Abstract
Objectives
In adult hypertensive patients, blood pressure variability is considered a risk factor for heart failure. The relationship between pulse pressure variability and the risk of heart failure remains unclear. This study aims to explore the impact of pulse pressure variability (PPV) on heart failure through a secondary analysis of the SPRINT randomized controlled trial.
Methods
The data were derived from the SPRINT (Systolic Blood Pressure Intervention Trial) study. The trial recruited participants 50 years or older, with SBP ≥ 130 mm Hg and at least one additional CVD risk factor. We calculated pulse pressure based on the systolic and diastolic blood pressure obtained during follow-up, and used the coefficient of variation to represent pulse pressure variability (PPV) for statistical analysis. We considered the incidence of acute decompensated heart failure as the outcome measure. We employed multivariable Cox regression analysis to examine the relationship between PPV and the risk of heart failure occurrence. Additionally, we used a restricted cubic spline model to analyze the dose–response relationship between PPV and the risk of heart failure occurrence.
Results
In this study, a total of 9429 participants were included. During a median follow-up time of 3.87 years, 188 new cases of heart failure were observed. The mean age of the study population was 67.9 ± 9.4 years and 3382 participants (35.5%) were females. The average PPCV was 13.85 ± 5.37%. The results from the multivariable Cox regression analysis indicated that the risk of heart failure increased by 3% for every 1% increase in PPCV (HR = 1.030 [95% CI 1.016–1.044]; P < 0.001).
Conclusions
The study found that PPV is an independent risk factor for the occurrence of heart failure. This underscores the importance of maintaining long-term stability in pulse pressure, in preventing the development of heart failure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-024-02164-0.
Keywords: Heart failure, Hypertension, Coefficient, Pulse pressure variability, Risk prediction
Introduction
Heart failure (HF) is a chronic late-stage complication of several cardiovascular diseases [1]. The global prevalence of HF is increasing, primarily due to the aging population. The diagnosis and treatment of coronary heart disease (CHD) and heart valve disease continue to improve [2]. Despite significant advances in the treatment, increased risk of mortality, hospitalization, and decreased quality of life are observed among HF patients [3]. Heart failure can be classified into three categories based on the left ventricular ejection fraction; Heart Failure with reduced Ejection Fraction (HFrEF, EF < 40%), Heart Failure with mildly reduced Ejection Fraction (HFmrEF, 40 ≤ EF ≤ 49%), and Heart Failure with preserved Ejection Fraction (HFpEF, EF ≥ 50%) [4]. Hypertension impacts overall health and is a major risk factor for cardiovascular diseases (CVDs) [5, 6]. The research has shown that angiotensin-converting enzyme is a key target for blood pressure control, providing an important therapeutic target for the treatment of hypertension [7]. There is interaction between different gene loci of angiotensin-converting enzyme in patients with primary hypertension [8]. In clinical practice, blood pressure (BP) control is an important strategy for preventing and managing HF [6, 9–11]. However, traditional BP assessment methods generally rely on a single measurement or just an average, which may not adequately capture the variability of BP levels. A measure of blood pressure variability (BPV), which includes variability measured during follow-up, day-to-day activities, and in the 24-h clinics has been adopted to assess the effectiveness of BP control. In addition to BP levels, BPV is considered a critical risk factor in cardiovascular health assessments [12–15]. An animal experimental study found that continuous infusion of angiotensin II for 14 days significantly increases blood pressure variability in rats [16]. A meta-analysis on blood pressure variability and the risk of cardiovascular diseases revealed that long-term variability in blood pressure is associated with poor cardiovascular outcomes and increased mortality rates, exceeding the impact of mean blood pressure levels [17]. Previous studies have explored the relationship between BPV and increased risk of HF in patients with hypertension [18–20]. The patients with large blood pressure fluctuations had an increased risk of developing heart failure. This underscores the clinical significance of maintaining stable blood pressure. Therefore, blood pressure variability is currently being used to assess the fluctuation in BP. However, most studies only utilize variability in systolic or diastolic blood pressure. Since pulse pressure is the difference between systolic and diastolic blood pressure, it can reflect the magnitude of blood pressure fluctuation within one cardiac cycle [21]. Therefore, monitoring blood pressure and pulse pressure can comprehensively assess cardiovascular health. A prospective cohort study of patients with ischemic heart failure showed a J-shaped relationship between pulse pressure and all-cause mortality in ischemic heart failure patients, where higher pulse pressure was associated with poorer outcomes only in patients with systolic blood pressure (SBP) ≥ 110 mmHg [22]. Other studies have shown that in acute HFrEF patients, there is an almost linear negative correlation between mortality rate and pulse pressure (PP); conversely, in HFpEF patients, a J-shaped relationship exists between mortality rates and PP, with the best prognosis observed at the lowest levels of PP [23]. Most studies focused on the relationship between systolic blood pressure, diastolic blood pressure, and heart failure. However, research on the exact relationship between PP and heart failure and the role of PP in heart failure risk assessment remains unexplored. Since when monitoring blood pressure, we focus on blood pressure variability, in studying the relationship between pulse pressure and heart failure, paying attention to pulse pressure variability is also crucial. Pulse pressure variability (PPV) can serve as a new assessment metric for exploring the connection between pulse pressure and heart failure. The pulse pressure coefficient of variation (PPCV) can be used to measure the extent of pulse pressure variation over time, reflecting its fluctuations or instability. Therefore, this study aims to investigate the relationship between PPV and incident heart failure (including HFrEF, HFmrEF, and HFpEF). By further exploring the association between PPV and heart failure outcomes, we aim to develop innovative methods for assessing blood pressure control and improve strategies for the prevention and treatment of heart failure.
Methods
Data availability
We registered an account on the BioLINCC website (https://biolincc.nhlbi.nihgov/home/). We uploaded our study protocol and IRB approval and requested to use the data. We then retrieved the original data on HF after signing the NHLBI Research Materials Distribution Agreement (RMDA). Following the full preparation of the application materials, the process took approximately 3 days. This study was approved by the Ethics Committee of Zhejiang Chinese Medical University.
SPRINT design
The design and methodology of the Systolic Blood Pressure Intervention Trial (SPRINT) have been previously reported [10, 24, 25] and the trial protocol is outlined in Supplement 1. In brief, SPRINT is a multicenter, randomized, controlled, open-label trial comparing intensive systolic BP control (goal < 120 mmHg) and standard systolic BP control (goal < 140 mmHg) in 9361 participants across 102 sites in the United States. The inclusion criteria were as follows; participants aged 50 years or older, with a systolic BP ranging from 130 to 180 mmHg, and an elevated risk of cardiovascular events. Increased cardiovascular risk was defined by one or more of the following criteria: the presence of clinical or subclinical cardiovascular disease (excluding stroke); chronic kidney disease (excluding polycystic kidney disease), with an estimated glomerular filtration rate (eGFR) between 20 and 60 ml/min/1.73 m2, calculated using a four-variable modified equation for kidney disease; a 10-year cardiovascular disease risk of 15% or higher, based on the Framing-ham risk score; or being 75 years of age or older. The trial evaluated several safety and efficacy endpoints, including serious adverse events, compound cardiovascular events, and pre-specified renal outcomes. The primary outcome of interest was the new acute decompensated HF in the study population. The study was conducted in accordance with the principles of the Declaration of Helsinki, and was approved by the institutional review board. Informed consent was obtained from all participants.
