leone M, Proietti-Cecchini A, Busch V, Mea E, Bussone G, May A (2009) Hypothalamic deep-brain stimulation modulates thermal sensitivity and pain thresholds in cluster headache. Pain 146(1–2):84–90 [DOI] [PubMed] [Google Scholar]
- 92.Kagan R, Kainz V, Burstein R, Noseda R (2013) Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photophobia. Neuroscience 248:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaspo GA (2013) Dental Sleep Medicine and the Use of Oral Devices. In: Orofacial Pain: A Clinician's Guide. Springer, Cham, Switzerland, p 35–64
- 94.Katagiri A, Okamoto K, Thompson R, Bereiter DA (2013) Posterior hypothalamic modulation of light-evoked trigeminal neural activity and lacrimation. Neuroscience 246:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaushal R, Taylor BK, Jamal A, Zhang L, Ma F, Donahue R, Westlund K (2016) GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAα1 receptors in the medial prefrontal cortex. Neuroscience 334:148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kc E, Islam J, Kim HK, Park YS (2023) GFAP-NpHR mediated optogenetic inhibition of trigeminal nucleus caudalis attenuates hypersensitive behaviors and thalamic discharge attributed to infraorbital nerve constriction injury. J Headache Pain 24(1):137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kc E, Islam J, Lee G, Park YS (2024) Optogenetic approach in Trigeminal Neuralgia and potential concerns: preclinical insights. Mol Neurobiol 61(3):1769–1780 [DOI] [PubMed] [Google Scholar]
- 98.Khusnullina AA (2016) Neuroimaging of Chronic Pain. Bangor University (United Kingdom) [Google Scholar]
- 99.Kim DJ, Jassar H, Lim M, Nascimento TD, DaSilva AF (2021) Dopaminergic regulation of reward system connectivity underpins pain and emotional suffering in migraine. J Pain Res 14:631–643 [DOI] [PMC free article] [PubMed]
- 100.Kobayashi M (2011) Macroscopic connection of rat insular cortex: anatomical bases underlying its physiological functions. Int Rev Neurobiol 97:285–303 [DOI] [PubMed] [Google Scholar]
- 101.Kobayashi M (2018) Mechanisms of orofacial sensory processing in the rat insular cortex. J Oral Biosci 60(3):59–64 [Google Scholar]
- 102.Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M (2016) The role of trigeminal nucleus caudalis orexin 1 receptors in orofacial pain transmission and in orofacial pain-induced learning and memory impairment in rats. Physiol Behav 157:20–27 [DOI] [PubMed] [Google Scholar]
- 103.Krolewski DM, Medina A, Kerman IA, Bernard R, Burke S, Thompson RC, Bunney WE Jr, Schatzberg AF, Myers RM, Akil H (2010) Expression patterns of corticotropin-releasing factor, arginine vasopressin, histidine decarboxylase, melanin‐concentrating hormone, and orexin genes in the human hypothalamus. J Comp Neurol 518(22):4591–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krudewig R, Langer B, Vögler O, Markschies N, Erl M, Jakobs KH, Van Koppen CJ (2000) Distinct internalization of M2 muscarinic acetylcholine receptors confers selective and long-lasting desensitization of signaling to phospholipase C. J Neurochem 74(4):1721–1730 [DOI] [PubMed] [Google Scholar]
- 105.Lambru G, Lanteri-Minet M (2019) Neuromodulation in headache and facial pain management: principles, rationale and clinical data. Springer Nature, Cham, Switzerland
- 106.Leone M, Bussone G (2009) Pathophysiology of trigeminal autonomic cephalalgias. Lancet Neurol 8(8):755–764 [DOI] [PubMed] [Google Scholar]
- 107.Leone M, Ferraro S, Cecchini AP (2021) The neurobiology of cluster headache. Handb Clin Neurol 182:401–414 [DOI] [PubMed] [Google Scholar]
- 108.Leone M, Franzini A, Bussone G (2001) Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med 345(19):1428–1429 [DOI] [PubMed] [Google Scholar]
