Abstract
Background
Our previous small-sample study indicated that serum levels of interleukin enhancer binding factor 2 (ILF2) may have the potential for gastric cancer (GC) detection. The present study was conducted to further validate the diagnostic value of serum ILF2 protein for GC.
Methods
Serum specimens and clinical data were collected from patients with GC (n = 99) or benign gastric disease (BGD) (n = 49) and healthy controls (HC) (n = 51). Serum ILF2 levels were measured using enzyme-linked immunosorbent assay. The diagnostic performance of ILF2 was evaluated using the area under the receiver operating characteristic curve (AUC). The independence and synergy of ILF2 in GC diagnosis were analyzed by modeling with conventional blood indicators.
Results
The median serum ILF2 level was higher in the GC group (227.8ng/mL) than in the BGD group (72.0ng/mL) and the HC group (56.8ng/mL) (p < 0.001), and no significant difference across GC subgroups. The AUCs of ILF2 were 0.915 (95%CI 0.873–0.957) for GC vs. HC, 0.854 (95%CI 0.793–0.915) for GC vs. BGD, 0.885 (95%CI 0.841–0.929) for GC vs. BGD + HC, and 0.888 (95% CI 0.830–0.945) for TNM I stage GC vs. BGD + HC, outperforming conventional blood indicators (corresponding AUCs ranging from 0.641 to 0.782). ILF2 was independent of and synergistic with conventional blood indicators in GC diagnosis, and a simple diagnostic model based on ILF2 and red blood cell count improved the diagnostic performance, with positive rates of approximately 90% in various subgroups of GC.
Conclusions
Serum ILF2 protein is a novel and potential serum biomarker for the detection of GC, especially for early GC.
Keywords: Gastric cancer, ILF2, Early diagnosis, Serum biomarker
Introduction
Gastric cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer-related death, with more than 1.08 million new cases and 0.76 million deaths annually [1]. The prognosis for GC remains poor, with a 5-year survival rate close to 30% [2]. Gastroscopy and biopsy [3] are effective but invasive and expensive methods for diagnosing GC [4], leading to a need for less invasive and more affordable diagnostic options.
Serum tumor biomarkers are clinically convenient approaches for cancer detection. In previous studies, traditional serum biomarkers have been extensively evaluated in the diagnosis of GC, such as carbohydrate antigen 72 − 4 (CA72-4) [5], carcinoembryonic antigen (CEA), carbohydrate antigen 19 − 9 (CA19-9), cancer antigen 125 (CA125), AFP (alpha-fetoprotein) [6], and carbohydrate antigen 242 (CA242) [7]. However, these traditional biomarkers are limited in the clinical application of GC due to low sensitivity and specificity [8, 9]. The discovery of novel biomarkers with improved diagnostic performance for GC is urgently needed.
Recent studies have identified some new types of tumor biomarkers [10], such as circulating tumor cells (CTC) [11], circulating tumor DNA (ctDNA) [12], and exosomes [13], which provide potential new approaches for GC detection [14]. However, these novel biomarkers have low serum levels and require further validation due to lack of standardized detection methods and limited real-world applications [15]. Proteins are important biomarkers in cancer liquid biopsy as they provide more detailed information than DNA/RNA in these samples [16]. Several protein biomarkers have been identified for GC, such as src kinase-associated phosphoprotein 1 (SKAP1) [17], carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) [18], as well as specific pepsinogen (PG), gastrin-17 (G-17) [19], and gastrokine 1 (GKN1) [20], but further clinical studies are needed to confirm their accuracy [21].
Previously, we identified a potential serum protein biomarker of GC, interleukin enhancer binding factor 2 (ILF2), using our aptamer-based serum proteomic data of GC and transcriptomic data of GC from public database, and the preliminary data showed that serum ILF2 levels were significantly elevated in GC patients compared with normal controls [22], suggesting that serum ILF2 protein may be a valuable biomarker for the detection of GC. Therefore, in this study, we further evaluated the diagnostic value of serum ILF2 protein in GC, especially in early stage GC.
Materials and methods
Subjects
The subjects of this study were patients with GC or benign gastric disease (BGD) and healthy controls (HC) who received medical services at the First Affiliated Hospital of Nanchang University between 2013 and 2022. The inclusion criteria for the subjects were as follows: (1) GC patients were hospitalized for surgical treatment and did not had previously received antitumor therapy for GC; (2) BGD patients were hospitalized for the endoscopic or medical treatment of benign gastric diseases, including gastrointestinal stromal tumor (GIST) (only those with low or very low risk), gastric polyp, leiomyoma, ectopic pancreas, chronic gastritis, and peptic ulcer; (3) all patients were definitively diagnosed by pathology. Those whose blood laboratory results might be affected by disease or treatment were excluded: (1) with a history of blood transfusion in the past three months; (2) with malignant tumors of other organs; (3) with hematologic diseases or acute infectious diseases likely to affect the results of blood cell analysis. The HC group consisted of healthy individuals who underwent a health examination and did not have a history of gastric disease, epigastric symptoms, or abnormal routine laboratory and imaging findings. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
Collection of serum samples and clinical data
Leftover serum specimens (originally drawn for blood biochemistry) of the subjects were collected from the clinical laboratory department and stored at -80 °C. Clinical data of the subjects were collected from the hospital information system, including demographic data (age and sex), clinical laboratory data (blood cell analysis and conventional serum tumor markers), and pathological data (such as tumor histological type, pathological TNM tumor stage).