The participants were randomly assigned in a 1:1 ratio to either the intensive or standard systolic BP reduction groups based on clinical location. SPRINT’s intervention algorithm and formula have been previously described [24]. BP was measured regularly at baseline, monthly for the first 3 months, and every 3 months thereafter. The BP value was the average of three sitting measurements after 5 min of rest [26]. The SPRINT trial did not provide participants with home BP devices. Most BP outcome trials relied on office BP measurements, as home readings may introduce errors. In SPRINT, medication titration to achieve goals was based on office readings rather than home BP measurements. Based on previous studies, the target systolic BP ranges of 110–130 mmHg were for the intensive treatment group and 120–140 mmHg were for the standard treatment group. During follow-up, the participants’ systolic and diastolic BP data were collected.
Research redesign
Current analysis population
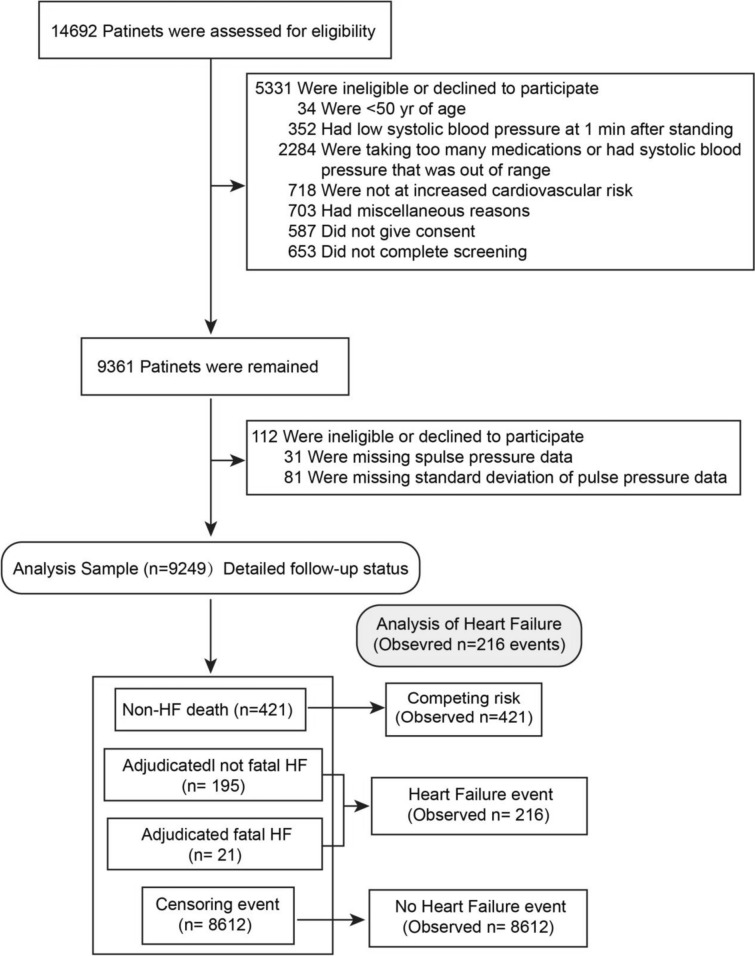
We excluded participants for whom the PPV could not be calculated (112 of 9631 randomized [1.2%]), or whose baseline covariates were missing. Overall, the study population included 9,249 SPRINT participants: 4,633 in the intensive treatment group and 4,616 in the standard treatment group (Fig. 1). During the follow-up period, 211 cases of HF were reported according to the primary outcome of the SPRINT trial [10], and 216 cases of HF were observed after adjustment (which included five deaths due to HF).
Fig. 1.
Flow chart
Definition of heart failure
The main results of our analysis were defined in the original SPRINT agreement [24]. This protocol defined HF as a condition requiring hospitalization or emergency infusion due to cardiac insufficiency and is based on the ARIC research system [27]. Acute or subacute HF (with preserved or reduced left ventricular ejection fraction), and chronic stable HF were not considered HF outcomes of the SPRINT study. Instead of relying on just single outcomes such as dyspnea, edema, low ejection fraction, or high brain natriuretic peptide (BNP) values to identify HF, the reviewers used available data and clinical judgment to distinguish between “definite” and “probable” decompensated HF. The determination of HF in patients with advanced chronic kidney disease is particularly difficult and requires a comprehensive assessment of all available information.
Statistical analysis
Pulse pressure variability grouping
This study investigates the relationship between pulse pressure variability and heart failure using the coefficient of pulse pressure variability (PPCV) as a statistical measure.
The formula to calculate the pulse pressure coefficient of variation (PPCV) is as follows:
| 1 |
| 2 |
Through multiple follow-up visits, each patient’s blood pressure was recorded at different intervals. We calculated the pulse pressure for each patient at each follow-up visit using Formula (1) and compiled a comprehensive dataset of pulse pressure values. Subsequently, we determined the mean and standard deviation of pulse pressure for each patient and computed the pulse pressure coefficient of variation (PPCV) using Formula (2). This provided detailed information on blood pressure fluctuations, giving a more comprehensive assessment of cardiovascular health status of the patients. For example: suppose patient A had multiple follow-ups and recorded 15 blood pressure values. Using Formula (1), we derived 15 corresponding pulse pressure values. Subsequently, based on these 15 pulse pressure values, we calculated the average and standard deviation of the patient’s pulse pressure during the study period. Then, employing Formula (2), we compute the patient’s Pulse Pressure Coefficient of Variation (PPCV) value.
Currently, there is no standardized classification for PPCV. Therefore, we classified PPCV into three categories (levels): low (0 < PPCV ≤ 15.0%), medium (15.0% < PPCV < 20.0%), and high (PPCV ≥ 20.0%). This classification was based on a review of the literature on the cut-off values of the coefficient of variation. The rationale for this classification is that a coefficient of variation below 15% indicates minimal fluctuations, while a coefficient above 20% signifies significant variability [28, 29].