- 109.Leone M, Franzini A, Cecchini AP, Mea E, Broggi G, Bussone G (2010) Deep brain stimulation in trigeminal autonomic cephalalgias. Neurotherapeutics 7:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lerebours F, Boulanouar K, Barège M, Denuelle M, Bonneville F, Payoux P, Larrue V, Fabre N (2019) Functional connectivity of hypothalamus in chronic migraine with medication overuse. Cephalalgia 39(7):892–899 [DOI] [PubMed] [Google Scholar]
- 111.LeVan P, Maclaren J, Herbst M, Sostheim R, Zaitsev M, Hennig J (2013) Ballistocardiographic artifact removal from simultaneous EEG-fMRI using an optical motion-tracking system. NeuroImage 75:1–11 [DOI] [PubMed] [Google Scholar]
- 112.Levy D, Abdian L, Dekel-Steinkeller M, Defrin R (2018) Experimental evidence for weaker endogenous inhibition of trigeminal pain than extra-trigeminal pain in healthy individuals. Cephalalgia 38(7):1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindholm P (2017) Neural mechanisms of orofacial pain-effects of transcranial magnetic stimulation. Turku, Finland
- 114.Liu S, Tang Y, Shu H, Tatum D, Bai Q, Crawford J, Xing Y, Lobo MK, Bellinger L, Kramer P (2019) Dopamine receptor D2, but not D1, mediates descending dopaminergic pathway–produced analgesic effect in a trigeminal neuropathic pain mouse model. Pain 160(2):334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ljubisavljevic S, Zidverc Trajkovic J (2019) Cluster headache: pathophysiology, diagnosis and treatment. J Neurol 266:1059–1066 [DOI] [PubMed] [Google Scholar]
- 116.Lorenz J, Minoshima S, Casey K (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126(5):1079–1091 [DOI] [PubMed] [Google Scholar]
- 117.Lozić M, Tasić T, Martin A, Greenwood M, Šarenac O, Hindmarch C, Paton JF, Murphy D, Japundžić-Žigon N (2016) Over-expression of V1A receptors in PVN modulates autonomic cardiovascular control. Pharmacol Res 114:185–195 [DOI] [PubMed] [Google Scholar]
- 118.Lupi C, Benemei S, Guerzoni S, Pellesi L, Negro A (2019) Pharmacokinetics and pharmacodynamics of new acute treatments for migraine. Expert Opin Drug Metab Toxicol 15(3):189–198 [DOI] [PubMed] [Google Scholar]
- 119.Malu OO, Bailey J, Hawks MK (2022) Cluster headache: rapid evidence review. Am Family Phys 105(1):24–32 [PubMed] [Google Scholar]
- 120.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11(7):308–314 [DOI] [PubMed] [Google Scholar]
- 121.Matsumoto N (1996) Pain pathways from the trigeminal system and their functions. Dent J Iwate Med Univ 21(2):117–135. 10.20663/iwateshigakukaishi.21.2_117 [Google Scholar]
- 122.May A (2005) Cluster headache: pathogenesis, diagnosis, and management. Lancet 366(9488):843–855 [DOI] [PubMed] [Google Scholar]
- 123.May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ (1998) Hypothalamic activation in cluster headache attacks. Lancet 352(9124):275–278 [DOI] [PubMed] [Google Scholar]
- 124.May A, Burstein R (2019) Hypothalamic regulation of headache and migraine. Cephalalgia 39(13):1710–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.May A, Leone M, Boecker H, Sprenger T, Juergens T, Bussone G, Tolle TR (2006) Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci 26(13):3589–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.May A, Schwedt TJ, Magis D, Pozo-Rosich P, Evers S, Wang SJ (2018) Cluster headache. Nat Rev Dis Primers 4(1):1–17 [DOI] [PubMed] [Google Scholar]
- 127.McEwen BS (2000) The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886(1–2):172–189 [DOI] [PubMed] [Google Scholar]
- 128.McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87(3):873–904 [DOI] [PubMed] [Google Scholar]
- 129.McEwen BS, Akil H (2020) Revisiting the stress concept: implications for affective disorders. J Neurosci 40(1):12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2018) Deep in the brain: changes in subcortical function immediately preceding a migraine attack. Hum Brain Mapp 39(6):2651–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2020) Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache. Cephalalgia 40(5):448–460 [DOI] [PubMed] [Google Scholar]
