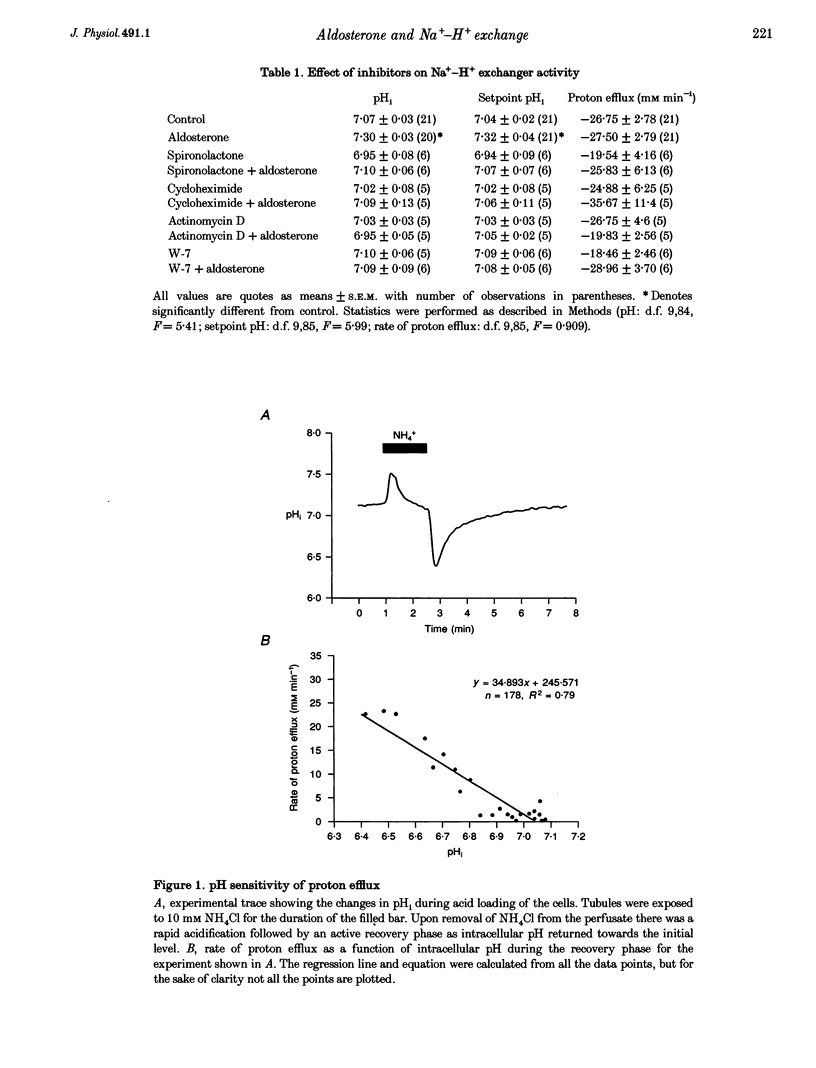
Abstract
1. In the amphibian early distal tubule aldosterone activates the Na(+)-H+ exchangers, resulting in an increase in intracellular pH (pHi). Since this activation is rapid (within 30 min), it may be mediated by either a genomic or non-genomic pathway. 2. pHi was measured in single microperfused early distal tubule segments using the fluorescent probe 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF). 3. A 30 min incubation in aldosterone increased both resting pHi and the setpoint of the Na(+-H+ exchanger. These changes were prevented by the mineralocorticoid receptor antagonist, spironolactone. 4. Actinomycin D and cycloheximide, inhibitors of transcription and translation, respectively, were without effect on resting pHi, but inhibited activation of the Na(+)-H+ exchanger by aldosterone. 5. The effect of aldosterone upon pHi and setpoint was also prevented by the calcium-calmodulin antagonist, W-7. 6. These results indicate that, although the response to aldosterone is rapid, aldosterone binds to a specific mineralocorticoid receptor which then triggers gene activation followed by de novo protein synthesis. Furthermore, since calmodulin is a known activator of the Na(+)-H+ exchanger, and the response is inhibited by W-7, it is suggested that this protein may be calmodulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper G. J., Hunter M. Na(+)-H+ exchange in frog early distal tubule: effect of aldosterone on the set-point. J Physiol. 1994 Sep 15;479(Pt 3):423–432. doi: 10.1113/jphysiol.1994.sp020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen C., Meyer C., Christ M., Theisen K., Wehling M. Novel membrane receptors for aldosterone in human lymphocytes: a 50 kDa protein on SDS-PAGE. Cell Mol Biol (Noisy-le-grand) 1994 May;40(3):351–358. [PubMed] [Google Scholar]
- Geheb M., Hercker E., Singer I., Cox M. Subcellular localization of aldosterone-induced proteins in toad urinary bladders. Biochim Biophys Acta. 1981 Mar 6;641(2):422–426. doi: 10.1016/0005-2736(81)90499-5. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Oberleithner H., Giebisch G. The amphibian diluting segment. Am J Physiol. 1988 May;254(5 Pt 2):F615–F627. doi: 10.1152/ajprenal.1988.254.5.F615. [DOI] [PubMed] [Google Scholar]
- Horisberger J. D., Rossier B. C. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992 Mar;19(3):221–227. doi: 10.1161/01.hyp.19.3.221. [DOI] [PubMed] [Google Scholar]
- Hurst A. M., Hunter M. Acute changes in channel density of amphibian diluting segment. Am J Physiol. 1990 Dec;259(6 Pt 1):C1005–C1009. doi: 10.1152/ajpcell.1990.259.6.C1005. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Messner G., Wang W. Mechanism of hydrogen ion transport in the diluting segment of frog kidney. Pflugers Arch. 1984 Nov;402(3):272–280. doi: 10.1007/BF00585510. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom S. C., O'Neil R. G. Mineralocorticoid regulation of apical cell membrane Na+ and K+ transport of the cortical collecting duct. Am J Physiol. 1985 Jun;248(6 Pt 2):F858–F868. doi: 10.1152/ajprenal.1985.248.6.F858. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]
- Vogel H. J. The Merck Frosst Award Lecture 1994. Calmodulin: a versatile calcium mediator protein. Biochem Cell Biol. 1994 Sep-Oct;72(9-10):357–376. [PubMed] [Google Scholar]
- Wehling M., Käsmayr J., Theisen K. Rapid effects of mineralocorticoids on sodium-proton exchanger: genomic or nongenomic pathway? Am J Physiol. 1991 May;260(5 Pt 1):E719–E726. doi: 10.1152/ajpendo.1991.260.5.E719. [DOI] [PubMed] [Google Scholar]
- Wehling M. Nongenomic aldosterone effects: the cell membrane as a specific target of mineralocorticoid action. Steroids. 1995 Jan;60(1):153–156. doi: 10.1016/0039-128x(94)00027-a. [DOI] [PubMed] [Google Scholar]