Statistical methods
Continuous variables were summarized using the mean ± SD if they were normally distributed and as median (interquartile spacing) if they were not normally distributed. Categorical variables were expressed as percentages. The difference in the baseline features among the three groups were assessed using the one-way ANOVA for normally distributed continuous variables, Kruskal–Wallis test for non-normally distributed continuous variables, and χ2 test for categorical variables, respectively. A histogram was used to show the distribution of the pulse pressure variation coefficient. Univariate and multivariate Cox regression models were constructed to evaluate the relationship between PPCV and HF outcomes in the entire cohort, after which the main outcomes were evaluated according to the pulse pressure coefficient of variation. The Cox model was adjusted for age, baseline diastolic blood pressure, baseline serum creatinine, history of chronic kidney disease, history of cardiovascular disease, 10-year risk of arteriosclerotic cardiovascular disease (CVD), and the number of antihypertensive drugs used. We used a restricted cubic spline to explore the potential dose–response pattern between PPCV and the risk of heart failure, utilizing 3 knots to smooth the curve [30]. Since death is a competing risk factor for HF outcomes, a competing mortality risk model was constructed for the entire study population [31]. Additionally, we performed a sensitivity analysis on the multivariate Cox model. The results were presented as hazard ratios (HR) and 95% confidence intervals (CI). A two-sided p value of less than 0.05 was considered statistically significant. All analyses were performed using the R software version 4.2.1.
Results
Baseline characteristics
The study excluded 112 patients, for whom the PPCV could not be calculated, resulting in a final sample size of 9249 eligible patients (Fig. 1). Normality testing revealed that the mean age of the study population was 67.9 ± 9.4 years, with 3382 women (35.5%) participating. Based on different follow-up times for each patient, each patient had a varying number of blood pressure readings. The average number of blood pressure readings per patient is 14.6 times. Among them, 8288 patients (89.6%) had blood pressure readings 10 times or more, while 5253 patients (56.8%) had readings 15 times or more. The mean SBP was 139.5 ± 15.5 mmHg and the mean DBP was 78.1 ± 11.9 mmHg. Supplement 2, S3 shows the frequency distribution of the PPCV in the study population, with an average PPCV of 13.85 ± 5.37%.
Participants with a PPCV greater than 20.0% were more likely to be female, have higher total cholesterol levels, be on blood pressure medications, and have HF compared to participants with a PPCV of 0 to 15.0%. Among patients in the highest PPCV group, there was notable prevalence of chronic kidney disease and were current smokers. Additionally, the baseline and mean DBP increased with higher PPCV (Table 1). Statistically significant differences (p < 0.001) were observed in the mean pulse pressure, mean coefficient of variation of pulse pressure, mean standard deviation of pulse pressure, and blood pressure between the HF group and the non-HF group (Supplement 2, S4).
Table 1.
Baseline characteristics of participants grouped according to PPCV
| Characteristics | PPCV GROUP | All patients (n = 9429) | P* | ||
|---|---|---|---|---|---|
| PPCV ≤ 15.0% (n = 6165) | 15.0% < PPCV < 20.0% (n = 2143) | PPCV ≥ 20.0% (n = 941) | |||
| RISK10YRS, Mean ± SD | 20.11 ± 10.59 | 19.90 ± 11.50 | 19.48 ± 11.63 | 20.00 ± 10.92 | 0.222 |
| Baseline SBP, mmHg, Mean ± SD | 137.88 ± 13.57 | 142.05 ± 17.48 | 145.98 ± 20.41 | 139.67 ± 15.60 | < 0.001 |
| Baseline DBP, mmHg, Mean ± SD | 77.16 ± 11.38 | 79.67 ± 12.33 | 80.90 ± 13.76 | 78.12 ± 11.93 | < 0.001 |
| Mean DBP, mmHg, Mean ± SD | 71.49 ± 9.18 | 73.04 ± 9.09 | 74.14 ± 9.34 | 72.12 ± 9.22 | < 0.001 |
| SCREAT, mg/dL, Mean ± SD | 1.08 ± 0.33 | 1.06 ± 0.37 | 1.05 ± 0.38 | 1.07 ± 0.35 | 0.041 |
| AGE, years, Mean ± SD | 68.05 ± 9.26 | 67.79 ± 9.50 | 67.32 ± 10.09 | 67.91 ± 9.40 | 0.071 |
| CHR, mg/dL, Mean ± SD | 188.03 ± 41.18 | 192.36 ± 43.23 | 194.63 ± 45.41 | 189.70 ± 42.18 | < 0.001 |
| BMI, kg/m2, Mean ± SD | 29.40 ± 5.71 | 29.99 ± 7.04 | 30.65 ± 7.36 | 29.67 ± 6.24 | < 0.001 |
| PPCV, %, Mean ± SD | 11.10 ± 2.43 | 17.10 ± 1.43 | 24.45 ± 7.36 | 13.85 ± 5.37 | < 0.001 |
| SBPCV, %, Mean ± SD | 7.91 ± 2.36 | 11.07 ± 2.57 | 14.00 ± 3.79 | 9.26 ± 3.32 | < 0.001 |
| DBPCV, %, Mean ± SD | 9.36 ± 3.04 | 10.84 ± 3.26 | 12.14 ± 4.05 | 9.99 ± 3.35 | < 0.001 |
| Intensive, n (%) | < 0.001 | ||||