Measurement of serum ILF2 levels
Serum levels of ILF2 protein were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (SAB, USA) according to the manufacturer’s instructions. Briefly, as follows: 100 µL of serum diluted 1:50 was incubated with capture antibodies in a 96-well plate at 37 °C for 2 h, followed by the addition of 100 µL of biotinylated antibody and incubation for 1 h, then the avidin conjugated to horseradish peroxidase (HRP) was added and incubated for 1 h after three washes. The liquid was discarded and the wells were washed five times. Tetramethylbenzidine (TMB) substrate was then added and incubated for 15 min in the dark, followed by the measurement of optical density (OD) values of each well at 450 nm using a JS-THERMO Varioskan Flash (Thermo Fisher Scientific, USA). Simultaneously, a standard curve was generated and used to determine the concentration of ILF2 in serum based on the OD values.
Statistical analysis
Data analysis was performed using R studio software (version 4.4.0). For numerical variables, Student t test or Wilcoxon rank sum test was utilized for comparisons between two groups, and one-way ANOVA or Kruskal-Wallis test was applied for comparisons between three groups, depending on data distribution and homogeneity of variance. Categorical variables were analyzed using chi-squared test, continuity-corrected chi-squared test, or Fisher’s exact test depending on data characteristics. The receiver operating characteristic (ROC) curve was used to analyze the diagnostic value of the variables, and the area under the curve (AUC) and diagnostic validity metrics (sensitivity, specificity, accuracy, etc.) were used to evaluate the diagnostic performance. The two-tailed p-value was used, and a p-value < 0.05 was considered statistically significant.
Results
Clinical characteristics of the subjects
A total of 199 Chinese subjects and their clinical data were collected, including 99 cases of GC, 49 cases of BGD, and 51 cases of HC. GC consisted mainly of tubular adenocarcinoma (TAC) (75.8%) and poorly cohesive carcinoma (PCC) (20.2%); benign gastric diseases included low or very low risk gastrointestinal stromal tumor (GIST) (n = 17, 34.7%), gastric polyp (n = 16, 32.6%), leiomyoma (n = 7, 14.3%), chronic gastritis (n = 4, 8.2%), ectopic pancreas (n = 3, 6.1%), and gastric ulcer (n = 2, 4.1%). Table 1 shows the clinical data of these subjects. There were significant differences in red blood cell count (RBC), hemoglobin (Hb), hematocrit (HCT), red blood cell distribution width coefficient of variation (RDW), and CEA among the three groups.
Table 1.
Demographic, clinical and pathological characteristics of the subjects
| Result [n (%), Mean ± SD, or Median (interquartile range)] | p | ||||||
|---|---|---|---|---|---|---|---|
| n | GC | n | BGD | n | HC | ||
| Sex, Male/Female | 99 |
69 (69.7) / 30 (30.3) |
49 |
22 (44.9) / 27 (55.1) |
51 |
22 (43.1) / 29 (56.9) |
0.001 |
| Age (year) | 99 |
65.0 (56.0, 71.0) |
49 |
53.0 (48.0, 60.0) |
51 |
33.0 (25.0, 39.5) |
< 0.001 |
| Blood cell analysis | |||||||
| WBC (×109∕L) | 99 | 5.6 ± 1.7 | 49 | 5.7 ± 1.4 | 51 | 5.8 ± 1.2 | 0.606 |
| RBC (×1012∕L) | 99 | 4.1 ± 0.7 | 49 | 4.5 ± 0.7 | 51 | 4.8 ± 0.5 | < 0.001 |
| Hb (g/L) | 99 | 121.4 ± 23.1 | 49 | 134.4 ± 18.9 | 51 | 140.8 ± 25.1 | < 0.001 |
| HCT (%) | 99 | 0.37 ± 0.07 | 49 | 0.41 ± 0.05 | 50 | 0.43 ± 0.04 | < 0.001 |
| MCV (fl.) | 99 | 89.9 ± 8.2 | 49 | 89.3 ± 7.1 | 51 | 89.9 ± 6.6 | 0.896 |
| MCH (pg) | 99 | 29.4 ± 3.5 | 49 | 29.4 ± 3.0 | 51 | 30.3 ± 1.9 | 0.067 |
| MCHC (g/L) | 99 | 325.1 ± 16.6 | 49 | 328.2 ± 13.0 | 51 | 328.8 ± 47.4 | 0.677 |
| RDW (%) | 99 | 14.0 ± 2.4 | 49 | 13.3 ± 1.6 | 51 | 12.7 ± 0.6 | < 0.001 |
| PLT (×109/L) | 99 | 227.6 ± 86.6 | 48 | 239.9 ± 67.5 | 51 | 226.3 ± 57.7 | 0.523 |
| Tumor markers | |||||||