- 132.Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2021) Brainstem functional oscillations across the migraine cycle: a longitudinal investigation. NeuroImage: Clin 30:102630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Millan MJ (2002) Descending control of pain. Prog Neurobiol 66(6):355–474 [DOI] [PubMed] [Google Scholar]
- 134.Möller M, Mehnert J, May A (2020) Hypothalamic activation discriminates painful and non-painful initiation of the trigeminal autonomic reflex–an fMRI study. Cephalalgia 40(1):79–87 [DOI] [PubMed] [Google Scholar]
- 135.Montagna P (2006) Hypothalamus, sleep and headaches. Neurol Sci 27:s138–s143 [DOI] [PubMed] [Google Scholar]
- 136.Moon HC, Heo WI, Kim YJ, Lee D, Won SY, Kim HR, Ha SM, Lee YJ, Park YS (2017) Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve. J Pain Res 10:2355–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moradi M, Yazdanian M, Haghparast A (2015) Role of dopamine D2-like receptors within the ventral tegmental area and nucleus accumbens in antinociception induced by lateral hypothalamus stimulation. Behav Brain Res 292:508–514 [DOI] [PubMed] [Google Scholar]
- 138.Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ (2021) Targets for migraine treatment: beyond calcitonin gene-related peptide. Curr Opin Neurol 34(3):363–372 [DOI] [PubMed] [Google Scholar]
- 139.Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D (2014) Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS ONE 9(4):e95508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mrad Y (2023) The role of tryptophan dysregulation in a mouse model of migraine. Université Clermont Auvergne, Clermont-Ferrand, France
- 141.Mungoven TJ, Marciszewski KK, Macefield VG, Macey PM, Henderson LA, Meylakh N (2022) Alterations in pain processing circuitries in episodic migraine. J Headache Pain 23(1):9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Napeñas JJ, Zakrzewska JM (2011) Diagnosis and management of trigeminal neuropathic pains. Pain Manage 1(4):353–365 [DOI] [PubMed] [Google Scholar]
- 143.Ong W-Y, Stohler CS, Herr DR (2019) Role of the prefrontal cortex in pain processing. Mol Neurobiol 56(2):1137–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Onuțu AH, Dîrzu DS, Petrișor C (2018) Serotonin reuptake inhibitors and their role in chronic pain management. In: Serotonin. IntechOpen, London, United Kingdom
- 145.Park J-W, Chu MK, Kim J-M, Park S-G, Cho S-J (2016) Analysis of trigger factors in episodic migraineurs using a smartphone headache diary applications. PLoS ONE 11(2):e0149577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel NM, Jozsa F, Das JM (2022) Neuroanatomy, spinal trigeminal nucleus. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, Florida, United States [PubMed]
- 147.Pohl H, Sandor PS, Michels L, Gantenbein AR (2021) Cluster headache Pathophysiology—A disorder of Network Excitability? Clin Translational Neurosci 5(2):16 [Google Scholar]
- 148.Price S, Daly DT (2019) Neuroanatomy, trigeminal nucleus. StatPearls, Tampa, FL [PubMed]
- 149.Pringsheim T (2002) Cluster headache: evidence for a disorder of circadian rhythm and hypothalamic function. Can J Neurol Sci 29(1):33–40 [DOI] [PubMed] [Google Scholar]
- 150.Qin Z, Su J, He X-W, Ban S, Zhu Q, Cui Y, Zhang J, Hu Y, Liu Y-S, Zhao R (2020) Disrupted functional connectivity between sub-regions in the sensorimotor areas and cortex in migraine without aura. J Headache Pain 21:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiu E, Wang Y, Ma L, Tian L, Liu R, Dong Z, Xu X, Zou Z, Yu S (2013) Abnormal brain functional connectivity of the hypothalamus in cluster headaches. PLoS ONE 8(2):e57896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rainero I, Roveta F, Vacca A, Noviello C, Rubino E (2020) Migraine pathways and the identification of novel therapeutic targets. Expert Opin Ther Targets 24(3):245–253 [DOI] [PubMed] [Google Scholar]