| Standard treatment | 3244 (52.6) | 1008 (47.0) | 364 (38.7) | 4616 (49.9) | |
| Intensive treatment | 2921 (48.4) | 1135 (53.5) | 577 (61.3) | 4633 (50.1) | |
| Inclusionfrs, n (%) | < 0.001 | ||||
| No | 2285 (37.1) | 877 (40.9) | 404 (44.2) | 3566 (38.6) | |
| Yes | 3880 (62.9) | 1266 (59.1) | 537 (56.8) | 5683 (61.4) | |
| Noagents, n (%) | 0.046 | ||||
| No | 5563 (90.2) | 1941 (90.6) | 873 (92.8) | 8377 (90.6) | |
| Yes | 602 (9.8) | 202 (9.4) | 68 (7.2) | 872 (9.4) | |
| Smoke CAT, n (%) | < 0.001 | ||||
| Never | 2759 (44.7) | 918 (42.8) | 398 (42.3) | 7661 (82.8) | |
| Former | 2718 (44.1) | 877 (40.9) | 347 (36.9) | 3942 (42.6) | |
| Current | 683 (11.1) | 345 (16.1) | 194 (20.6) | 1222 (13.2) | |
| Missing | 5 (0.1) | 3 (0.2) | 2 (0.2) | 10 (0.11) | |
| SUB CKD, n (%) | < 0.001 | ||||
| No | 4509 (73.1) | 1506 (70.3) | 625 (66.4) | 6640 (71.8) | |
| Yes | 1656 (26.9) | 637 (29.7) | 316 (33.6) | 2609 (28.2) | |
| Female, n (%) | < 0.001 | ||||
| Male | 4376 (71.0) | 1179 (45.0) | 412 (42.1) | 5976 (64.6) | |
| Female | 1789 (29.0) | 964 (55.0) | 529 (57.9) | 3282 (35.5) | |
| Heart failure, n (%) | < 0.001 | ||||
| Censored | 6045 (98.1) | 2090 (97.5) | 898 (95.4) | 9033 (97.7) | |
| Event | 120 (1.9) | 53 (2.5) | 43 (4.6) | 216 (2.3) | |
SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, SCREAT serum creatinine, CHR Cholesterol, BMI body mass index, PPCV Coefficient of pulse pressure variation, SBPCV Coefficient of Systolic Blood Pressure variation, DBPCV Coefficient of Diastolic Blood Pressure variation; INTENSIVE is Assigned to intensive BP arm; INCLUSIONFRS is Framingham 10-year CVD risk > 15%; N AGENTS is Number of medications prescribed; NOAGENTS is participants on no anti-hypertensive agents; SMOKE CAT is Baseline smoking status; ASPIRIN is Daily Aspirin Use; SUB CKD is Subgroup with CKD (eGFR < 60); SUB CVD is subgroup with history of clinical/sub-clinical CVD; SUB CLINICALCVD is subgroup with history of clinical CVD; SUB SUBCLINICALCVD is subgroup with history of sub-clinical CVD
*The above p-value for quantitative data was obtained through analysis of variance (ANOVA), while for categorical data, it was obtained through chi-square test. RISK10YRS is Framingham estimation of 10-year CVD risk
Cumulative incidence of HF in each PPCV group
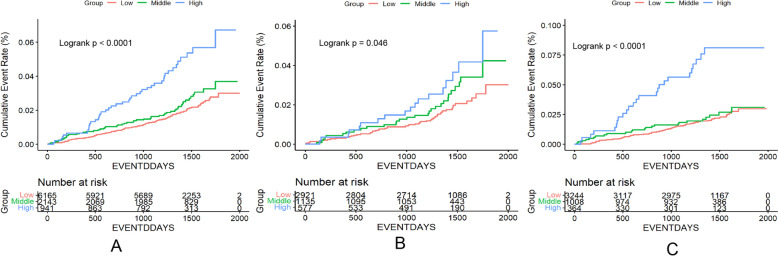
During a median follow-up of 3.87 years (1412 days), 188 participants experienced HF events, resulting in an incidence rate of 0.525 per 100 person-years (95% CI 0.255, 1.080). The cumulative incidences of HF across the three groups were 1.95%, 2.47%, and 4.57%, respectively (Supplement 2, S5). Log-rank tests revealed significant differences in the cumulative incidence among the three groups. Notably, the high PPCV group exhibited a higher cumulative incidence of HF compared to the low PPCV group, a trend that was consistent in both the intensive and standard treatment groups (Fig. 2).
Fig. 2.
HF incidence by PPCV category. A is totality, B is INTENSIVE = Intensive treatment, C is INTENSIVE = Standard treatment. Incidence of heart failure by pulse pressure variability groups: pulse pressure variability groups were based on pulse pressure coefficient of variation as Low (PPCV ≤ 15.0%), Middle (15.0% < PPCV < 20.0%), and High (PPCV ≥ 20.0%). P < 0.0001 for differences among curves using the log-rank test
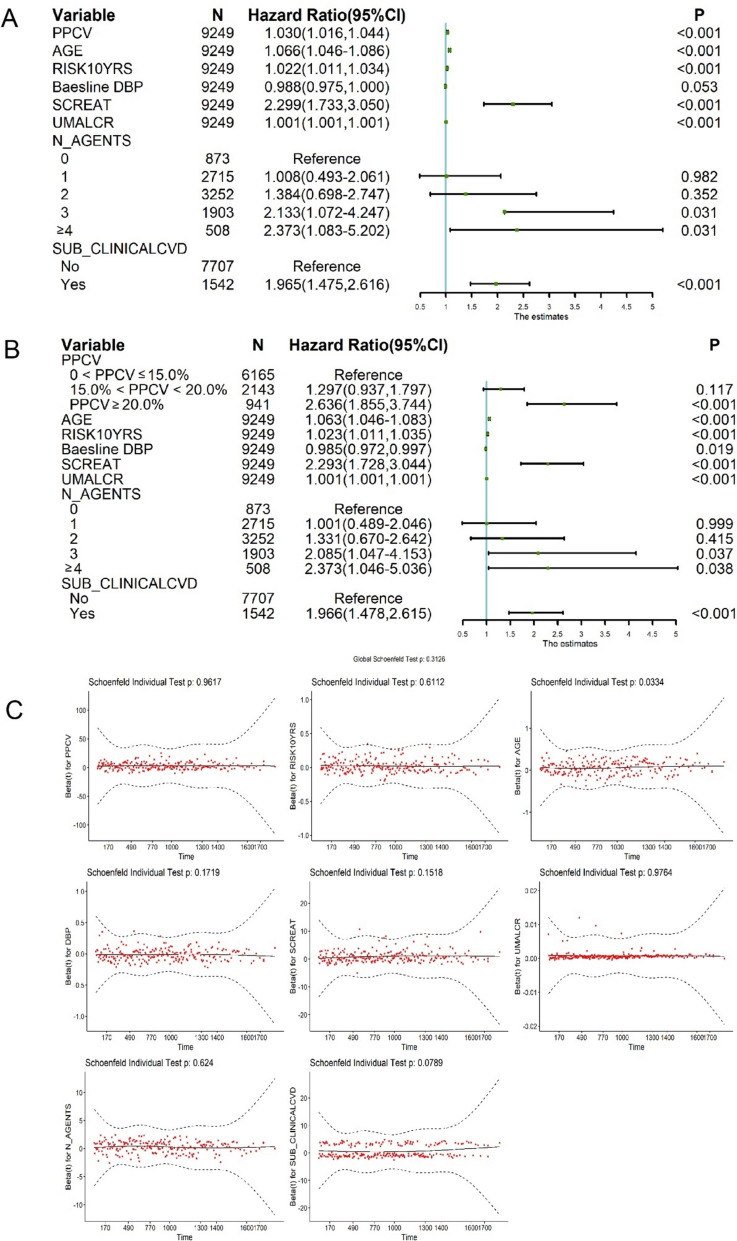
Multivariate cox regression analysis of the relationship between PPCV and HF
In univariate Cox model, the risk of heart failure increased by 3.2% times for every 1% increase in PPCV (HR = 1.032 [95% Cl 1.018–1.046]; P < 0.001; Table 2). We then developed a multivariate Cox model, adjusting for baseline demographics, medical history, and baseline DBP, with HF as the dependent variable and PPCV grouping or with each 1% increase in PPCV as the independent variable (Fig. 3). The risk of developing new HF was 1.297 and 2.636 times higher in the moderate and high PPCV groups, compared to the low PPCV group, respectively (95% CI 0.937–1.797 and 95% CI 1.855–3.744, respectively; Table 2, Fig. 3B). After multivariate adjustment, the risk of heart failure increased by 3.0% for every 1% increase in PPCV (HR = 1.030 [95% CI 1.016–1.044]; P < 0.001; Table 2; Fig. 3A). Furthermore, we also performed a residual analysis of the regression model with PPCV as a continuous variable to further validate our findings (Fig. 3C). To account for potential impact of all-cause mortality on HF outcomes, we also constructed a competing risk model for total population mortality, which yielded consistent results indicating that higher PPCV remains significantly associated with an increased risk of HF (Supplement 2, S6).