| AFP (ng/mL) | 83 | 2.5 (1.7, 3.8) | 46 | 2.6 (1.9, 3.4) | 26 | 2.1 (1.8, 2.9) | 0.499 |
| CEA (ng/mL) | 97 | 2.1 (1.4, 3.2) | 46 | 1.5 (0.9, 2.3) | 27 | 1.3 (0.7, 1.7) | < 0.001 |
| CA19-9 (µg/mL) | 97 | 9.3 (6.0, 13.6) | 48 | 10.7 (7.2, 17.1) | 26 | 6.8 (5.4, 11.8) | 0.054 |
| CA125 (µg/mL) | 96 | 9.5 (6.8, 14.5) | 47 | 9.5 (7.3, 13.0) | 26 | 12.9 (8.2, 19.4) | 0.238 |
| Lauren’s type | 96 | / | / | / | |||
| Intestinal | 72 (75.0) | / | / | ||||
| Diffuse | 16 (16.7) | / | / | ||||
| Mixed | 8 (8.3) | / | / | ||||
| Histological type | 99 | / | / | / | |||
| TAC | 75 (75.8) | / | / | ||||
| PCC | 20 (20.2) | / | / | ||||
| MAC | 3 (3.0) | / | / | ||||
| PAC | 1 (1.0) | / | / | ||||
| pTNM stage | 97 | / | / | / | |||
| I | 43 (44.3) | / | / | ||||
| II | 19 (19.6) | / | / | ||||
| III | 33 (34.0) | / | / | ||||
| IV | 2 (2.1) | / | / | ||||
GC, gastric cancer; BGD, benign gastric disease; HC, healthy control; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width (coefficient of variation); PLT, platelet; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19 − 9; CA125, cancer antigen 125; TAC, tubular adenocarcinoma; PCC, poorly cohesive carcinoma; MAC, mucinous adenocarcinoma; PAC, papillary adenocarcinoma; pTNM, pathological TNM
Serum levels of ILF2 protein
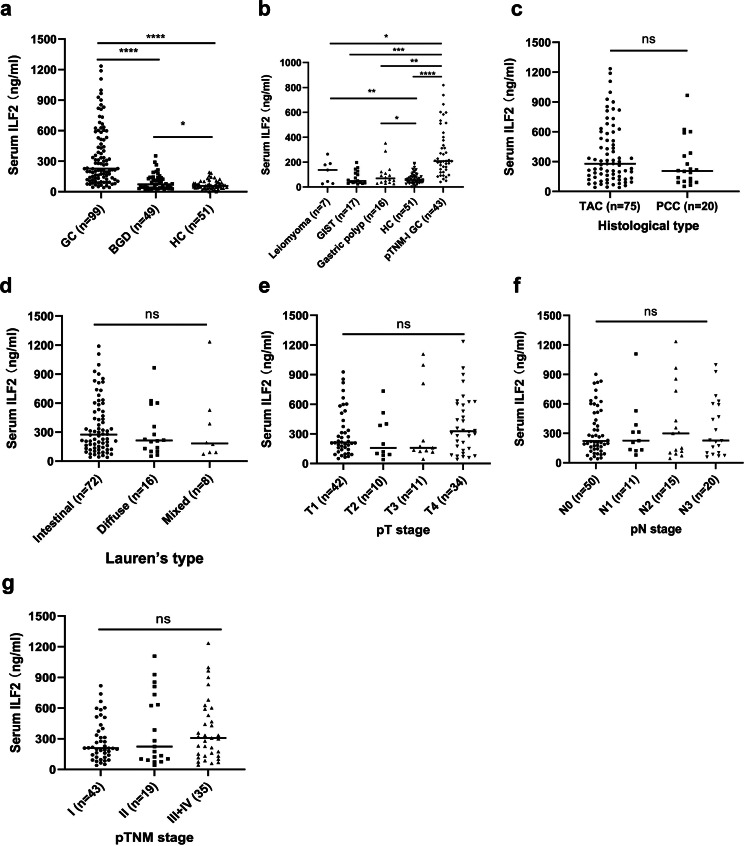
The serum ILF2 protein level [median (interquartile range)] in the GC group was 227.8 (131.2, 521.8) ng/mL, which was significantly higher than that in the BGD group [72.0 (36.5, 127.4) ng/mL] and the HC group [56.8 (33.9, 84.7) ng/mL] (p < 0.001) (Fig. 1a). In addition, the ILF2 levels were higher in the BGD group than in the HC group (p < 0.05). In various types of BGD (more than five cases), serum ILF2 levels were significantly lower than that in TNM stage I GC, but similar to the HC group (Fig. 1b), with 213.5 (147.4, 420.8) ng/mL in pTNM stage I GC, 137.2 (35.9, 183.1) ng/mL in leiomyoma, 49.29 (33.9, 111.8) ng/mL in GIST, and 68.71 (38.4, 112.8) ng/mL in gastric polyps. Additionally, serum ILF2 levels were higher in the leiomyoma and gastric polyp subgroups than in the healthy control group (p < 0.05). However, the ILF2 levels did not differ significantly within different histological types, Lauren’s types, pT stages, pN stages, and pTNM stages (Fig. 1c-g).
Fig. 1.
Serum ILF2 protein levels in different groups of subjects and subgroups of gastric cancer. a: ILF2 levels in three groups of subjects; b: ILF2 levels in different types of BGD and their comparisons with early stage GC and HC groups; c-g ILF2 levels in various subgroups of gastric cancer. In the subgroup analysis, the following subgroups were not shown due to small sample size: chronic gastritis (n = 4), ectopic pancreas (n = 3) and gastric ulcer (n = 2) for BGD (b), and MAC (n = 3) and PAC (n = 1) for histological type (c); there were missing samples in the following subgroups: 3 cases for Lauren’s type (d), 2 cases for pT stage (e), 3 cases for pN stage (f), and 2 cases for pTNM stage (g). ILF2, interleukin enhancer-binding factor 2; GC, gastric cancer; BGD, benign gastric disease; HC, healthy control; GIST, gastrointestinal stromal tumor; TAC, tubular adenocarcinoma; PCC, poorly cohesive carcinoma; pT, pathological T; pN, pathological N; pTNM, pathological TNM. *p < 0.05; **** p < 0.0001; ns, no significance
Diagnostic performance of ILF2 protein in GC
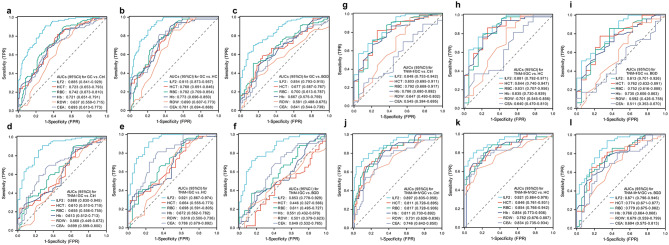
Using the ROC curve, we analyzed the diagnostic value of ILF2 and other significant blood laboratory indicators for GC. The results showed that ILF2 had high AUCs in differentiating GC patients, including early GC, from BGD patients and HC individuals, outperforming HCT, RBC, Hb, RDW and CEA, and the AUCs were similar between different stages of GC (Fig. 2).