- 153.Rasskazoff SY, Slavin KV (2013) Neuromodulation for cephalgias. Surg Neurol Int 4(Suppl 3):S136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418(6901):935–941 [DOI] [PubMed] [Google Scholar]
- 155.Robert C, Bourgeais L, Arreto C-D, Condes-Lara M, Noseda R, Jay T, Villanueva L (2013) Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci 33(20):8827–8840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ross EL (2000) The evolving role of antiepileptic drugs in treating neuropathic pain. Neurology 55(5 Suppl 1):S41-46; discussion S54 [PubMed] [Google Scholar]
- 157.Sánchez-del-Rio M, Reuter U (2004) Migraine aura: new information on underlying mechanisms. Curr Opin Neurol 17(3):289–293 [DOI] [PubMed] [Google Scholar]
- 158.Saper CB (2002) The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25(1):433–469 [DOI] [PubMed] [Google Scholar]
- 159.Saper CB, Lowell BB (2014) The hypothalamus. Curr Biol 24(23):R1111–R1116 [DOI] [PubMed] [Google Scholar]
- 160.Schulte LH, Allers A, May A (2017) Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology 88(21):2011–2016 [DOI] [PubMed] [Google Scholar]
- 161.Schulte LH, Haji AA, May A (2020) Phase dependent hypothalamic activation following trigeminal input in cluster headache. J Headache Pain 21:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139(7):1987–1993 [DOI] [PubMed] [Google Scholar]
- 163.Schulte LH, May A (2017) Of generators, networks and migraine attacks. Curr Opin Neurol 30(3):241–245 [DOI] [PubMed] [Google Scholar]
- 164.Sclocco R, Beissner F, Desbordes G, Polimeni JR, Wald LL, Kettner NW, Kim J, Garcia RG, Renvall V, Bianchi AM (2016) Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philosophical Trans Royal Soc A: Math Phys Eng Sci 374(2067):20150189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shafiei I, Vatankhah M, Zarepour L, Ezzatpanah S, Haghparast A (2018) Role of D1-and D2-like dopaminergic receptors in the nucleus accumbens in modulation of formalin-induced orofacial pain: involvement of lateral hypothalamus. Physiol Behav 188:25–31 [DOI] [PubMed] [Google Scholar]
- 166.Shimizu T, Hosomi K, Maruo T, Goto Y, Yokoe M, Kageyama Y, Shimokawa T, Yoshimine T, Saitoh Y (2017) Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an H-coil and figure-8 coil. J Neurosurg 127(5):1172–1180 [DOI] [PubMed] [Google Scholar]
- 167.Shivani VKS, Roy B (2014) MMPI Profile of Individuals with Migraine. Eastern J Psychiatry, 17(2):1–8
- 168.Siemian JN, Arenivar MA, Sarsfield S, Borja CB, Erbaugh LJ, Eagle AL, Robison AJ, Leinninger G, Aponte Y (2021) An excitatory lateral hypothalamic circuit orchestrating pain behaviors in mice. Elife 10:e66446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Silberstein S (2004) Migraine pathophysiology and its clinical implications. Cephalalgia 24(2suppl):2–7 [DOI] [PubMed] [Google Scholar]
- 170.Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog Clin Neurosci 8(4):383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sohn JH, Lee MJ, Cho SJ (2021) Update on treatment of cluster headache. J Korean Neurol Association 39:113–120 [Google Scholar]
- 172.Sprenger T, Valet M, Hammes M, Erhard P, Berthele A, Conrad B, Tolle T (2004) Hypothalamic activation in trigeminal autonomic cephalgia: functional imaging of an atypical case. Cephalalgia 24(9):753–757 [DOI] [PubMed] [Google Scholar]
- 173.Starr PA, Barbaro NM, Raskin NH, Ostrem JL (2007) Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg 106(6):999–1005 [DOI] [PubMed] [Google Scholar]
- 174.Steinbusch HW, Sauren Y, Groenewegen H, Watanabe T, Mulder AH (1986) Histaminergic projections from the premammillary and posterior hypothalamic region to the caudate-putamen complex in the rat. Brain Res 368(2):389–393 [DOI] [PubMed] [Google Scholar]