Table 2.
Univariate and multivariate cox regression tables of PPCV
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PPCV constructed as continuous variables | ||||||
| PPCV | 1.032 | 1.018–1.046 | < 0.001 | 1.030 | 1.016–1.044 | < 0.001 |
| AGE | 1.099 | 1.083–1.117 | < 0.001 | 1.066 | 1.046–1.086 | < 0.001 |
| RISK10YRS | 1.046 | 1.036–1.056 | < 0.001 | 1.022 | 1.011–1.034 | < 0.001 |
| Baseline DBP | 0.955 | 0.944–0.966 | < 0.001 | 0.988 | 0.975–1.000 | 0.053 |
| SCREAT | 3.502 | 2.785–4.405 | < 0.001 | 2.299 | 1.733–3.050 | < 0.001 |
| UMALCR | 1.001 | 1.001–1.001 | < 0.001 | 1.001 | 1.001–1.001 | < 0.001 |
| N_AGENTS* | ||||||
| 1 | 1.115 | 0.553–2.246 | 0.762 | 1.008 | 0.493–2.061 | 0.982 |
| 2 | 1.805 | 0.931–3.503 | 0.081 | 1.384 | 0.698–2.747 | 0.352 |
| 3 | 3.542 | 1.835–6.836 | < 0.001 | 2.133 | 1.072–4.247 | 0.031 |
| ≥ 4 | 3.582 | 1.676–7.653 | 0.001 | 2.373 | 1.083–5.202 | 0.031 |
| SUB_CLINICALCVD* | ||||||
| Yes | 3.162 | 2.401–4.166 | < 0.001 | 1.965 | 1.475–2.616 | < 0.001 |
| PPCV as a grouping variable | ||||||
| PPCV* | ||||||
| 15.0% < PPCV < 20.0% | 1.250 | 0.905–1.728 | 0.175 | 1.297 | 0.937–1.797 | 0.117 |
| PPCV ≥ 20.0% | 2.507 | 1.770–3.552 | < 0.001 | 2.636 | 1.855–3.744 | < 0.001 |
| AGE | 1.099 | 1.083–1.117 | < 0.001 | 1.063 | 1.044–1.083 | < 0.001 |
| RISK10YRS | 1.046 | 1.036–1.056 | < 0.001 | 1.023 | 1.011–1.035 | < 0.001 |
| Baseline DBP | 0.955 | 0.944–0.966 | < 0.001 | 0.985 | 0.972–0.997 | 0.019 |
| SCREAT | 3.502 | 2.785–4.405 | < 0.001 | 2.293 | 1.728–3.044 | < 0.001 |
| UMALCR | 1.001 | 1.001–1.001 | < 0.001 | 1.001 | 1.001–1.001 | < 0.001 |
| N_AGENTS* | ||||||
| 1 | 1.115 | 0.553–2.246 | 0.762 | 1.001 | 0.489–2.046 | 0.999 |
| 2 | 1.805 | 0.931–3.503 | 0.081 | 1.330 | 0.670–2.642 | 0.415 |
| 3 | 3.542 | 1.835–6.836 | < 0.001 | 2.085 | 1.047–4.153 | 0.037 |
| ≥ 4 | 3.582 | 1.676–7.653 | 0.001 | 2.295 | 1.046–5.036 | 0.038 |
| SUB_CLINICALCVD* | ||||||
| Yes | 3.162 | 2.401–4.166 | < 0.001 | 1.966 | 1.478–2.615 | < 0.001 |
We conducted univariate Cox regression analysis on all baseline variables. Variables with a p value less than 0.2 were selected for further Cox regression analysis. Subsequently, variables with a p-value greater than 0.05 and a change in the hazard ratio (HR) value for PPCV (after eliminating the variable) less than 10% were eliminated. Finally, we identified PPCV, AGE, RISK10YRS, Baseline DBP, SCREAT, UMALCR, N_AGENTS, SUB_CLINICALCVD as the significant variable. * N_AGENTS with 0 prescription drugs as the control group, SUB_CLINICALCVD with no history of clinical CVD as the control group, and PPCV with 0 < PPCV ≤ 15.0% as the control group
Fig. 3.