Fig. 2.
Receiver operating characteristic (ROC) curves of serum ILF2 protein and significant blood laboratory indicators for the diagnosis of GC. a-c: Total GC group vs. control, HC and BGD groups; d-f: TNM-I GC subgroup vs. control, HC and BGD groups; g-i: TNM-II GC subgroup vs. control, HC and BGD groups; j-l: TNM-III + IV GC subgroups vs. control, HC and BGD groups. GC, gastric cancer; BGD, benign gastric disease; HC, healthy control; AUC, area under the curve; Ctrl, control (BGD + HC). ILF2, interleukin enhanced-binding factor 2; HCT, hematocrit; RBC, red blood cell; Hb, hemoglobin; RDW, red blood cell distribution width (coefficient of variation); CEA, carcinoembryonic antigen
Independence and synergy of serum ILF2 protein in the diagnosis of GC
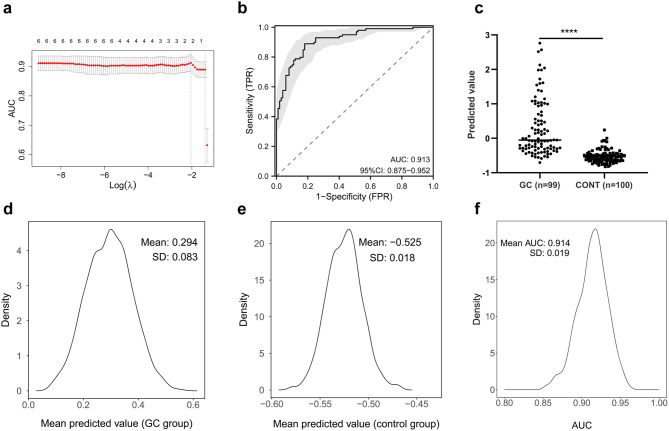
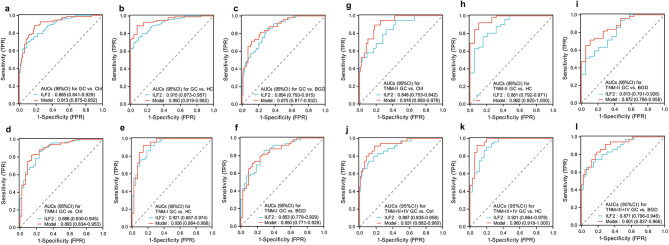
The least absolute shrinkage and selection operator (LASSO) logistic regression analysis was performed using the “glmnet” R package to evaluate the independence of serum ILF2 and its synergic effect with significant blood laboratory indicators (HCT, RBC, Hb, RDW, and CEA) in the diagnosis of GC, in which 10-fold cross-validation and bootstrap resampling analyses were utilized to assess the robustness of the results. The LASSO logistic regression analysis identified two key indicators (Fig. 3a), ILF2 and RBC, and resulted in a diagnostic model: Y = 0.003ILF2–0.158RBC, which had good discriminatory power between the GC group and the control group (Fig. 3b, c). The mean predicted values of the bootstrapped samples showed a normal distribution and a small standard deviation, especially in the control group (Fig. 3d, e). The mean AUC of the bootstrapped samples was high (0.914) and its standard deviation was very small (0.019), with a coefficient of variation of only 2.1% (Fig. 3f). The model outperformed ILF2 in overall diagnostic performance for GC (Fig. 4a-c). The improved diagnostic performance of the model was observed in TNM-II and TNM-III + IV GC, but not in TNM-I GC (Fig. 4d-l). These results suggest that ILF2 is independent of the above blood indicators in the diagnosis of GC, and the combination of ILF2 with RBC improves the performance in the diagnosis of advanced GC and shows good robustness in the internal validation by bootstrap resampling.
Fig. 3.
Variable selection and internal validation of the model. a: Variable selection using the least absolute shrinkage and selection operator logistic regression and 10-fold cross-validation; b: The receiver operating characteristic curve of the model; c: Predicted values of the model for the GC and control (BGD + HC) groups; d, e: The distribution of mean predicted values of bootstrapped samples in the GC and control groups; f: The distribution of AUC values of bootstrapped samples. AUC, area under the curve; GC, gastric cancer; CONT, control; BGD, benign gastric disease; HC, health control; **** p < 0.0001
Fig. 4.
Receiver operating characteristic (ROC) curves of serum ILF2 protein and the model for the diagnosis of GC. a-c: Total GC group vs. control, HC and BGD groups; d-f: TNM-I GC subgroup vs. control, HC and BGD groups; g-i: TNM-II GC subgroup vs. control, HC and BGD groups; j-l: TNM-III + IV GC subgroup vs. control, HC and BGD groups. AUC, area under the curve; Ctrl, control (BGD + HC); GC, gastric cancer; BGD, benign gastric disease; HC, healthy control; ILF2, interleukin enhancer-binding factor 2
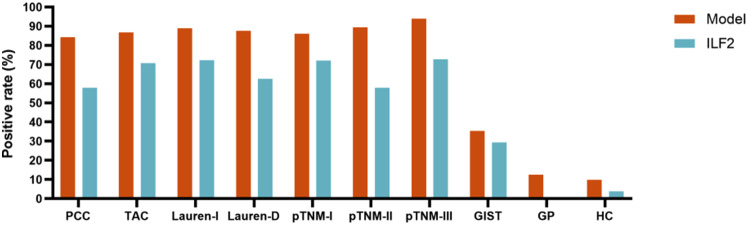
The diagnostic accuracy metrics of the model, ILF2, and RBC (with the best diagnostic performance among these blood indicators) for the diagnosis of GC were calculated and shown in Table 2, and the model exhibited improved diagnostic accuracy for GC compared with ILF2 or RBC. The positive rates of the model and ILF2 were 84-94% and 57-73%, respectively, in various GC subgroups (with more than 15 cases), but were very low in the gastric polyp subgroup and the HC group (Fig. 5).