- 175.Strother LC, Srikiatkhachorn A, Supronsinchai W (2018) Targeted orexin and hypothalamic neuropeptides for migraine. Neurotherapeutics 15(2):377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tardiolo G, Bramanti P, Mazzon E (2019) Migraine: experimental models and novel therapeutic approaches. Int J Mol Sci 20(12):2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Terrier L-M, Hadjikhani N, Destrieux C (2022) The trigeminal pathways. J Neurol 269(7):3443–3460 [DOI] [PubMed] [Google Scholar]
- 178.Tsagareli N, Tsiklauri N, Kvachadze I, Tsagareli MG (2020) Endogenous opioid and cannabinoid systems contribute to antinociception produced by administration of NSAIDs into the insular cortex of rats. Biomed Pharmacother 131:110722 [DOI] [PubMed] [Google Scholar]
- 179.Turnbull AV, Rivier CL (1999) Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev, 79(1):1–71 [DOI] [PubMed]
- 180.Vadivelu N, Huang Y, Mancini P, Gruenbaum S, Vadivelu A, Dabu-Bondoc S (2014) The neurobiology of orofacial pain. In: A clinician’s guide, orofacial pain, p 1–8
- 181.Van Eden CG, Buijs RM (2000) Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog Brain Res 126:49–62 [DOI] [PubMed] [Google Scholar]
- 182.Vila-Pueyo M, Hoffmann J, Romero-Reyes M, Akerman S (2019) Brain structure and function related to headache: Brainstem structure and function in headache. Cephalalgia 39(13):1635–1660 [DOI] [PubMed] [Google Scholar]
- 183.Vollesen AL, Benemei S, Cortese F, Labastida-Ramírez A, Marchese F, Pellesi L, Romoli M, Ashina M, Lampl C, Federation SoASotEH (2018) Migraine and cluster headache–the common link. J Headache Pain 19:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wagner K, Roeder Z, DesRochers K, Buhler AV, Heinricher MM, Cleary DR (2013) The dorsomedial hypothalamus mediates stress-induced hyperalgesia and is the source of the pronociceptive peptide cholecystokinin in the rostral ventromedial medulla. Neuroscience 238:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wei DY-T, Ong JJY, Goadsby PJ (2018) Overview of trigeminal autonomic cephalalgias: nosologic evolution, diagnosis, and management. Ann Indian Acad Neurol 21(Suppl 1):S39–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA (2015) Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J Neurosci 35(6):2508–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xie Y, Dorsky RI (2017) Development of the hypothalamus: conservation, modification and innovation. Development 144(9):1588–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang F-C, Chou K-H, Fuh J-L, Lee P-L, Lirng J-F, Lin Y-Y, Lin C-P, Wang S-J (2015) Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatry 86(4):437–445 [DOI] [PubMed] [Google Scholar]
- 189.Young CK, Brown AR, Robinson JH, Tuor UI, Dunn JF, Bland BH, Teskey GC (2011) Functional MRI response and correlated electrophysiological changes during posterior hypothalamic nucleus deep brain stimulation. NeuroImage 56(1):35–44 [DOI] [PubMed] [Google Scholar]
- 190.Youssef AM, Gustin SM, Nash PG, Reeves JM, Petersen ET, Peck CC, Murray GM, Henderson LA (2014) Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. PAIN® 155(3):467–475 [DOI] [PubMed] [Google Scholar]
- 191.Yu N, Huang L, Zhou Y, Xue T, Chen Z, Han G (2019) Near-infrared‐light activatable nanoparticles for deep‐tissue‐penetrating wireless optogenetics. Adv Healthc Mater 8(6):1801132 [DOI] [PubMed] [Google Scholar]
- 192.Zakrzewska JM (2010) Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother 11(8):1239–1254 [DOI] [PubMed] [Google Scholar]
- 193.Zangen A, Roth Y, Voller B, Hallett M (2005) Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 116(4):775–779 [DOI] [PubMed] [Google Scholar]
- 194.Zhu J-N, Yung W-H, Chow BK-C, Chan Y-S, Wang J-J (2006) The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev 52(1):93–106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.