Multivariate cox regression forest map and residual plot of the relationship between PPCV and HF outcomes. A PPCV is continuous variable; B PPCV is classified variable. C residual plot of PPCV as a continuous variable. We conducted univariate Cox regression analysis on all baseline variables. Variables with a p value less than 0.2 were selected for further Cox regression analysis. Subsequently, variables with a p-value greater than 0.05 and a change in the hazard ratio (HR) value for PPCV (after eliminating the variable) less than 10% were eliminated. Finally, we identified PPCV, AGE, RISK10YRS, Baseline DBP, SCREAT, UMALCR, N_AGENTS, SUB_CLINICALCVD as the significant variable
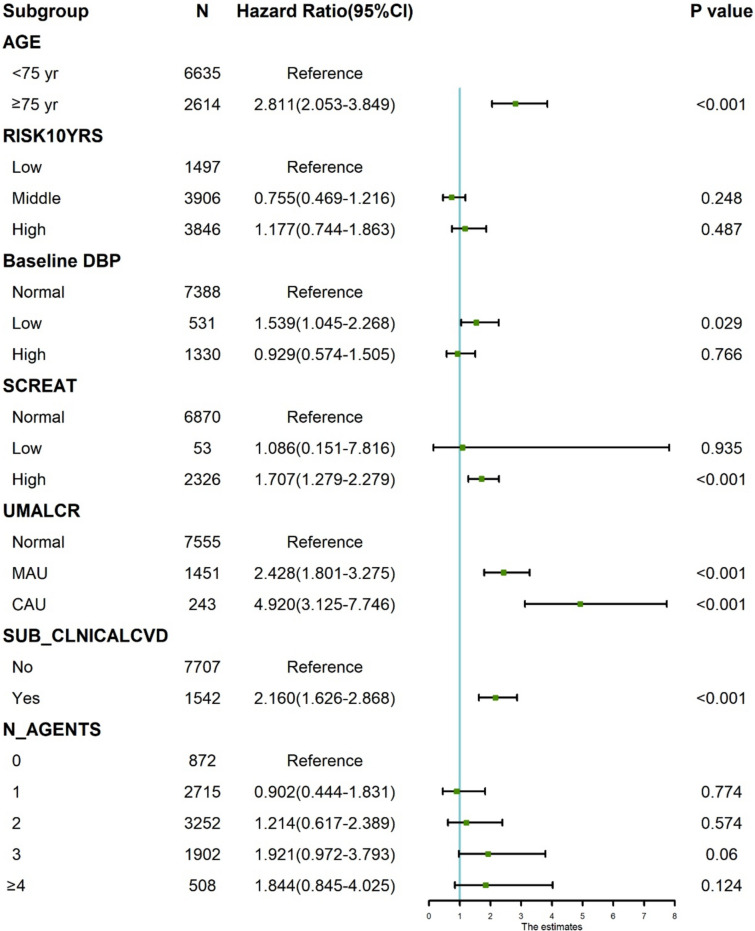
We performed multivariate regression analysis to examine the association between age, baseline DBP, baseline serum creatinine, history of chronic kidney disease, history of CVD, 10-year risk of arteriosclerotic CVD, and taking antihypertensive drugs, with the incidence of HF. Multivariate regression analysis showed that age, history of chronic kidney disease, baseline DBP, baseline serum creatinine level, and cardiovascular history had a significant impact on the incidence of HF (Fig. 4). After controlling for other relevant factors, we found a statistically significant association with HF in participants over 75 years of age, with low baseline DBP, high baseline serum creatinine, history of chronic kidney disease (microproteinuria versus hyper proteinuria), and history of CVD (p < 0.05). Furthermore, we included additional indicators of BP variability in the Cox regression models mentioned above for analysis. The results indicate that PPCV (HR: 1.028, 95% CI 1.010–1.046; p = 0.002) still exhibits a significant predictive capability for the risk of heart failure occurrence (Supplement 2, S7). In addition, we divided PPCV, SBPCV, and DBPCV into deciles and calculated the incidence of heart failure events within each decile to complement the completeness of our results (Supplement 2 S8).
Fig. 4.
Dummy Forest plot of multivariate cox regression model of PPCV. AGE was based on age as < 75 year and ≥ 75 year; RISK10YRS was based on Framingham estimation of 10-year CVD risk as Low (risk10yrs < 10.0%), Middle (risk10 years ≥ 10.0% to ≤ 20.0%), and High(risk10 years > 20.0%); Baseline DBP was based on diastolic blood pressure as Normal (DBP ≥ 60 to ≤ 90 mmHg), Low (DBP < 60 mmHg), and High (DBP > 90 mmHg); SCREAT was based on serum creatinine(SC), as Normal (SC ≥ 0.5 to ≤ 1,2 mg/dL), Low (SC < 0.5 mg/dL), and High(SC > 1.2 mg/dL); UMALCR was based on Urine Albumin/Creatinine ratio (uACR) as Normal (uACR ≤ 30 mg/g), MAU(micro albuminuria, uACR > 30 to ≤ 300 mg/g), and CAU (clinical albuminuria, uACR > 300 mg/g); SUB_CLNICALCVD was based on subgroup with history of clinical CVD as No and Yes; N_AGENTS was based on number of anti-hypertensive medications prescribed as 0, 1, 2, 3, ≥ 4 (including 4, 5, 6). The model included PPCV of HR (95%Cl) was 1.026 (1.011–1.041), and P value was < 0.001
The restrictive cubic spline cox proportional hazards model was used to analyze the relationship between PPCV and the risk of new-onset HF
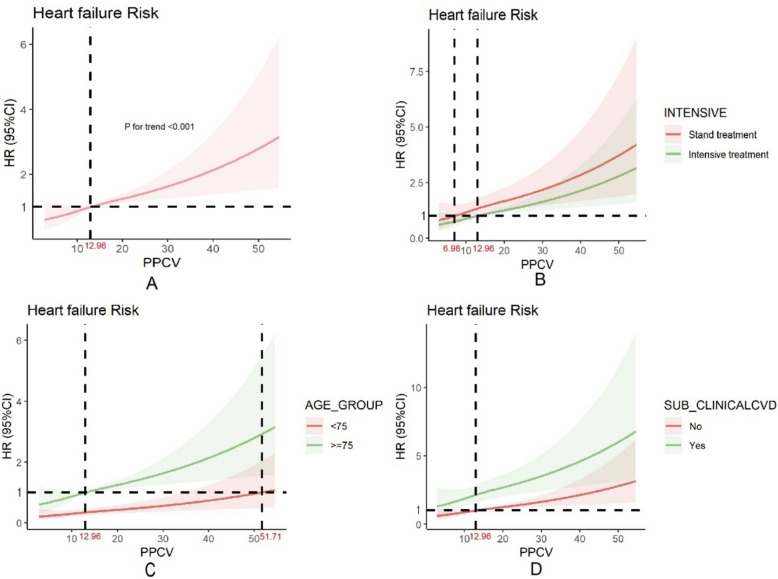
The global and nonlinear associations between PPCV and new-onset HF were significant (p < 0.001). The results of the restricted cubic spline Cox proportional hazards model showed that the risk of HF gradually increased with PPCV after adjusting for covariates (Fig. 5). This relationship was consistent across both the pre-specified baseline subgroups and in various intervention groups.
Fig. 5.
Limiting cubic splines of PPCV for heart failure outcomes. A is not-group, Adjusted relative hazard of heart failure by the continuous level pulse pressure coefficient of variation of (PPCV). The reference point is PPCV of 12.96%. The solid lines represent the hazard ratios across the spectrum of PPCV. The shaded regions represent the upper and lower bounds of the 95% confidence interval. P-values reflect adjusted trends (accounting for Baseline DBP, RISK10YRS, SCREAT, AGE, UMALCR, N_AGENTS, SUB_CLINICALCVD). B is INTENSIVE group, the reference point is PPCV of 6.98% and 12.96%. C is AGE group, the reference point is PPCV of 12.96%3 and 51.71%. D is SUB_CLNICALCVD group, the reference point is PPCV of 12.96%. Other group graphs are also based on the agreement model and reflect the same trend
Sensitivity analysis
Among the factors influencing HF, we found no significant interactions between PPCV and age, baseline DBP, history of chronic kidney disease, baseline serum creatinine, history of CVD, 10-year risk of arteriosclerotic CVD, or the number of antihypertensive drugs taken (p > 0.1; Supplement 2, S9).