Table 2.
Diagnostic validity metrics of the model, ILF2 protein and RBC for GC
| Cut-off value | SEN (%) |
SPE (%) |
ACC (%) |
PPV (%) |
NPV (%) |
PLR | NLR | DOR | |
|---|---|---|---|---|---|---|---|---|---|
| Model | -0.43 | ||||||||
| GC vs. Ctrl | 88.9 | 82.0 | 85.4 | 83.0 | 88.2 | 4.94 | 0.14 | 35.29 | |
| GC vs. BGD | 88.9 | 71.4 | 83.1 | 86.3 | 76.1 | 3.11 | 0.16 | 19.44 | |
| GC vs. HC | 88.9 | 92.2 | 90.0 | 95.7 | 81.0 | 11.33 | 0.12 | 94.42 | |
| ILF2 | 158.30 (ng/mL) | ||||||||
| GC vs. Ctrl | 69.7 | 91.0 | 80.4 | 88.5 | 75.2 | 7.74 | 0.33 | 23.45 | |
| GC vs. BGD | 69.7 | 85.7 | 75.0 | 90.8 | 58.3 | 4.88 | 0.35 | 13.94 | |
| GC vs. HC | 69.7 | 96.1 | 78.7 | 97.2 | 62.0 | 17.77 | 0.32 | 55.53 | |
| RBC | 4.11 (×1012∕L) | ||||||||
| GC vs. Ctrl | 50.5 | 88.0 | 69.3 | 80.6 | 64.2 | 4.21 | 0.56 | 7.52 | |
| GC vs. BGD | 50.5 | 85.7 | 62.2 | 87.7 | 46.2 | 3.54 | 0.58 | 6.10 | |
| GC vs. HC | 50.5 | 90.2 | 64.0 | 90.9 | 48.4 | 5.15 | 0.55 | 9.36 |
SEN, sensitivity; SPE, specificity; ACC, accuracy; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio. ILF2, interleukin enhancer-binding factor 2; GC, gastric cancer; Ctrl, control (BGD + HC); BGD, benign gastric disease; HC, healthy control. RBC, red blood cell
Fig. 5.
Positive rates of the model and ILF2 protein in the GC and BGD subgroups and the HC group (only subgroups with more than 15 cases are shown). ILF2, interleukin enhancer binding factor 2; PCC, poorly cohesive carcinoma; TAC, tubular adenocarcinoma; Lauren-I, Lauren intestinal type; Lauren-D, Lauren diffuse type; GIST, gastrointestinal stromal tumor; GP, gastric polyp; HC, healthy control
Discussion
In the present study, we further investigated the diagnostic value of serum ILF2 protein for gastric cancer based on our preliminarily findings. The levels of ILF2 in the serum samples of GC and BGD patients and HC individuals were determined by ELISA and were significantly elevated in the GC group compared with the BGD and HC groups, and showed good diagnostic value for gastric cancer, including early-stage gastric cancer, and moreover, the diagnostic value of ILF2 was independent of conventional laboratory indicators and significantly improved in combination with RBC.
Currently, the early diagnosis of GC is suboptimal, leading to frequent diagnosis at advanced stages [23] and a median overall survival of less than one year [24]. We evaluated the serum levels and diagnostic performance of ILF2 protein in different stages of GC and found that ILF2 was valuable for the early detection of GC. The serum levels of ILF2 were not significantly different among TNM stages of GC and demonstrated similar diagnostic performance between early and advanced GC, with an AUC of 0.888 (95%CI 0.830–0.945) for TNM-I stage GC and much better than the simultaneously analyzed significant blood indicators (HCT, RBC, HB, RDW, and CEA, their AUCs ranging from 0.560 to 0.699). Compared with previous reports, ILF2 is also superior to some novel serum protein biomarkers for the diagnosis of early GC, such as anosmin 1 (ANOS1) (AUC = 0.713) [25], mRNA export factor (RAE1) specific autoantibody (AUC = 0.745) [26], and inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) (AUC = 0.839) [27].
Anemia is frequent and has some diagnostic and prognostic value in GC patients [28, 29]. In addition, traditional serum tumor markers (CEA, CA19-9, etc.) are widely used in the clinical detection of GC [6, 8], although their sensitivity and specificity are limited [30]. Therefore, in the present study, we simultaneously evaluated the diagnostic value of RBC-related indicators and traditional serum tumor markers and compared them with ILF2 protein. We found that RBC and some of its derivatives (HCT, Hb, and RDW) had diagnostic value for GC, especially for advanced GC. Among the traditional serum tumor markers, only CEA showed weak value for the diagnosis of GC. However, both RBC-related indicators and traditional serum tumor markers are inferior to ILF2 in diagnostic performance.
In order to understand the independence and synergy of ILF2 protein with RBC-related indicators and CEA in GC diagnosis, we used multivariate analysis (LASSO regression) to select variables from ILF2 and blood indicators for the establishment of a diagnostic model, and found that only ILF2 and RBC remained in the model, indicating that ILF2 is an independent and synergistic indicator for the diagnosis of GC. The diagnostic model improved the diagnostic performance for GC compared to single ILF2 and RBC, especially for advanced GC, with AUC 0.893 for TNM-I GC and > 0.9 for advanced GC, and positive rates approximately 90% for GC in different conditions, which was good enough for the simple model with only two variables. Compared with some of the reported diagnostic models, our model has either fewer variables or higher performance, or both [31–34]. The high diagnostic performance and ease of application indicate that our model has promising clinical usefulness.
The traditional modeling approach is to randomly divide subjects into a training set and a test set, where the training set is used to construct a diagnostic model and the test set is used to internally validate the generalizability of the model. Due to insufficient sample size, we did not use this training-and-test approach in our modeling, but instead built the model using the full dataset and internally validated the model using the bootstrap resampling approach. The internal validation by bootstrap resampling showed good robustness of the model at different levels (Fig. 3d-f). It has been reported that modeling with bootstrap validation can yield a better model than the traditional training-and-test method [35], while our internal validation method by bootstrap resampling in this study provides an alternative method for evaluating diagnostic models built in a relatively small cohort. However, external validation in an independent case cohort is essential to assess whether new diagnostic models are overestimated.
ILF2 protein was first identified as a 45 kDa transcription factor (also known as NF45) required for the expression of the interleukin-2 gene in T cells [36, 37]. Subsequent studies have shown that ILF2, as a component of the ILF2-ILF3 complex, is involved in various cellular processes and functions in different cell types, such as regulating mRNA abundances [38], mRNA processing and translation [39], DNA break repair [40], regulation of microRNA processing [41, 42], and regulation of cell growth during mitosis [43]. Notably, ILF2 gene and/or protein are involved in cancer development and progression through multiple mechanisms, such as regulating cell cycle and apoptosis [44, 45], participating in tumor metabolism [46], and maintaining tumor mitochondrial homeostasis [47]. In GC, ILF2 protein was overexpressed in tumor tissues and closely associated with tumor metastasis and poor prognosis [48, 49], and was involved in the DNA damage response mechanism of GC [50]. Our previous study showed that the expression level of ILF2 could regulate the growth of GC cells [22]. All these findings support ILF2 as a valuable biomarker for GC.
In our previous study, we analyzed serum ILF2 protein levels and its diagnostic performance in a small sample consisting only of GC and HC. In the present study, we not only increased the sample size of GC and HC, but also added benign gastric disease controls, which resulted in the evaluation of the diagnostic value of ILF2 for GC in a more representative study sample. However, the sample size of this study is still relatively small and further studies with a larger sample size and a more representative BGD group are warranted. Additionally, the fact that this study was conducted on inpatients may not be representative of the composition of gastric patients in the real world, which may introduce bias into the study results. Furthermore, the diagnostic performance of serum ILF2 protein and the model may be overestimated due to the lack of validation in independent cohorts. Therefore, multi-center and multi-cohort validation studies are needed to more comprehensively and accurately evaluate the value of ILF2 and the model in GC detection.
Conclusions
In conclusion, this study validated elevated levels of ILF2 protein in the serum of patients with GC and demonstrated that ILF2 had good diagnostic value for GC, including early and advanced GC, outperforming conventional hematological indicators (serum tumor markers and RBC-derived indicators). Furthermore, the diagnostic performance of ILF2 was independent of and synergistic with conventional hematological indicators, and the combination of ILF2 with RBC could significantly improve the diagnostic efficiency. As a promising novel serum diagnostic biomarker for GC, ILF2 deserves further studies to validate its performance in the diagnosis of GC.
Acknowledgements
This work was supported by the Key Laboratory Project of Digestive Diseases in Jiangxi Province (2024SSY06101), and Jiangxi Clinical Research Center for Gastroenterology (20223BCG74011).
Abbreviations
- AFP
Alpha-fetoprotein
- AUC
Area under the curve
- BGD
Benign gastric disease
- CA125
Cancer antigen 125
- CA19-9
Carbohydrate antigen 19 − 9
- CA242
Carbohydrate antigen 242
- CA72-4
Carbohydrate antigen 72 − 4
- CEA
Carcinoembryonic antigen
- CEACAM5
Carcinoembryonic antigen-related cell adhesion molecule 5
- CTC
Circulating tumor cells
- ctDNA
Circulating tumor DNA
- ELISA
Enzyme-linked immunosorbent assay
- G-17
Gastrin-17
- GC
Gastric cancer
- GIST
Gastrointestinal stromal tumor
- GKN1
Gastrokine 1
- Hb
Hemoglobin
- HC
Healthy control
- HCT
Hematocrit
- HRP
Horseradish peroxidase
- ILF2
Interleukin enhancer-binding factor 2
- LASSO
Least absolute shrinkage and selection operator
- OD
Optical density
- PCC
Poorly cohesive carcinoma
- PG
Pepsinogen
- RBC
Red blood cell count
- RDW
Red blood cell distribution width coefficient of variation
- ROC
Receiver operating characteristic
- SKAP1
Src kinase-associated phosphoprotein 1
- TAC
Tubular adenocarcinoma
- TMB
Tetramethylbenzidine
Author contributions
KHZ, TW and SSL designed the study. SSL conducted the study. MSL and JKW participated in the experiments. DFG, QW and YHL participated in the data analysis. SSL drafted the manuscript, and KHZ, TW revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82160494).
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This research was approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University. All examinations in humans were conducted according to the Declaration of Helsinki and its amendments. All procedures performed in studies involving human participants were approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University. All methods were carried out in accordance with relevant guidelines and regulations. The Ethics Committee of the First Affiliated Hospital of Nanchang University waived the requirement for patient informed consent because the assay for ILF2 used previously collected frozen leftover serum specimens from biochemical assays, not specifically drawn blood specimens.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ting Wang, Email: tingwang@ncu.edu.cn.