Discussion
We conducted a secondary analysis of the SPRINT trial to explore the relationship between long-term monitoring of pulse pressure variability and the occurrence of heart failure in hypertensive patients. The patients with the highest PPV (PPCV ≥ 20.0%) had a 2.636 times greater risk of developing HF than those with the lowest PPV (0 < PPCV ≤ 15%). In addition, PPV was associated with the risk of new-onset HF in a dose-dependent manner. Overall, we found that the PPV is a powerful additional determinant of HF risk and that long-term control of PPV within a certain range is beneficial for preventing the occurrence of HF. The increase in pulse pressure variability may reflect reduced arterial elasticity or instability in cardiac pumping function, which could lead to an increased burden on the heart and consequently raise the risk of heart failure. Therefore, long-term monitoring and controlling of pulse pressure variability help to assess the stability of the cardiovascular system, thereby reducing the risk of heart failure occurrence.
Our study provides novel insights into the relationship between long-term repeated measurements of pulse pressure and new-onset HF in patients with hypertension. Understanding this relationship can help physicians identify patients with hypertension who are at high risk of developing heart failure. Previously, we used BPV in clinics to describe the degree of fluctuation in BP over a period, which includes systolic pressure variability, diastolic pressure variability, and pulse pressure variability. A previous study followed 3,820,191 healthy individuals for an average of 5 years and found that those with high SBP variability were 1.16 times more likely to develop new HF during follow-up than those with normal SBP variability [32]. In a previous study, Muntner et al. showed that the risk of new-onset HF in hypertensive patients with elevated BPV during the follow-up period was 1.56 times that in patients without elevated BPV [20]. Similarly, Nuyujukian et al. found that both systolic and diastolic blood pressure variability increased the risk of HF in patients with type 2 diabetes [33]. However, the relationship between long-term pulse pressure variability and the risk of new HF currently remains unclear. Our study found that as pulse pressure variability increases, the risk of heart failure in hypertensive patients also increases. Elevated PPV was identified as an independent risk factor for new-onset HF in hypertensive patients, regardless of age, cardiovascular history, baseline DBP, number of antihypertensive drugs taken, or presence of renal stratification. In recent years, many epidemiological studies on HF risk factors indicated a need to explore more risk factors closely related to HF pathogenesis, in addition to the well-known risk factors such as age, cardiovascular history, hypertension, and hyperglycemia. Our study is the first to demonstrate the predictive value of PPV together with traditional risk factors in identifying new risk factors for HF in patients with hypertension. Blood pressure exhibits variability, so associated pulse pressure also fluctuates. In addition to monitoring the blood pressure levels, the pulse pressure value is equally important in providing a comprehensive assessment of cardiovascular health. In this study, Pulse Pressure Variability (PPV) demonstrates superior predictive capabilities compared to Blood Pressure Variability (BPV). In clinical practice, assessment of both BPV and PPV will aid in blood pressure management, and provide insights into the heart health and overall blood flow status [34].
Since patients enrolled in the SPRINT had different BP goals at the start of the trial, the intensive treatment group had a lower target range for systolic BP (less than 120 mmHg) compared to the standard treatment group, which is consistent with other studies [24]. As such, it is not surprising that the PPCV was generally higher in the intensive treatment group than in the standard treatment group. However, we found that the dummy variables for intervention in the restricted cubic model were associated with a lower risk of HF in the intensive group than in the standard group for the same coefficient of pulse pressure variation, suggesting that intensive systolic pressure control may reduce the association between the coefficient of variation and HF risk. Therefore, the association between PPV and HF outcomes may be stronger than that reported in the present study. Moreover, our study was based on a relatively long-term calculation of PPV (up to 54 months of follow-up), highlighting the benefits of long-term stable control of pulse pressure for heart failure outcomes. Our study also found that after adjusting for covariates, the study population with a low baseline DBP had a higher risk of new-onset HF than the population with high baseline DBP. This suggests that patients with hypertension should also pay attention to decreasing DBP when strengthening SBP control; otherwise, blind use of antihypertensive drugs may increase the risk of HF. This phenomenon is known as the “J-curve effect”. Previous studies have shown that intensified antihypertensive treatment can lead to a “J-curve effect” in patients with coronary heart disease and hypertension [35]. It is important to guide the adjustment of antihypertensive drugs in clinical settings.
Although clinical guidelines for heart failure do not emphasize the importance of long-term monitoring of pulse pressure variability [36], conversely, our findings suggest that long-term assessment of pulse pressure variability could be used for routine evaluation in hypertensive patients. More randomized controlled trials are required to establish the standard range of pulse pressure variability. Therefore, in addition to focusing on systolic pressure, diastolic pressure, and blood pressure variability, future studies on hypertensive patients should pay attention to long-term pulse pressure and its variability. This approach can better reflect the long-term control of blood pressure and vascular changes, enabling the assessment of the risk of cardiovascular events. This approach would facilitate medication based on both blood pressure levels and variability. In particular, with the rise of wearable devices and innovative products [37, 38], long-term monitoring of PPV is becoming more convenient and accurate, and accessible even in settings with limited resources. However, it’s crucial to consider potential differences in BP measurement methods when using wearable devices for unattended monitoring. The use of these devices may yield different BPV measurements compared to traditional attended measurements, which could affect the interpretation of PPV values. Further research is needed to assess the comparability of PPV values obtained from various measurement modalities to ensure reliability and validity of long-term PPV monitoring in clinical practice.
This study has several limitations. First, there is no established cut-off values for the categorization of the coefficient of variation of blood pressure (PPCV), therefore, we could only refer to the classification in other fields. Second, the interaction of the different antihypertensive drugs used in the study with fluctuating BP requires to be explored further, and confounding factors cannot be entirely ruled out. Finally, information on SBP control after the trial was limited to routine outpatient SBP values for a subgroup of participants, extracted from the EHR. This method is known to be inconsistent with the standardized BP measurement protocol used during the trial [39], potentially introducing measurement bias. We are merely suggesting a direction for future research; more exploration of the relationship between pulse pressure variability and heart failure is needed.
Conclusion
Overall, this study showed that increased PPV was significantly associated with an increased risk of new-onset HF in a dose-dependent manner among hypertensive patients. These findings support the use of PPV as a measure of BP control. Efforts should be made to reduce the risk of HF in this patient-group by ensuring a stable PP through regular BP measurements during routine care or through self-monitoring. This study further indicates that long-term monitoring of PPV provides a new target for early prevention of HF in public health settings. This highlights the potential benefits of incorporating long-term monitoring and assessment of PPV in community health screenings.
Supplementary Information
Acknowledgements
We thank the SPRINT (Systolic Blood Pressure Intervention Trial) investigators for making the study data available for public use through the National Heart, Lung, and Blood Institute BioLINCC Biologic Specimen and Data Repository.
Abbreviations
- BP
Blood pressure
- BPV
Blood pressure variability
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- EHR
Electronic health record
- HF
Heart failure
- HfrEF
Heart failure with reduced ejection fraction
- HfmrEF
Heart failure with mildly reduced ejection fraction
- HfpEF
Heart failure with preserved ejection fraction
- HR
Hazard ratio
- PP
Pulse pressure
- PPCV
Coefficient of pulse pressure variation
- PPV
Pulse pressure variability
- SBP
Systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Author contributions
HM and CJ drafted the initial manuscript. HM and MW process and analyze data, HM and CJ reviewed and edited the manuscript. Oscar contributed in editing the article in English. HM, MW, CQ, RC, LW, YS, and CJ approved the submitted manuscript.
Funding
This work was supported by the Research Center for the Development of Medicine and Health Science and Technology of the National Health Commission (2023YFC3606200) and Zhejiang Provincial Health Commission (No. 2023ZL360 and No. 2024KY1195).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the BioLINCC data repository, https://biolincc.nhlbi.nih. gov/home/.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhejiang Chinese Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huan Ma, Minyan Wang and Chu Qin have contributed equally to this work.
Contributor Information
Wei Mao, Email: maoweilw@163.com.
Conghua Ji, Email: jchi2005@126.com.
References
- 1.Tao J, Sang D, Zhen L, Zhang X, Li Y, Wang G, et al. Elevated urine albumin-to-creatinine ratio increases the risk of new-onset heart failure in patients with type 2 diabetes. Cardiovasc Diabetol. 2023;22(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388(10060):2665–712. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad H, Khan H, Haque S, Ahmad S, Srivastava N, Khan A. Angiotensin-converting enzyme and hypertension: a systemic analysis of various ACE inhibitors, their side effects, and bioactive peptides as a putative therapy for hypertension. J Renin Angiotensin Aldosterone Syst. 2023;2023:7890188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Song TT, Dong CW. Association between Interactions among ACE gene polymorphisms and essential hypertension in patients in the Hefei Region, Anhui, China. J Renin Angiotensin Aldosterone Syst. 2023;2023:1159973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinho-Gomes AC, Azevedo L, Bidel Z, Nazarzadeh M, Canoy D, Copland E, et al. Effects of blood pressure-lowering drugs in heart failure: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37(9):1757–67. [DOI] [PubMed] [Google Scholar]
- 12.Yano Y, Reis JP, Lewis CE, Sidney S, Pletcher MJ, Bibbins-Domingo K, et al. Association of blood pressure patterns in young adulthood with cardiovascular disease and mortality in Middle Age. JAMA Cardiol. 2020;5(4):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68(13):1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alaarag A, Amin O. Blood pressure variability in patients with angina and non-obstructive coronary artery disease. J Hum Hypertens. 2021;35(12):1074–80. [DOI] [PubMed] [Google Scholar]
- 15.Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens. 2016;34(2):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Matsuzaki M, Kawagoe Y, Kitamura K, Tsuruda T, Kaikita K, et al. Analysis of mechanisms for increased blood pressure variability in rats continuously infused with angiotensin II. J Renin Angiotensin Aldosterone Syst. 2023;2023:4201342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, et al. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26(10):1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arashi H, Ogawa H, Yamaguchi J, Kawada-Watanabe E, Hagiwara N. Impact of visit-to-visit variability and systolic blood pressure control on subsequent outcomes in hypertensive patients with coronary artery disease (from the HIJ-CREATE substudy). Am J Cardiol. 2015;116(2):236–42. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163(5):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homan TD, Bordes SJ, Cichowski E. Physiology, Pulse Pressure. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Stephen Bordes declares no relevant financial relationships with ineligible companies. Disclosure: Erica Cichowski declares no relevant financial relationships with ineligible companies: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
- 22.Qiu W, Xiao X, Cai A, Gao Z, Li L. Pulse pressure and all-cause mortality in ischaemic heart failure patients: a prospective cohort study. Ann Med. 2022;54(1):2701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonapace S, Rossi A, Laroche C, Crespo-Leiro MG, Piepoli MF, Coats AJS, et al. Brachial pulse pressure in acute heart failure. Results of the Heart Failure Registry. ESC Heart Fail. 2019;6(6):1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, Cutler JA, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. 2021;384(20):1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71(5):848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bfpj H. Estimation of temporal and spatial distribution of potential vegetation net primary productivity in China since 2000. Acta Ecol Sin. 2022;42(24):10288–96. [Google Scholar]
- 29.Huang SYCQF. Analysis of vegetation variation characteristics of MODIS EVI in Zhongnan Mountain from 2001 to 2020. Geogr Sci Res. 2023;12(6):727–37. [Google Scholar]
- 30.Li C, Zhu Y, Ma Y, Hua R, Zhong B, Xie W. Association of cumulative blood pressure with cognitive decline, dementia, and mortality. J Am Coll Cardiol. 2022;79(14):1321–35. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon S, Lee SR, Choi EK, Lee SH, Han KD, Lee SY, et al. Visit-to-visit variability of metabolic parameters and risk of heart failure: a nationwide population-based study. Int J Cardiol. 2019;293:153–8. [DOI] [PubMed] [Google Scholar]
- 33.Nuyujukian DS, Koska J, Bahn G, Reaven PD, Zhou JJ. Blood pressure variability and risk of heart failure in ACCORD and the VADT. Diabetes Care. 2020;43(7):1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang KS, Medeiros ED, Shah AD. Wide pulse pressure: a clinical review. J Clin Hypertens. 2020;22(11):1960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu W. Could intensive anti-hypertensive therapy produce the “J-curve effect” in patients with coronary artery disease and hypertension after revascularization? Eur Rev Med Pharmacol Sci. 2016;20(7):1350–5. [PubMed] [Google Scholar]
- 36.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. [DOI] [PubMed] [Google Scholar]
- 37.Zhou ZB, Cui TR, Li D, Jian JM, Li Z, Ji SR, et al. Wearable continuous blood pressure monitoring devices based on pulse wave transit time and pulse arrival time: a review. Materials. 2023;16(6):2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Sheng S, Liu Y, Wen J, Song C, Chen Z, et al. Automatic and continuous blood pressure monitoring via an optical-fiber-sensor-assisted smartwatch. PhotoniX. 2023;4(1):21. [Google Scholar]
- 39.Drawz PE, Agarwal A, Dwyer JP, Horwitz E, Lash J, Lenoir K, et al. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern Med. 2020;180(12):1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We registered an account on the BioLINCC website (https://biolincc.nhlbi.nihgov/home/). We uploaded our study protocol and IRB approval and requested to use the data. We then retrieved the original data on HF after signing the NHLBI Research Materials Distribution Agreement (RMDA). Following the full preparation of the application materials, the process took approximately 3 days. This study was approved by the Ethics Committee of Zhejiang Chinese Medical University.
The datasets generated and/or analyzed during the current study are available in the BioLINCC data repository, https://biolincc.nhlbi.nih. gov/home/.