Kun-He Zhang, Email: khzhang@ncu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA-CANCER J CLIN. 2021;2021/5(1):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Zheng R, Zhang S, Chen R, Wang S, Sun K et al. Gastric and esophageal cancer in China 2000 to 2030: Recent trends and short-term predictions of the future burden. CANCER MED-US. 2022 2022/4/1;11(8):1902-12. [DOI] [PMC free article] [PubMed]
- 3.Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J SURG ONCOL. 2022;2022/6(1):1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dooley CP, Larson AW, Stace NH, Renner IG, Valenzuela JE, Eliasoph J et al. Double-contrast barium meal and upper gastrointestinal endoscopy. A comparative study. ANN INTERN MED. 1984 1984/10/1;101(4):538 – 45. [DOI] [PubMed]
- 5.Xu Y, Zhang P, Zhang K, Huang C. The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer. BBA-REV CANCER. 2021;1876(2):188634. 2021/12/1. [DOI] [PubMed] [Google Scholar]
- 6.Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;2017/11(9):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanan Z, Juan W, Jun W, Xin M, Kejian W, Fangyu W. Application of serum gastric function markers and digestive tumor indices to the diagnosis of early gastric cancer and precancerous lesions. SAUDI MED J. 2023;2023/8(1):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;2014/1(1):26–33. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Yang Q, Liu B, Zhu Z. Serum proteomics for gastric cancer. CLIN CHIM ACTA. 2014;431:179–84. [DOI] [PubMed]
- 10.Ma S, Zhou M, Xu Y, Gu X, Zou M, Abudushalamu G, et al. Clinical application and detection techniques of liquid biopsy in gastric cancer. MOL CANCER. 2023;2023/1(11):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskandarion MR, Eskandarieh S, Tutunchi S, Shakoori FA, Shirkoohi R. Investigating the role of circulating tumor cells in gastric cancer: a comprehensive systematic review and meta-analysis. CLIN EXP MED. 2024;24(1):59. 2024/3/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M et al. Circulating tumor DNA as an early cancer detection tool. PHARMACOL THERAPEUT. 2020;207:107458. [DOI] [PMC free article] [PubMed]
- 13.Guan XL, Guan XY, Zhang ZY. Roles and application of exosomes in the development, diagnosis and treatment of gastric cancer. WORLD J GASTRO ONCOL. 2024;16(3):630–42. 2024/3/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang T, Mei L, Yang X, Sun T, Wang Z, Ji Y. Biomarkers of gastric cancer: current advancement. HELIYON. 2022;8(10):e10899. 2022/10/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Wu H, Chong W, Shang L, Jing C, Li L. Liquid biopsy in gastric cancer: predictive and prognostic biomarkers. CELL DEATH DIS. 2022;13(10):903. 2022/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng D, Zhang Y, Zhang R, Yi J, Dong J, Sha L, et al. Circulating proteins and metabolite biomarkers in gastric Cancer: a systematic review and Meta-analysis. ARCH MED RES. 2023;2023/2(154):124–34. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Yu Q, Li Y, Zhang M, Peng Z, Wang S et al. SKAP1 is a novel biomarker and therapeutic target for gastric Cancer: evidence from expression, functional, and bioinformatic analyses. INT J MOL SCI. 2023;24(14):11870. [DOI] [PMC free article] [PubMed]
- 18.Zhang L, Zhang C, Liu N. CEACAM5 targeted by miR-498 promotes cell proliferation, migration and epithelial to mesenchymal transition in gastric cancer. TRANSL ONCOL. 2022 2022/10/1;24:101491. [DOI] [PMC free article] [PubMed]
- 19.Shen H, Xiong K, Wu X, Cheng S, Lou Q, Jin H, et al. The diagnostic value of serum Gastrin-17 and Pepsinogen for Gastric Cancer Screening in Eastern China. GASTROENT RES PRACT. 2021;2021(2021/1/20):6894248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon JH, Park YG, Nam SW, Park WS. The diagnostic value of serum gastrokine 1 (GKN1) protein in gastric cancer. CANCER MED-US. 2019;8(12):5507–14. 2019/9/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repetto O, Vettori R, Steffan A, Cannizzaro R, De Re V. Circulating proteins as diagnostic markers in gastric Cancer. INT J MOL SCI. 2023;24(23):16931. [DOI] [PMC free article] [PubMed]
- 22.Liu SS, Wan QS, Lv C, Wang JK, Jiang S, Cai D, et al. Integrating trans-omics, cellular experiments and clinical validation to identify ILF2 as a diagnostic serum biomarker and therapeutic target in gastric cancer. BMC Cancer. 2024;24(1):465. 2024/4/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao W, Li Y, Zhu M, Li C, Li P. LncRNA NORAD Promotes Proliferation And Inhibits Apoptosis Of Gastric Cancer By Regulating miR-214/Akt/mTOR Axis. ONCOTARGETS THER. 2019 2019/1/20;12:8841-51. [DOI] [PMC free article] [PubMed] [Retracted]
- 24.Patel TH, Cecchini M. Targeted therapies in Advanced Gastric Cancer. CURR TREAT OPTION ON. 2020;21(9):70. [DOI] [PubMed]
- 25.Kanda M, Suh YS, Park DJ, Tanaka C, Ahn SH, Kong SH, et al. Serum levels of ANOS1 serve as a diagnostic biomarker of gastric cancer: a prospective multicenter observational study. Gastric Cancer. 2020;2020/3(123):203–11. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Q, He P, Zheng C, Chen Z, Qi S, Zhou D, et al. Identification and evaluation of novel serum autoantibody biomarkers for early diagnosis of gastric cancer and precancerous lesion. J CANCER RES CLIN. 2023;149(11):8369–78. 2023/9/1. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Jin J, Jing H, Lu Y, Zhu Q, Shu C et al. ITIH4 is a novel serum biomarker for early gastric cancer diagnosis. CLIN CHIM ACTA. 2021;523:365 – 73. [DOI] [PubMed]
- 28.Li WH, Zhang JY, Liu WH, Chen XX. Role of the initial degree of anaemia and treatment model in the prognosis of gastric cancer patients treated by chemotherapy: a retrospective analysis. BMC Cancer. 2020;20(1):414. 2020/5/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Liu Z, Gao G, Mo Y, Zhou H, Huang W et al. Efficacy of circulating microRNA-130b and blood routine parameters in the early diagnosis of gastric cancer. ONCOL LETT. 2021;22(4):725. [DOI] [PMC free article] [PubMed]
- 30.He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC GASTROENTEROL. 2013;13:87. [DOI] [PMC free article] [PubMed]
- 31.Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang L, et al. Six serum-based miRNAs as potential diagnostic biomarkers for gastric Cancer. CANCER EPIDEM BIOMAR. 2017;2017(2/1):188–96. [DOI] [PubMed] [Google Scholar]
- 32.Lee IS, Ahn J, Kim K, Okugawa Y, Toiyama Y, Hur H et al. A blood-based transcriptomic signature for noninvasive diagnosis of gastric cancer. BRIT J CANCER. 2021;125(6):846–53. [DOI] [PMC free article] [PubMed]
- 33.Chu LY, Wu FC, Guo HP, Xie JJ, Qu QQ, Li XH et al. Combined detection of serum EFNA1 and MMP13 as diagnostic biomarker for gastric cancer. SCI REP-UK. 2024;14(1):15957. [DOI] [PMC free article] [PubMed]
- 34.Yi Y, Nan R, Lu J, Liang D, Zhao S, Wang X et al. Screening of novel serum biomarkers for gastric cancer in coastal populations using a protein microarray. CANCER SCI. 2023;114(8):3396–410. [DOI] [PMC free article] [PubMed]
- 35.Brunelli A, Rocco G. Internal validation of risk models in lung resection surgery: bootstrap versus training-and-test sampling. J THORAC CARDIOV SUR. 2006;2006/6(1):1243–7. [DOI] [PubMed] [Google Scholar]
- 36.Marchesini M, Ogoti Y, Fiorini E, Aktas SA, Nezi L, D’Anca M, et al. ILF2 is a Regulator of RNA splicing and DNA damage response in 1q21-Amplified multiple myeloma. Cancer Cell. 2017;32(1):88–100. 2017/7/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kao PN, Chen L, Brock G, Ng J, Kenny J, Smith AJ et al. Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J BIOL CHEM. 1994;269(32):20691–9. [PubMed]
- 38.Hia F, Yang SF, Shichino Y, Yoshinaga M, Murakawa Y, Vandenbon A, et al. Codon bias confers stability to human mRNAs. EMBO REP. 2019;20(11):e48220. 2019/11/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J VIROL. 2006;2006(14):6936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shamanna RA, Hoque M, Lewis-Antes A, Azzam EI, Lagunoff D, Pe’Ery T, et al. The NF90/NF45 complex participates in DNA break repair via nonhomologous end joining. MOL CELL BIOL. 2011;2011(1):4832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto S, Aoki K, Higuchi T, Todaka H, Morisawa K, Tamaki N, et al. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. MOL CELL BIOL. 2009;2009(1):3754–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Li X, Wang C, Zhang M, Yang H, Lv K. lncRNA AK085865 promotes macrophage M2 polarization in CVB3-Induced VM by regulating ILF2-ILF3 complex-mediated miRNA-192 Biogenesis. MOL THER-NUCL ACIDS; 2020;21:441 – 51. [DOI] [PMC free article] [PubMed]
- 43.Guan D, Altan-Bonnet N, Parrott AM, Arrigo CJ, Li Q, Khaleduzzaman M et al. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. MOL CELL BIOL 2008;28(14):4629–41. [DOI] [PMC free article] [PubMed]
- 44.Liu Y, Li Z, Zhang J, Liu W, Guan S, Zhan Y, et al. DYNLL1 accelerates cell cycle via ILF2/CDK4 axis to promote hepatocellular carcinoma development and palbociclib sensitivity. BRIT J CANCER. 2024;2024/7(1131):243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng S, Jiang X, Ding C, Du C, Owusu-Ansah KG, Weng X et al. Expression and critical role of interleukin enhancer binding factor 2 in Hepatocellular Carcinoma. INT J MOL SCI. 2016;17(8):1373. [DOI] [PMC free article] [PubMed]
- 46.Xi Z, Huang H, Hu J, Yu Y, Ma X, Xu M, et al. LINC00571 drives tricarboxylic acid cycle metabolism in triple-negative breast cancer through HNRNPK/ILF2/IDH2 axis. J EXP CLIN CANC RES. 2024;43(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Liu Y, Chang J, Qi J, Liu R, Hou Y, et al. ILF2 cooperates with E2F1 to maintain mitochondrial homeostasis and promote small cell lung cancer progression. CANCER BIOL MED. 2019;2019/11(1164):771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Shen L, Zhang X, Chen Z, Huang P, Huang C, et al. LncRNA ELF3-AS1 inhibits gastric cancer by forming a negative feedback loop with SNAI2 and regulates ELF3 mRNA stability via interacting with ILF2/ILF3 complex. J EXP CLIN CANC RES. 2022;41(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin ZH, Jiang XW, Shi WB, Gui QL, Yu DF. Expression and clinical significance of ILF2 in gastric Cancer. DIS MARKERS. 2017;2017(1/20):2017–4387081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arai H, Wada R, Ishino K, Kudo M, Uchida E, Naito Z. Expression of DNA damage response proteins in gastric cancer: comprehensive protein profiling and histological analysis. INT J ONCOL. 2018;2018/3(1):978–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.